Probiotics in shellfish aquaculture
Einar Ringø
Norwegian College of Fishery Science,Faculty of Biosciences,Fisheries and Economics,UiT,The Arctic University of Norway,Tromsø9037,Norway
Keywords:
ABSTRACT
1.Introduction
In the 1970s and 1980s antibiotics were commonly used in disease control.However,the indiscriminate use of antibiotics used to treat infectious diseases led to selective pressure of antibiotic resistance,a property that may be transferred to other bacteria(Cabello,2006;Romero,Feijoó,&Navarrete,2012).Moreover,it is generally accepted that antibiotics administration in finfish and shellfish modulate the gut microbiota(Ringøet al.,2016),which in turn exerts negative effects on humans(Greenless,2013;Salyers,Gupta,&Wang,2014).Based on this fact,the European Union in 2003 banned the use of antibiotics in production.The use of probiotics is one of the alternative approaches to immunoprophylactic control in aquaculture,and is considered as a supplementary strategy or alternative to vaccines and chemicals.
There is a long history of health claiming microorganisms.According to Bottazzi(1983),the Roman historian Plinius in 76 B.C.recommended administration of fermented milk products for treating gastroenteritis.The wordprobioticstems from the Greek rootsproandbios,or“profile”(Schrezenmeir & de Vrese,2001),and several definitions of probiotics have been put forward since the first definition was given by Lilly and Stillwell(1965),but the most widely used is the definition by World Health Organisation's(WHO);“live microorganisms that when administrated in adequate amounts,confer a health benefit to the host”.During the last decades,several reviews have addressed on probiotics and their impacts in shellfish aquaculture as growth promoters,nutritional,environmental capacity,as immunostimulants and advantage as prophylactic against infectious diseases(Ayisi,Apraku,&Afriyie,2017;Cordero,Esteban,& Cuesta,2014;Farzanfar,2006;Hoseinifar,Dadar,van Doan,& Harikrishnan,2019;Hoseinifar,Sun,Wang,& Zhou,2018;Kuebutornye,Abarike,& Lu,2019;Kumar,Roy,Meena,& Sarkar,2016;Li et al.,2018;Ninawe & Selvin,2009;Shefat,2018;Soltani et al.,2019;van Hai et al.,2009a;van Hai and Fotedar,2010).
Even though information was presented in the above mention reviews,the present review address to present an update on probiotics in shellfish aquaculture,and on probiotics data not mention in the aforementioned reviews.In order to avoid overlaps,studies discussed in the aforementioned reviews are only briefly presented in the text and Tables.
In shellfish aquaculture,several probiotics species are used;Lactobacillus,Enterococcus,Bacillus,Aeromonas,Alteromonas,Arthrobacter,Bifidobacterium,Clostridium,Microbacterium,Paenibacillus,Phaeobacter,Pseudoalteromonas,Pseudomonas,Rhodosporidium,Roseobacter,Streptomyces andVibrio.To my knowledge,the first studies on the use of probiotics in shellfish aquaculture was carried out by Maeda and Liao(1992,pp.25-29)and Nogami and Maeda(1992),using bacterial strain PM-4 originally isolated from a crustacean culture pond,but since then numerous studies have been carried out(Table 1-3).
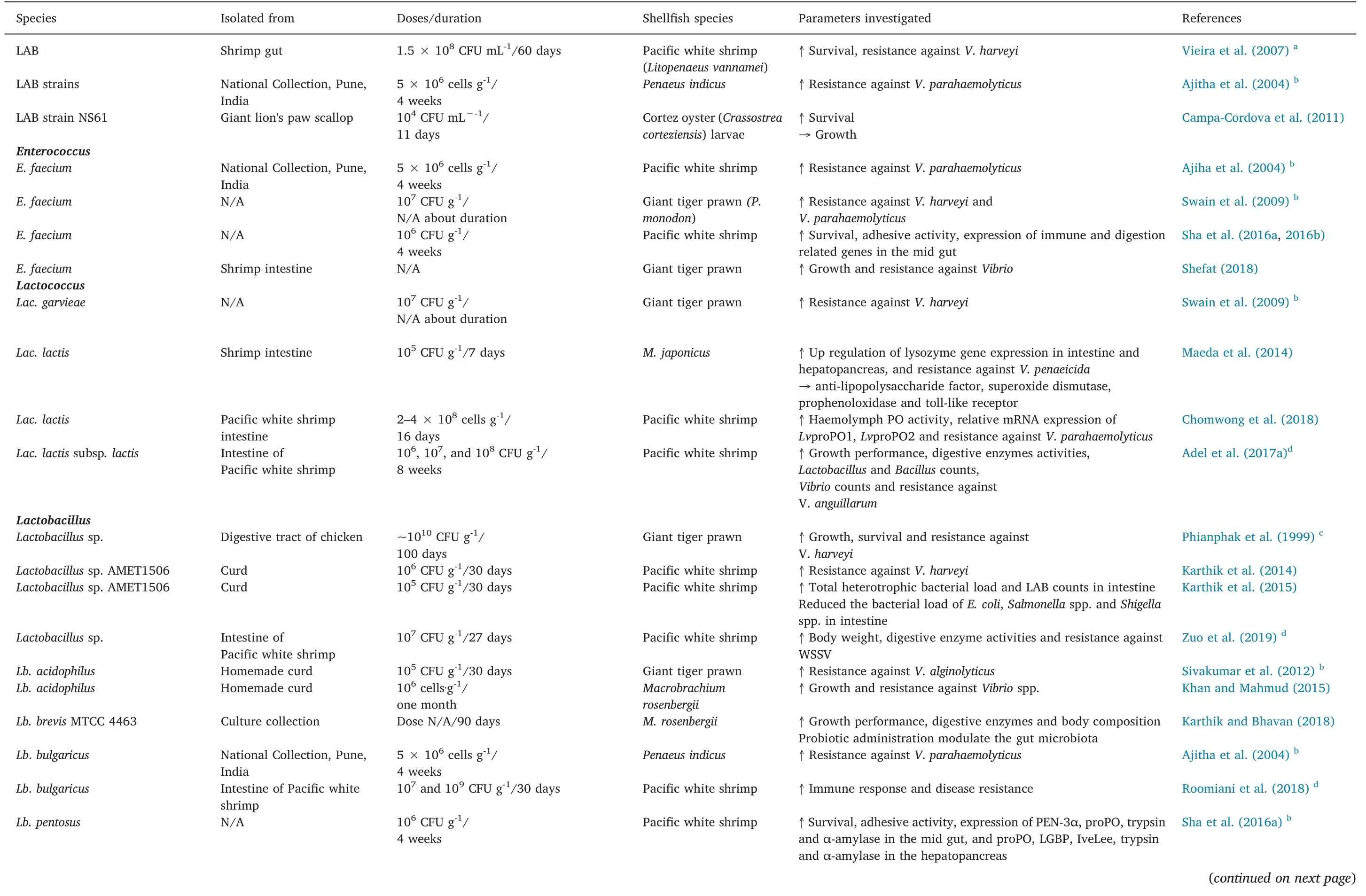
Table 1 Effect of lactic acid bacteria(LAB)on growth performance,immune response and disease resistance in shrimp culture.
