Post-dilatation improves stent apposition in patients with ST-segment elevation myocardial infarction receiving primary percutaneous intervention: A multicenter, randomized controlled trial using optical coherence tomography
Jun Jiang, Nai-liang Tian, Han-bin Cui, Chang-ling Li, Xian-bao Liu, Liang Dong, Yong Sun, Xiao-min Chen,Shao-liang Chen, Bo Xu, Jian-an Wang
1 Department of Cardiology, the Second Aff iliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
2 Department of Cardiology, Nanjing First Hospital, Nanjing, China
3 Department of Cardiology, Ningbo First Hospital, Ningbo, China
4 Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Beijing, China
KEY WORDS: ST-segment elevation myocardial infarction; Post-dilatation; Incomplete strut apposition; Optical coherence tomography
INTRODUCTION
Acute ST-segment elevation myocardial infarction(STEMI) is still the leading cause of death worldwide including China.[1]Drug eluting stent (DES) implantation with or without manual thrombus aspiration effectively opens infarct related arteries in acute STEMI patients.[2]However, the usual lipid rich and thrombus laden culprit lesions may lead to incomplete strut apposition (ISA) and higher rate of stent failure.[3]Post-dilatation effectively improves stent expansion and apposition but carries the risk of distal embolization and slow flow.[4]The present randomized trial assessing the rates of ISA and strut coverage with optical coherence tomography (OCT)tested the hypothesis that post-dilatation improves stent strut apposition in STEMI patients after manual thrombus aspiration (TA) and DES implantation during primary percutaneous coronary intervention (pPCI).
METHODS
Study design
The POST-STEMI study (POST-dilatation to improve outcomes in patients with acute ST-segment Elevation Myocardial Infarction undergoing primary percutaneous intervention after thrombus aspiration with optical coherence tomography assessment) was a prospective, randomized,controlled trial carried out in 3 Chinese hospitals. Written informed consents were acquired from all patients, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki was approved by the local ethics committee of each participating hospital.
Between July 30, 2014, and August 24, 2015, a total of 41 patients diagnosed with STEMI referred for pPCI meeting clinical and angiographic inclusion/exclusion criteria were enrolled among all patients with STEMI scheduled to receive PCI at any of the participating centers.Clinical inclusion criteria for the trial were: patients aged>18 years, admitted for STEMI with symptom duration >20 minutes and <12 hours; ST-segment elevation >1 mm in >2 contiguous leads, or new onset left bundle branch block;and provision of written informed consent. The angiographic inclusion criteria were: reference vessel size ≥2.5 mm and≤4.0 mm; Thrombolysis In Myocardial Infarction (TIMI)f low grade ≤1; and no severe tortuosity or calcif ication that would impede stent delivery or expansion.
Clinical exclusion criteria were: cardiogenic shock;contraindication to any of the trial medications; history of bleeding diathesis or known coagulopathy, intracerebral mass, aneurysm, hemorrhagic stroke; stroke or transient ischemic attack within 6 months or any permanent neurologic def icit; gastrointestinal bleed within 2 months, or major surgery within 6 weeks; recent or known platelet count< 100×109/L or hemoglobin level <10 g/dL; and scheduled surgical procedure that would necessitate discontinuation of ADP receptor antagonists during the first 6 months post enrollment. Angiographic exclusion criteria were: true bifurcation lesion requiring two-stent technique; left main lesion; in stent thrombosis; anticipated stent length > 38 mm.
Figure 1 summarizes the patient flowchart and study design. Patients were randomized into one of the two groups after manual thrombus aspiration and visually optimal Promus Element stent implantation (20 in the control group and 21 in the post-dilatation group). Angiogram criteria for optimal stent deployment were: post-stent diameter stenosis less than 30% based on the visual assessment by the physician. Randomization was conducted with sealed opaque consecutive envelopes containing the treatment group allocated to the patient and stratified by each center.There were no crossovers between groups.
Study endpoints
The primary endpoint was rate of ISA assessed by OCT at 7-month follow-up. ISA was defined as separation from the inner vessel wall > (stent strut thickness [81 μm for Promus Element stent] + ½thickness of the Blooming). Secondary endpoints were corrected TIMI frame count and f inal TIMI f low grade 3 immediately after pPCI procedure; and 12-month rates of major adverse cardiovascular events (MACE), target lesion restenosis (TLR), and target lesion failure (TLF).