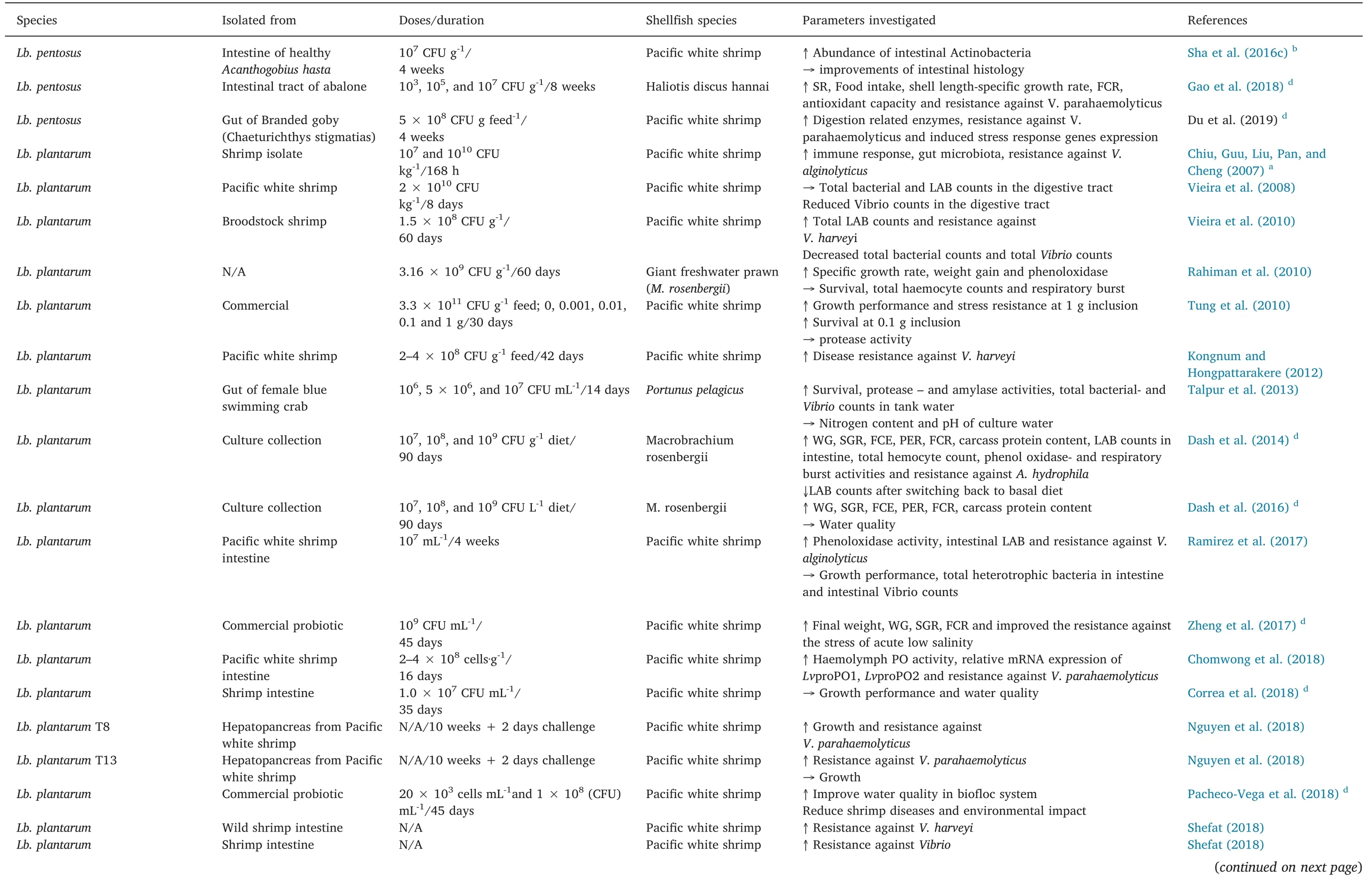
Table 1(continued)
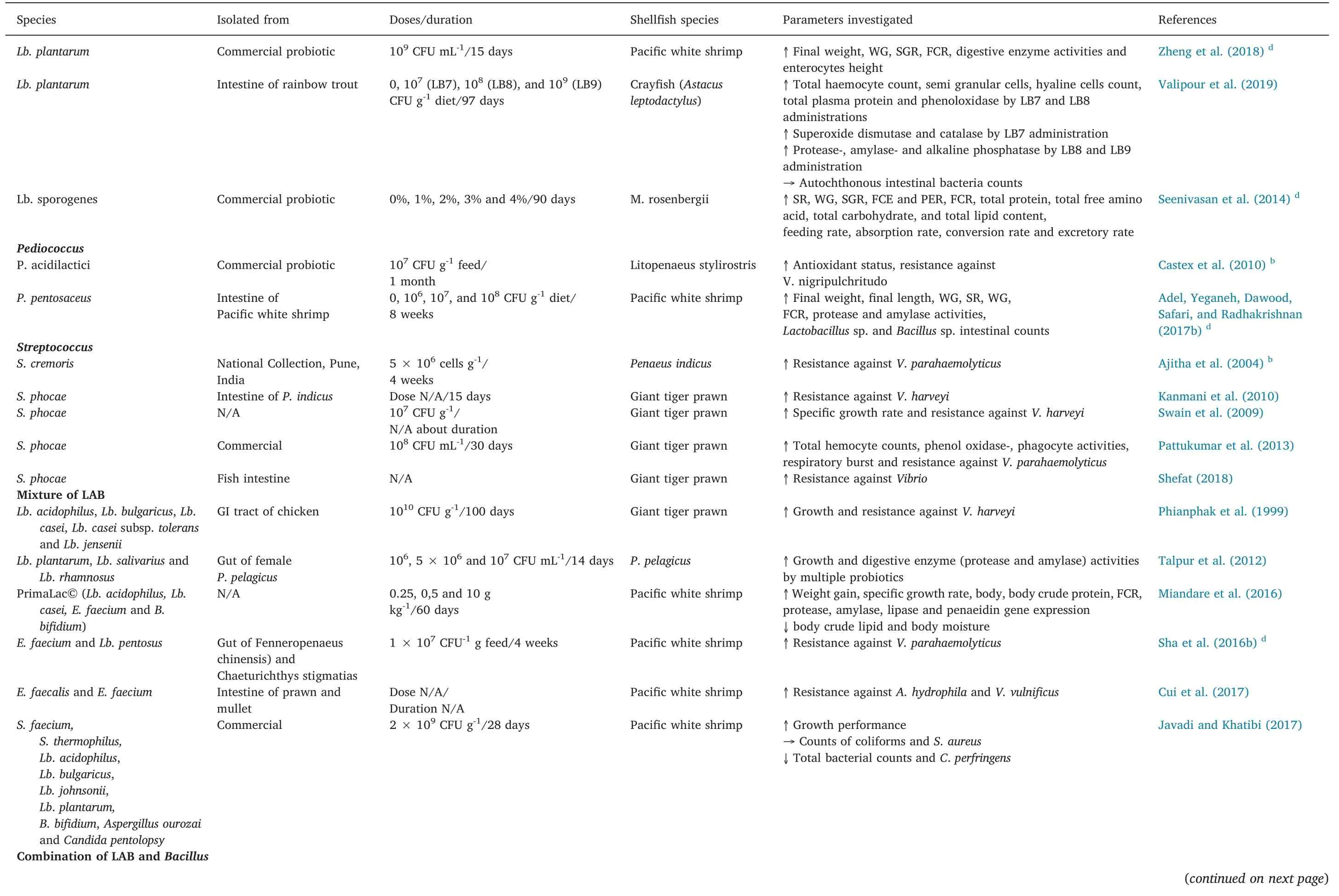
Table 1(continued)
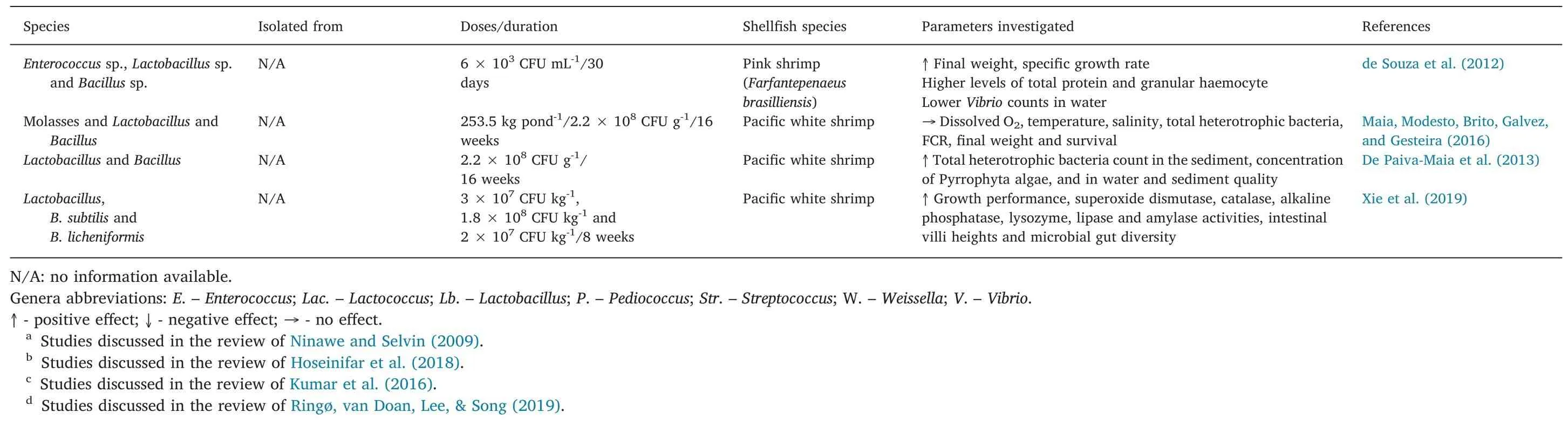
Table 1(continued)
Shellfish aquaculture plays an important role in the world economy,and according to FAO(2016)brackish and marine shrimp production have increased from less than 10.000 metric tonnes in 1970 to more than 4.000.000 metric tonnes in 2014,and most of the aquaculture shrimp production come from Pacific white shrimp(Litopenaeus vannamei)which accounts for 80% of the production.However,as pathogenic bacteria cause enormous economic loss,an alternative to chemotherapies and antibiotics is probiotics.The present review highlight probiotics as a key factor in sustainable shellfish aquaculture,and present information on the use of probiotics by their stimulating effect on growth performance,innate immune response and improved resistance towards pathogenic microbial infection.
2.Methods of probiotic administration
To my knowledge,the first application of probiotics in aquaculture was carried out by Kozasa(1986),but since then the environmentfriendly treatment has increased rapidly,and several comprehensive aquaculture reviews have been published(e.g.Gatesoupe,1999;Hoseinifar et al.,2018;Ringøet al.,2014;Ringøet al.,2018;van Hai,Buller,&Fotedar,2010;Verschuere,Rombaut,Sorgeloos,&Verstraete,2000).With regard to the use of probiotics,it is essential to investigate the best way of administration,optimal dose,and the technical solutions required;especially to keep the probiotics alive in dry pellets(Gatesoupe,1999).
Probiotic administrations mainly depends on several factors i.e.the probionts,supplementation form,vector of administration,dosage level and duration of application,and several different administration modes have been used:
i)Oral administration via diet or water/bath.Supplementation to the diet is the most widely used administration method.Generally,probiotics and cell wall components(parabiotics)are applied in the feed,added to the entire tank or pond water to confer protection against infection(Verschuere et al.,2000).In fish-and shellfish larvae,live food(e.g.Artemia)has proved to be an effi-cient carrier of probiotics(e.g.Giarma,Amanetidou,Toufexi,&Touraki,2017;van Hai et al.,2010).
ii)Administration of several probiotics in combination.In his pioneer review devoted to“Probiotics in man and animals,”Fuller(1989)wrote,“Probiotic preparations may consist of single strains or may contain any number up to eight strains.”However,since the early 1990s most probiotic studies in aquaculture used single administration,but during the last decade,supplementation of multiple probiotics in the diets to aquatic animals has gained interest(e.g.Mohapatra et al.,2014;Allameh et al.,2016;Zorriehzahra et al.,2016;Kesselring,Gruber,Standen,& Wein,2019;Mukherjee,Chandra & Ghosh,2019).The advantage of multiple-strain preparations is;they are active against wider range of conditions and species.
iii)Inactivated bacteria.For example,oral administration of heat-inactivatedLactobacillus delbrueckiissp.lactisandBacillus subtilis,individually or combined(Salinas et al.,2008).
iv)Spores,a structure produced by few bacteria genera is resistant to many environmental or induced factors that the bacteria may be subjected to.The spores help the bacteria to survive by being resistant to extreme changes in the bacteria's habitat including extreme temperatures,lack of moisture/drought,or being exposed to chemicals and radiation.Bacterial spores can also survive at low nutrient levels,and according to Elisashvili,Kachlishvili,and Chikindas(2019)spore-forming probiotic bacteria have received increased scientific and commercial interest.Several studies listed in Table 2,have used bacilli spores.
v)Culturing,storing and administration.Probiotics are usually added to feed as freeze-dried cultures,which are sometimes mixed withlipids to be added as top.