Quantitative coronary angiography (QCA) and procedures
The pPCI procedure was performed under the guidance of pure fluoroscopy including thrombus aspiration and DES implantation according to the standard of care. In post-dilatation group a non-compliant balloon (size=stent diameter+0.25 mm) was used and inflated at pressure >16 atm and dilatation duration≥20 seconds. Using the QAngio XA analysis software(Medis, Leiden, the Netherlands), two independent analysts from an angiographic core laboratory (Fuwai Hospital, Beijing, China) finished the QCA analysis.The measurements were performed for each pair of orthogonal views and averaged.

Figure 1. Study f lowchart summarizing the study design and patient randomization.
Optical coherence tomography assessment
Baseline OCT was performed after Promus Element stent implantation in the control group and after non-compliant balloon post-dilatation in the post-dilatation group. Several OCT runs were made as needed until a satisfactory result was acquired as the f inal run. OCT images were approached using C7XR Fourier-Domain system (St. Jude Medical, St.Paul, MN, USA). The procedure was conducted with a 6 French guiding catheter. Each imaging procedure/pullback was preceded by intracoronary administration of 0.2 mg nitroglycerin. Coronary “safety” angiograms were performed in-between pullbacks to monitor for adverse events. The images were digitally saved in the console and analyzed by two independent operators blinded to the angiographic findings, and procedural strategy in an independent core laboratory (Fuwai Hospital, Beijing, China), using the QIvus version 2.2 software (Medis, Leiden, the Netherlands).Discordant OCT analyses were resolved by discussion.
Follow-up
Patients were planned to receive angiographic and OCT follow-up at 7 months, and clinical follow-up up to 12 months. Major adverse cardiovascular events, including death, recurrent MI, heat failure, stent thrombosis based on ARC definition, or target lesion revascularization were recorded.
Statistical analysis
Categorical variables were presented as number and percentage. Quantitative variables were presented as mean (standard deviation [SD]) for normally distributed variables, and median (interquartile range) for nonnormally distributed variables. Normality of continuous variables was verified using the Kolmogorov-Smirnov test. Quantitative data were compared using the Student t tests or Wilcoxon signed rank test, and qualitative variables using the χ2or Fisher exact tests.
Sample size was calculated based on an expected testing group ISA rate of 0.7% under the hypothesis of a control group 7-month ISA rate of 1.5%; test significance level (α)=0.05 (2-sided); power (1-β)=90%; control group strut: test group strut =1:1= 3571:3571; assumption of stent implant length ≥24 mm (26 segment /stent and 16 strut /ring); average stent strut=26×16 strut=416 strut / stent;expected rate of stent strut analysis attrition=25%; and expected rate of clinical follow-up attrition=22%, it was calculated that 15 patients would be needed in each group.Taking into account that patients may lost to follow-up,unqualif ied images not suitable for analysis, an additional 5 patients were added in each group, for a total of 40 patients.A two-sided P value of <0.05 was considered statistically signif icant. All analyses were performed using SPSS version 22.0 (IBM, Armonk, NY, USA).
RESULTS
Baseline clinical characteristics and pre-procedure and 7-month follow-up quantitative angiographic parameters were similarly distributed between control and postdilatation groups, except for total stent length (26.0 [SD 5.8]vs. 21.9 [SD 6.5], respectively, P=0.01) (Tables 1 and 2).
While average stent diameter, stent volume and malapposed area were not significantly different between the 2 groups post pPCI, ISA rate was significantly higher in control than in post-dilatation group (4.5% vs. 2.5%,P=0.04) (Table 3). At 7-month follow-up, 14 patients in the control group and 20 patients in the post-dilatation group underwent successful angiographic and OCT assessment.The baseline characteristics of the patients who completed angiographic and OCT assessment were similarly distributed between groups. At 7-month follow-up, control group vs.post-dilatation group had signif icantly greater ISA rate (1.8%vs. 0.7%, P<0.0001) and distal reference lumen area (8.8 [SD 1.5] vs. 6.8 [SD 2.6], P=0.03).
All patients completed 12-month follow-up without signif icant between-group differences in rates of MACE or its individual components (Table 4).
DISCUSSION
While optimal post-dilation is an independent predictor of freedom from TLF after bioresorbable vascular scaffold implantation,[6]its role in STEMI patients receiving metallic DES is less well established. In the present study of STEMIpatients, post-dilatation following thrombus aspiration and stent implantation in pPCI signif icantly improved ISA both acutely and at 7 months follow-up with similar rates of complications up to 12 months follow-up.

Table 1. Baseline clinical characteristics
ISA is associated with incomplete endothelialization and fibrin deposits and poor vessel healing after stent implantation thereby increasing stent thrombosis risk.[7,8]ISA is often associated with delayed strut coverage. In a porcine model, percentage of struts remaining uncovered at 1 week was lowest in well-apposed segments followed by malapposed segments and highest in non-apposed side branch struts, while neointimal coverage was thickest on apposed struts followed by malapposed struts and least on non-apposed side branch struts. At 1 week, only 1.3% of Orsiro struts (60 μm thickness) remained uncovered, while for Promus Element, Cypher and BVS with greater strut thickness, 6.6%, 48.4% and 16.2% of their struts remained uncovered respectively.[9]
Thinner struts would interfere less with endothelial cells thus allowing faster stent integration into the vessel wall and reendothelialization.[10]ISA varies among stents being for instance signif icantly lower for everolimus- than sirolimuseluting stent (0.7% vs. 2.3%) at 7 months after primary PCI in a study of STEMI patients.[11]The ISA rate of everolimuseluting stent in that study is similar to that of our postdilatation group (0.65%), but lower than that of our control group, possibly because post-dilatation was used in 81.8% of patients under intravascular ultrasound guidance in that study.
ISA can be “acute”, i.e., immediately after stent implantation, and “late”, i.e., months after implantation.[12]In serial intravascular imaging studies, late ISA is further classified into persistent or acquired.[13]In our study, acute ISA was higher in the control group than the post-dilatation group and persisted in both groups albeit to a significantly lower extent in the post-dilatation group at 7 months follow up. Thinner struts and endothelium friendly polymer may facilitate strut apposition and embedding of second generation DES. Based on earlier IVUS studies, acute ISA is considered benign relative to late acquired ISA;[14]however,persistence of some acute incomplete apposed struts may underlie late ISA.
Although OCT can more accurately assess the status of strut apposition or coverage, it remains quite challenging to compare struts at different time points.[15]Recently, contour plot OCT reconstruction allowed successful evaluation and comparison of spatial distribution patterns of strut coverageand apposition at the strut level at different time points.[16,17]Using combined IVUS and OCT examination, Guagliumi et al[18]demonstrated significantly higher presence and magnitude (i.e., area and distance) of ISA in DES patients with vs. without thrombosis. Furthermore, they proposed that positive remodeling (a mechanism that led to ISA) and uncovered stent struts (another mechanism that could be a consequence of ISA) are independent predictors of DES thrombosis. Using a new system, Agrawal et al[12]classif ied the mechanisms of stent strut malapposition as localized lumen enlargement, vessel asymmetry, stent undersizing,strut underexpansion and stent deployment issue. They found that stent undersizing produced the largest ISA and may have clinical consequences. This system is only good for the classification of acute ISA and may help to inform individualized strategy to prevent and address ISA. For example, stent undersizing could be avoided through preintervention intravascular imaging evaluation.