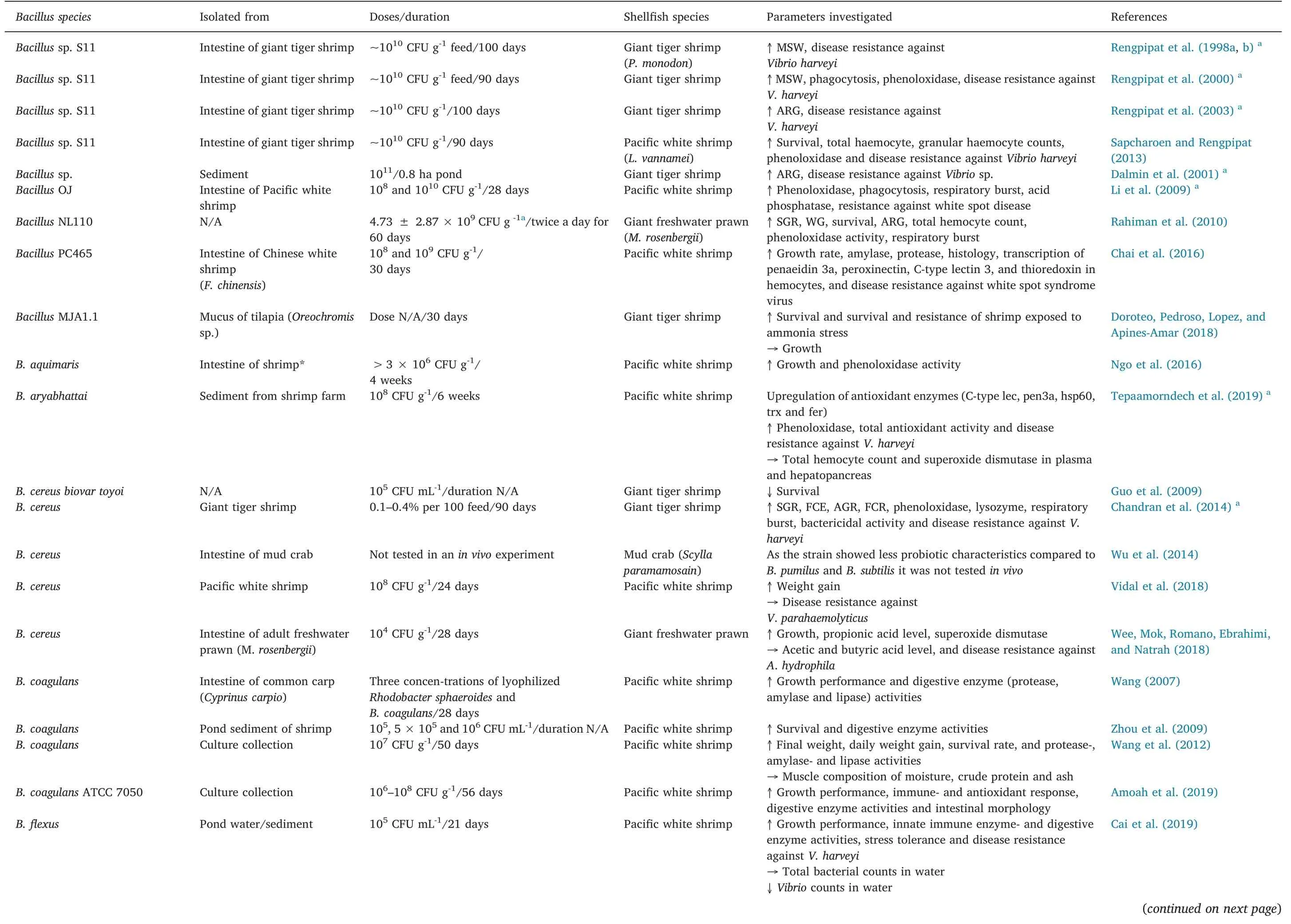
Table 2 Effect of Bacillus on growth performance,immune response and disease resistance in shrimp culture.
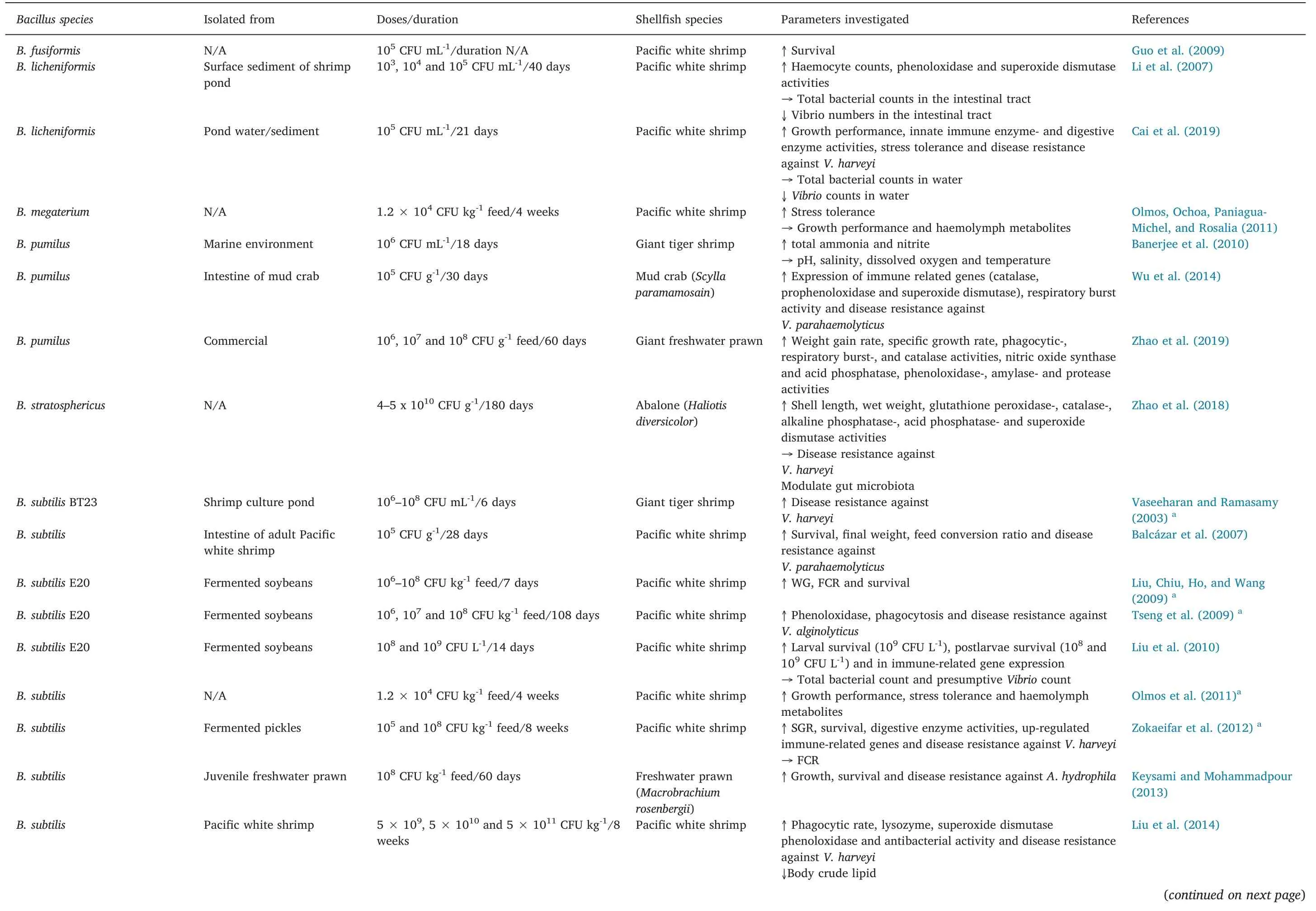
Table 2(continued)
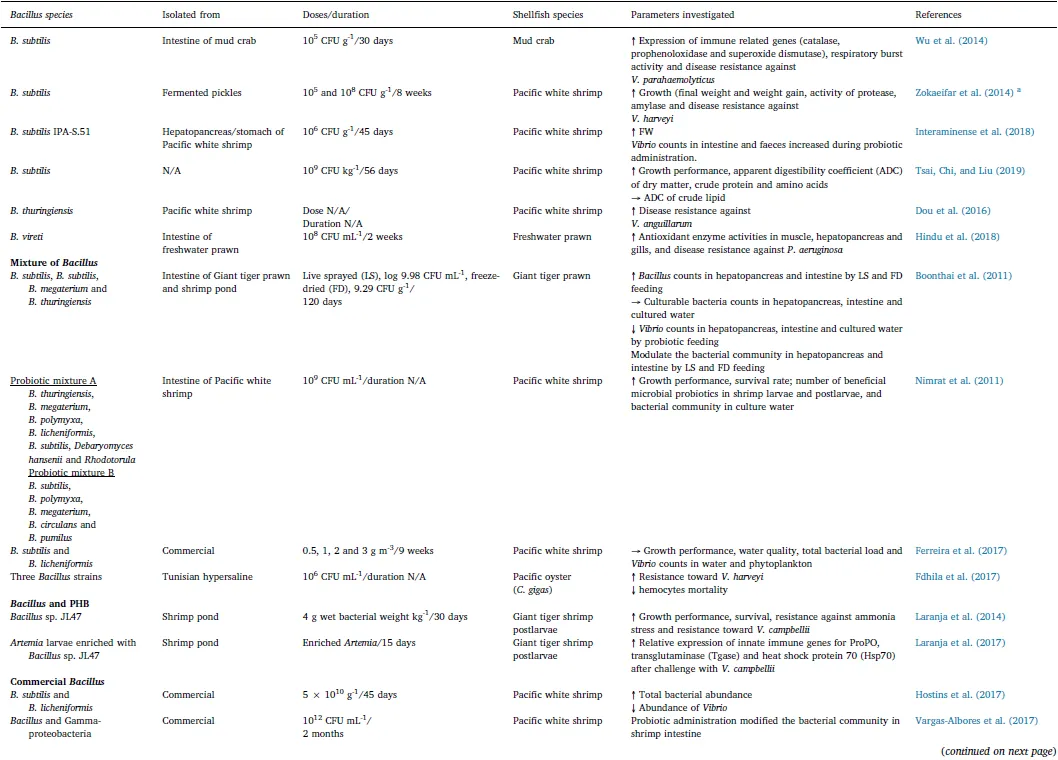
Table 2(continued)

Table 2(continued)
vi)Lyophilization or freeze drying,is a low temperature dehydration process,involving freezing of the product at low pressure,and removing the ice by sublimation.This method is from time to time used in probiotic studies of finfish and shellfish(e.g.Adel,El-Sayed,Yeganeh,Dadar,&Giri,2017a;Boonthai,Vuthiphandchai,& Nimrat,2011;De la Banda et al.,2012;Nimrat,Boonthai,&Vuthiphandchai,2011;Rengpipat,Phianphak,Piyatiratitivorakul,& Menasveta,1998a).
vii)Administration-continuously or regular intervals?Most studies carried out have continuously fed the host animal for a wide range of time,varying from 15 to 94 days(Hai,2015),but information is available on continuous or pulse-feeding of probiotic bacteria in Pacific white shrimp(Kesselring et al.,2019).The continuously application of probiotic cells(LAB,Bacillusspp.and certain Gramnegative spp.)may lead to high levels of colonization of the supplemented bacteria,and modulation of the gut microbiota.However,an important question arises;are the probiotics permanently colonisers in the GI tract?
viii)Co-administration of probiotics with prebiotics or plant products.
Important questions when discussing probiotics are;species isolated from the host,host specificity,strains from other species or commercial probiotics?
3.Mods of actions
Selection of potential probiotic strains is based on many different criteria,such as growth in mucus,acid and bile tolerance,survival in gastric juice,produce extracellular enzymes,produce antimicrobial substances which inhibitin vitrogrowth of pathogens,and bio-safety(hemolytic activity and antibiotic susceptibility).
i)Adhesion to the intestinal mucosa is considered an important selection criterion for persistent beneficial effects of probiotics,and is a prerequisite(Ouwehand et al.,1991;Rinkinen,Mättö,Salminen,Westermarck,& Ouwehand,2000).