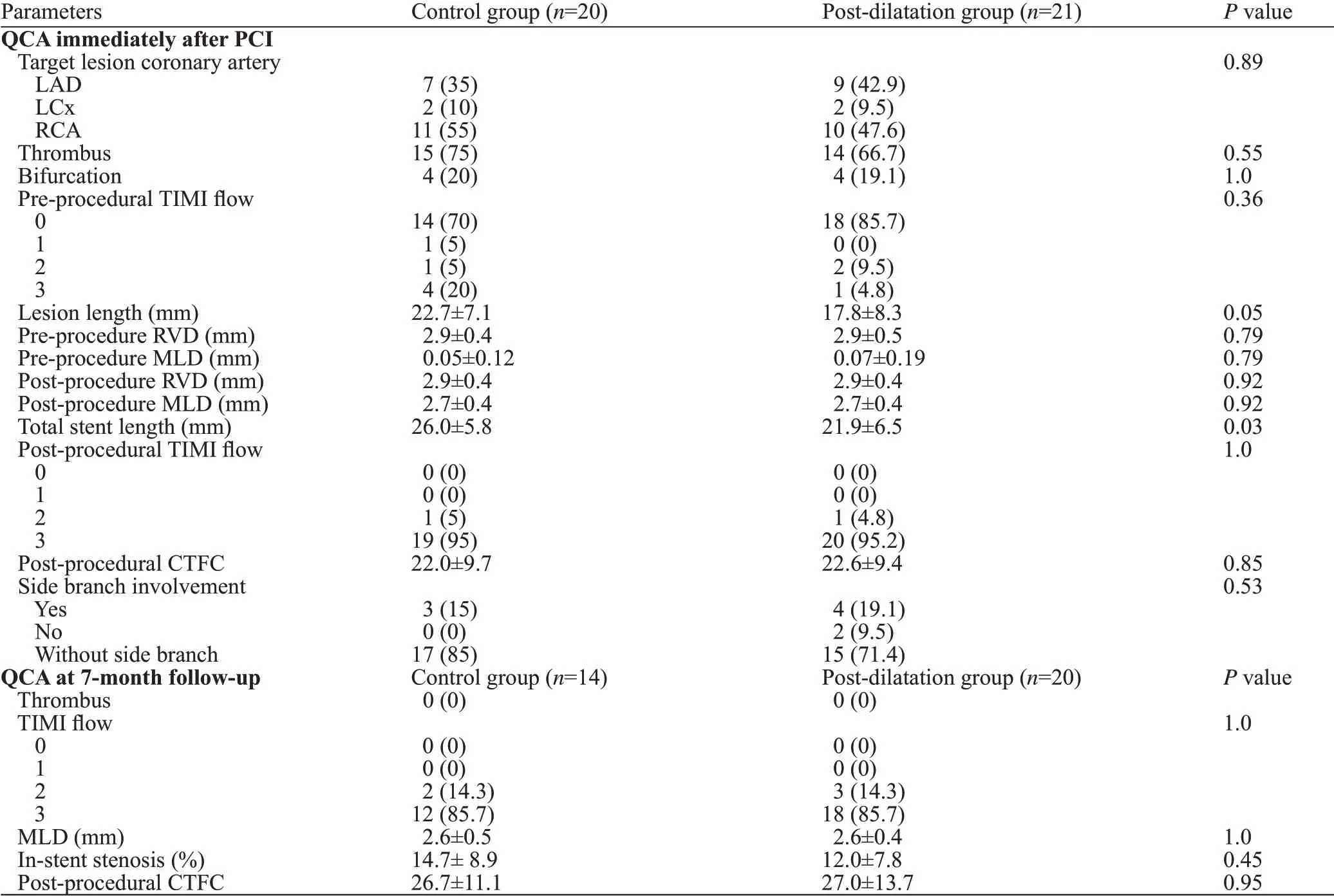
Table 2. Angiographic and procedural characteristics
Based on the mechanisms of acute ISA, several approaches can be adopted to minimize it. First, routine OCT guidance before and after stent implantation can help to choose the right stent size, find ideal landing zone and use post-dilatation to optimize stent expansion and apposition. Second, active utilization of new stent platforms that accommodate vessel remodeling over time (i.e., selfexpandable stents or biodegradable stents) and of new polymers or eluting drugs that lead to less inflammation(thus, less positive remodeling). In a substudy of the APPOSITION II trial, persistent and newly acquired ISA were more frequently observed in balloon-expandable stents than in self-expanding stents (33.9% vs.11.5%, P<0.01,14.8% vs. 2.7%, P<0.01, respectively). Newly acquired ISA was caused by tissue reabsorption, vasorelaxation and early recoil in balloon-expandable stents, and maybe only tissue reabsorption in self-expanding stents.[19]Third, high pressure post-dilatation with noncompliant balloons effectively improves ISA, especially under the guidance of OCT. In the present study, post-dilatation significantly improved ISA in STEMI patients even under routine angiographic guidance.Due to the high lipid and thrombus content in culprit lesions responsible for STEMI, post-dilatation may carry the risk of distal embolization and slow flow; however, this could be prevented by upstream manual thrombus aspiration. As shown in our study, post-procedural corrected TIMI frame count was similar between post-dilatation group and control group after suff icient manual thrombectomy. However, high pressure post-dilatation may also cause greater injury to the arterial wall resulting in more inflammation and a higher chance of restenosis. In the present study, despite lack of significant differences between post-dilatation group and control group in mean lumen area or area stenosis based on 7-month OCT analysis, distal reference lumen area was significantly smaller in post-dilatation group. Whether the improved ISA and smaller distal reference lumen area withpost-dilatation translates into long-term clinical benef its for STEMI patients warrants further study in large scale clinical trials.