ii)Competitive exclusion,probiotic organism colonizes the gut thereby inhibiting adherence and colonization of pathogenic bacteria(Ringøet al.,2010),by producing inhibitory substances which hinder pathogenic organism to adhere and colonize the GI tract(Ringøet al.,2018;Soltani et al.,2019).
iii)Substances produced by probiotics act as antagonist for quorum sensing mechanism.
iv)Enzymatic contribution to digestion,as shown in several studies in Tables 1-3.
v)Competition for iron.For pathogenic bacteria,the ability to acquire iron is vital to survival in the host.In consequence,many genes involved in iron acquisition are associated with bacterial virulence.Siderophores,low molecular weight substances,produced by probiotic candidates or beneficial gut endosymbionts reduce the availability ofiron for pathogenic bacteria,as siderophores has high affinity for ferric ion.
vi)Improved immunity,increase macrophage activity and antibody level.Probiotics which can enhance host immunity and disease resistance of finfish(Merrifield et al.,2010)and shrimps have gathered much interest during the last decade(Kumar et al.,2016;Tseng et al.,2009).Among probiotic bacteria,LAB andBacillusspecies are most frequently used,and they have shown to promotes the health of the host animal by stimulating the innate immune response and improving resistance towards pathogenic microbial infection(Laranja et al.,2017;Rengpipat,Rukpratanporn,Piyatiratitivorakul,& Menasveta,2000;Ringø et al.,2018;Soltani et al.,2019).
vii)Antiviral effect.The first study demonstrating bacteria with antiviral activity against infectious hematopoietic necrosis virus(IHNV)was carried out(Kamei,Yoshimizu,Ezura,& Kimura,1987).Since then,some studies were carried out,1988-1995.However,the topic lost interest.However,more recently,is has been revealed that probiotic administration controlling viral diseases(Lakshmi,Viswanath,B.,& SaiGopal,2013).
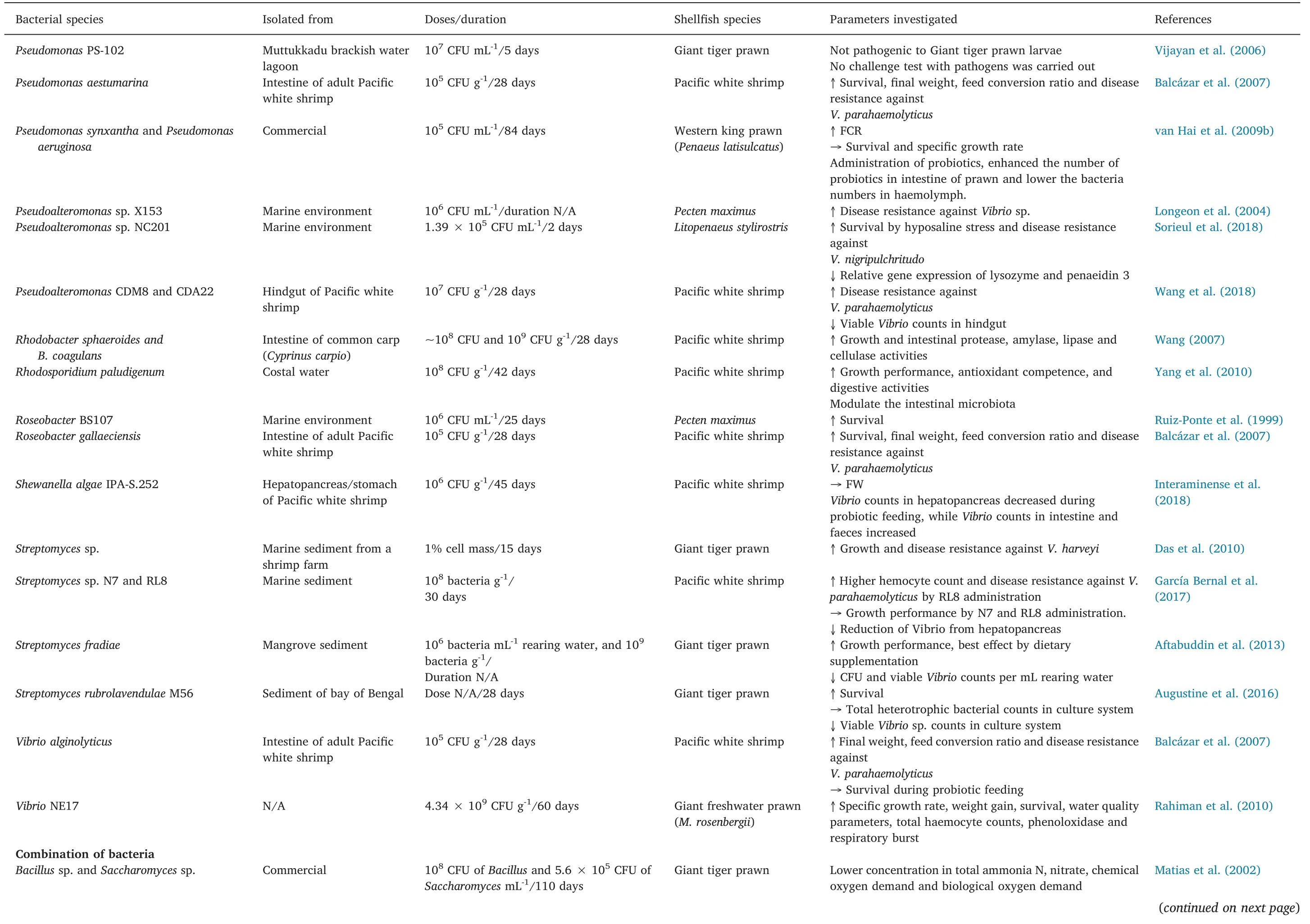
Table 3(continued)
viii)Improve water quality in ponds through modulation of the water microbiota(Cai et al.,2019),improve water physicochemical parameters(Zokaeifar et al.,2014),and control diseases(Kuebutornye et al.,2019).
4.Lactic acid bacteria
Lactic acid bacteria(LAB)have gained much attention as probiotics in aquaculture,for review see the comprehensive review of Ringøet al.(2018).They belong to phylum Firmicutes,class Bacilli and order Lactobacillales,and are Gram-positive,non-endosporing with rod-or coccid morphology.LAB are catalase-and oxidase negative,and most of them are non-motile with a growth optimum at pH 5.5-5.8.They are divided into homofermentative,produce lactic acid from sugars,or heterofermentative,produce lactic acid,acetic acid or alcohol and carbon dioxide.Another favourable trait of LAB is that they produce antimicrobial substances,bacteriocins(e.g.Cotter,Hill,& Ross,2005;Klaenhammer,1993;Ringøet al.,2018).
When discussing probiotics,host specificity of LAB is a topic to mention.Previously,adhesion of probiotic LAB was reported to be host specific,and Fuller(1989)stated,“The attachment to epithelial cells is very host specific which means in practical terms that a strain which is suitable as pig probiotic may not be active in chick and other animals”.However,several later studies have indicated that LAB originated from one host,adhere to mucus of other species(e.g.Nikoskelainen,Ouwehand,Salminen,& Bylund,2001b;Nikoskelainen,Salminen,Bylund,& Ouwehand,2001a;Rinkinen et al.,2000;Rinkinen,Westermarck,Salminen,& Ouwehand,2003;Tuomola,Ouwehand,&Salminen,1999).In addition,adhesion ability of LAB may be related to;adhesive and non-adhesive ability(Zhou et al.,2012),variation of mucin adhesion and cell surface characteristics depending on their isolation habitats indicated by Buntin,de Vos,and Hongpattarakere(2017),and hydrophobic properties(Grajek,Sip,Foksowicz-Flaczyk,Dobrowolska,& Wita,2016).
There are many reports regarding the advantages of using LAB as probiotics in shellfish aquaculture(Table 1).