Table 3. OCT f indings immediately after stent implantation and at 7-month follow-up
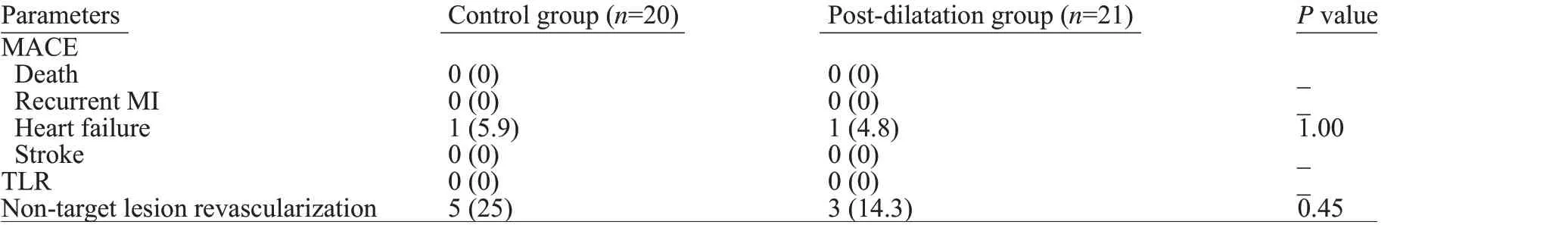
Table 4. Clinical outcomes at 12-month follow-up
The present study is limited by its relatively small sample size to assess safety of individual safety points, by its inclusion of only 3 centers in China, and by higher attrition rate in the control group for angiographic and OCT followup at 7 months.
CONCLUSIONS
Post-dilatation after manual thrombus aspiration in STEMI patients improves ISA both in the acute setting and at 7-month follow-up without impairing the f inal TIMI f low rate and with similar 12-month rates of MACE. Safety assessment in larger studies is warranted.
ACKNOWLEDGEMENTS
The authors thank Dr. Ming Zheng for his contribution to the completion of this study; and Zhongwei Sun for his advice on data analysis.
Funding:The POST-STEMI trial was funded by grants from National Natural Science Foundation of China (81100141 and 81570322 for JJ,81320108003 for JW), and jointly supported by Boston Scientif ic.
Ethical approval:The study was approved by the local ethics committee of each participating hospital.
Conf icts of interest:The authors have no conf licts of interest.
Contributors:JJ and JW contributed equally to this work. All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
 World journal of emergency medicine2020年2期
World journal of emergency medicine2020年2期
- World journal of emergency medicine的其它文章
- Availability and quality of procedural sedation and analgesia in emergency departments without emergency physicians: A national survey in the Netherlands
- Presenting patterns of dermatology conditions to an Australian emergency department
- Effect of neutrophil CD64 for diagnosing sepsis in emergency department
- Predictive role of interleukin-6 and CAT score in mechanical ventilation in patients with chronic obstructive pulmonary disease at the acute exacerbation stage in the emergency department
- Changes in peak inspiratory f ow rate and peak airway pressure with endotracheal tube size during chest compression
- Comparison of invasive dynamic blood pressure between superior mesenteric artery and common carotid artery in rats