4.1.Enterococcus faecium
Swain,Singh,and Arul(2009)administratedE.faeciumat 107CFU g-1to giant tiger prawn(Penaeus monodon)for unknown numbers of days and exposed the shrimp toVibrio harveyiandVibrio parahaemolyticus,and revealed improved resistance against the pathogens.In a later study,Sha et al.(2016a)revealed thatE.faeciumhad high adhesive activity,and improved expression of immune and digestion related genes in the mid gut of shrimp.
4.2.Lactobacillus sp
A mixture ofLactobacillusspp.isolated from chicken GI tracts improved the growth and survival rates of juvenile tiger shrimp when fed withLactobacillusstrains for 100 days(Phianphak,Rengpipat,Piyantiratitivorakul,& Menasveta,1999).Karthik,Jaffar,and Muthezhilan(2014)isolated aLactobacillussp.AMET1506 strain from curd which displayed strong antibacterial activity towards the pathogenic bacteria,V.harveyi.The lactobacilli was included in a diet at 106CFU g-1and fed to Pacific white shrimp for 30 days,and thereafter challenged withV.harveyiat a dose of 105CFU mL-1for 10 days.The shrimp fed the lactobacilli diet revealed improved weight gainvs.shrimp fed the control diet;not supplemented byLactobacillus.As expected,dietary supplementation ofLactobacillusincreased theLactobacilluscount in Pacific white shrimp intestines,but an important finding revealed was a significantly decrease in the bacterial load ofVibrio.Based on their results,the authors suggested thatLactobacillusAMET1506 could be a potential probiotic for Pacific white shrimp by modulation of the gut microbiota to control vibriosis in Pacific white shrimp farming.This suggestion was later confirmed as total heterotrophic bacterial load and LAB counts in intestine increase,while the bacterial load ofE.coli,Salmonellaspp.andShigellaspp.in intestine declined by feeding Pacific white shrimp 105Lactobacillussp.AMET1506(Karthik,Pushpam,Chelevan,& Vanitha,2015).Recently,Zuo,Shang,Shao,Li,and Sun(2019)conducted a 28-day feeding trial of Pacific white shrimp,and a WSSV infection trial to determine the effects ofLactobacillussp.on the growth,health status,and disease resistance.The results showed that during probiotic administration,the body weight of shrimp enhanced,and the activities of immune enzyme and digestive enzyme of shrimp fed with probiotics increased.After probiotic feeding,the cumulative mortality of the probiotics groups were significantly lower than the control group after WSSV infection.Moreover,electron microscopy evaluation of the midgut revealed that the intestinal mucosa was tight and the epithelium cells displayed an active secretory state in probiotics group.Furthermore,evaluation of the intestinal microbial communities revealed that probiotic administration affected the microbial community,and that the ability of intestinal microorganism to utilize carbon source was significantly enhanced,which may indicate that the digestive enzyme secreted by probiotics can improve digestion and absorption rate,thus promoting the rapid growth of shrimp.
4.3.Lactobacillus acidophilus
In a study with juvenile tiger shrimp,Lb.acidophilus04(105CFU g-1)was administered for one month and increased resistance(80%survival)was observed following exposure withVibrio alginolyticus;105CFU mL-1after 10 days challenge(Sivakumar,Sundararaman,&Selvakumar,2012).Later,Khan and Mahmud(2015)addressed the effect ofLb.acidophilus,and revealed improved growth and survival of the juvenile giant fresh water river prawn(Macrobrachium rosenbergii)infected with pathogenicVibriospp.
4.4.Lactobacillus brevis
Karthik and Bhavan(2018)conducted a 90-day feeding trial to determine the effects of probiotic effect ofLb.brevis,and revealed significant improvement of growth performance,digestive enzymes(protease,amylase and lipase)and body composition.Furthermore,probiotic administration modulated the gut microbiota.
4.5.Lactobacillus bulgaricus
In an early study,Ajitha,Sridhar,Sridhar,Singh,and Varghese(2004)supplemented Indian white shrimp(Penaeus indicus)diet with as single dose(5×106CFU g-1)ofLb.bulgaricus-57 for 4 weeks,and at the end of feeding trial was shrimp intra-muscular injected with 0.1 mL of an inoculum of 3×109V.alginolyticusper mL.The results revealed improved mortality rate(40%)compared control group(20%).In a recent study,Roomiani,Ahmadi,and Ghaeni(2018)administrated aLb.bulgaricusisolated from Pacific white shrimp intestine to Pacific white shrimp at two inclusion levels;107and 109CFU g-1,for 30 days and challenged withV.parahaemolyticus.There was significant enhancement of hemocyte counts,phenoloxidase activity,respiratory burst,and improved disease resistance against the pathogen,but it's worth noticing that the best results were revealed at inclusion level of 109CFU g-1.
4.6.Lactobacillus pentosus
The potential ofLb.pentosusas probiotics in shellfish aquaculture has been investigated in three studies(Gao et al.,2018;Sha et al.,2016c,2016a).Sha et al.(2016a)reported that administration of 107Lb.pentosusg-1,survival,adhesive activity,expression of PEN-3α,proPO,trypsin andα-amylase in the midgut,and proPO,LGBP,IveLee,trypsin andα-amylase in the hepatopancreas in Pacific white shrimp.Sha et al.(2016c)used aLb.pentosusstrain isolated from intestine of healthyAcanthogobius hastato evaluate the probiotic administration on bacterial community in intestines of Pacific white shrimp and intestinal histology.The abundance of intestinal Actinobacteria increased,while no improvements of intestinal histology were revealed.In a recent study,Gao et al.(2018)revealed that inclusion ofLb.pentosusat 103CFU g-1and 105CFU g-1,significantly improved survival rate,shell length-specific growth rate and feed conversion rate of abalone(Haliotis discus hannaiIno)compared to control.In groups fedLb.pentosus,total number of blood lymphocytes,lysozyme activity,acid phosphatase,superoxide dismutase,and expression levels of Mn-superoxide dismutase and thioredoxin peroxidase increased vs.the control,in contrast malondialdehyde content.The probiotic effect on disease resistance was tested by infection ofV.parahaemolyticus.At day seven all abalones in the control group were dead,while survival was significantly improved in theLb.pentosusadded groups.Based on their results,the authors concluded thatLb.pentosusoriginally isolated from the intestinal tract of abalone is a promising probiotic strain in abalone culture,but its potential in other shellfish species merits investigations.
4.7.Lactobacillus plantarum
Lb.plantarumhas gained popularity in fish and shellfish farming,and in an early study by Chiu Sundararaman and Selvakumar(2007)Lb.plantarumwere reported to enhance the immune responses and gene expression in Pacific white shrimp.The bacteria influenced both the cellular and humoral immune defences in the shrimp.Lb.plantarumenhanced the phenoloxidase-and prophenoloxidase activities,respiratory bursts,superoxide dismutase activity and clearance efficiency ofV.alginolyticus,peroxinectin mRNA transcription,and survival rate of Pacific white shrimp after challenged withV.alginolyticuswhen the diet containing 1010Lb.plantarumkg-1diet for 168 h.Similarly,Vieira et al.(2007)usedLb.plantarumas probiotic in Pacific white shrimp diet,and revealed that the dietary supplementation improved larval survival and enhanced resistance toV.harveyi.In a later study,Vieira et al.(2010)administrated aLb.plantarumto Pacific white shrimp for 60 days,and examined the following intestinal bacteria;total bacterial counts,totalVibriocounts and total LAB counts by cultivation.After 60 days,the probiotic supplemented diet significantly decreased total bacterial counts and totalVibriocounts,and enhanced total LAB counts.The latter finding is not surprisingly as feeding was not reverted back to a control diet without LAB supplementation.Therefore,in future studies the intestinal microbiota should be investigated post probiotic administration,for example 4 weeks.Moreover,probiotic administration significantly increased the resistance againstV.harveyi infection in Pacific white shrimp.After challenge,the bacterial counts in haemolymph and hepatopancreas was significantly lower by probiotic administration,while total hemocyte counts and serum agglutination activity were significantly higher.Subsequently,the effect ofLb.plantarumadministration fed to fresh water river prawn was investigated by Rahiman,Jesmi,Thomas,and Hatha(2010),and revealed significant enhancement of specific growth rate,weight gain and phenoloxidase.However,probiotic feeding did not significantly improved survival,total haemocyte counts or respiratory burst.
A commercial product containingLb.plantarum, at 3.3×1011CFU g-1feed,at inclusion of 0,0.001,0.01,0.1 and 1 g-1was fed to post larval Kuruma shrimp(Marsupenaeus japonicus)for 30 days(Tung,Koshio,& Traifalgar,2010).Growth performance and stress resistance were improved at 1 g inclusion,while 0.1 g-1showed highest relative survival.Moreover,no difference was noticed in protease activity between the treatments.
Kongnum and Hongpattarakere(2012)revealed thatLb.plantarumisolated from the shrimp intestines,no specification was given,significantly improved relative growth rate,feed conversion ratio,survival rate,and haemocytes count as well as disease resistance toV.harveyiin Pacific white shrimp.Talpur,Ikhwanuddin,Daniel Abdullah,and Ambok Bolong(2013)investigated the effect ofLb.plantarumadministration at three inclusion levels,106,5×106and 107CFU mL-1,on blue swimming crab(Portunus pelagius),and revealed best effect by 5×106administration;improved survival,protease-and amylase activities.Not surprisingly,the total bacterial-andVibriocounts in tank water was significantly lower in treated tanksvs.control,while probiotic administration revealed no clear effect on nitrogen content and pH of culture water.In three later studies using tiger shrimp,Dash et al.(2014,2015,2016)revealed that dietary inclusion ofLb.plantarumsignificant increased weight gain,specific growth rate,feed conversion efficiency,protein efficiency ratio,and carcass protein content,whereas feed conversion ratio significant decreased.During probiotic feeding,LAB counts significantly increased in the intestine with a concurrent decrease in Gram-negative bacteria counts.However,as the LAB counts decreased after switching back to basal diet,this clearly indicated that aLb.plantarumfrom a culture collection was not able to permanently colonize the intestine of Pacific white shrimp.On the other hand,immune parameters analysis revealed significantly improvement in total hemocyte count,phenol oxidase-and respiratory burst activities and resistance againstAeromonas hydrophila.
A dietary supplementation of aLb.plantarumisolated from Pacific white shrimp(Vieira et al.,2007)was administrated to Pacific white shrimp for 4 weeks days and challenged withV.alginolyticus(Ramirez et al.,2017).There was significant enhancement of phenoloxidase activity,intestinal LAB and resistance againstV.alginolyticus.However,growth performance,total heterotrophic bacteria in intestine and intestinalVibriocounts increased,which implicate thatLb.plantarum,even though the bacterium was isolated from the host,was not able to outcompeteVibrioalready present in the intestine,or that the administration period was too short.In later study using host-derivedLb.plantarum,Chomwong,Charoensapsi,Amparyup,and Tassanakajon(2018),revealed significant enhancement in haemolymph PO activity,relative mRNA expression ofLvproPO1,LvproPO2,and resistance againstV.parahaemolyticus.Even though information was presented by cultivation-and scanning electron microscopy analysis after 16 days of probiotic administration and 5 days post probiotic feeding,revealed presence of LAB in the intestine-These findings do not confirm permanent colonization,only indicating temporary colonization.However,no information was presented whether post probiotic administration affected the gut microbiota.Recently,Zheng,Duan,Dong,and Zhang(2017)administrated four treatments of a commercialLb.plantarum,fermentation supernatant(FS),live bacteria(LB),dead bacteria(DB)and cell-free extract(CE)ofLb.plantarumfor 45 days to Pacific white shrimp.The results revealed that probiotic administration significant enhanced final weight,WG,SGR,FCR,digestive enzyme activities and enterocytes height,improved resistance against stress,acute low salinity,but revealed no significant effect on gene expression of superoxide and lysozyme.Based on their results,the authors suggested that especially the CE diet might be a potential feed additive to overcome environmental stress of shrimp.Similar conclusion was shown in a later study,as Zheng,Duan,Dong,and Zhang(2018)administrated the FS,LB,DB and the CE diets for 15 days to Pacific white shrimp,and reported that probiotic administration significant enhanced final weight.Significantly highest weight gain and specific growth rate,and the highest lipase,amylase and pepsin activities in hepatopancreas and intestine were noticed for the CF diet.However,the above mention studies,did not evaluate the effect on gut microbiome and resistance towards pathogens,topics merits investigations.
Two strains ofLb.plantarumT8 and T13 was fed to Pacific white shrimp for 3 weeks,and then 7 weeks without probiotic feeding prior to challenge withV.parahaemolyticus(Nguyen et al.,2018).There was significant enhancement of growth and disease resistance towards the pathogen by feeding T8,while T13 revealed better disease resistance compared to the T8 treatment,but no growth improvement was noticed.The results are of interest,but further studies are needed as only a short period,3 weeks,of probiotic feeding was used.
Good management practice might masked the possible effect of probiotic supplementation on the water quality,as Correa et al.(2018)revealed that dietary inclusion ofLb.plantarumhad no effects on water quality and pathogens removal in Pacific white shrimp culture under biofloc system.In contrast,dietary inclusion ofLb.plantarumsignificantly improved water quality and reduced shrimp diseases,as well as environmental impact(Pacheco et al.,2018).
DietaryLb.plantarumat inclusion levels of 0,107(LB7),108(LB8),and 109(LB9)CFU g-1diet was fed to clawed crayfish(Astacus leptodactylus)for 97 days(Valipour,Nedaei,Noori,Khanipour,&Hoseinifar,2019).There was significant enhancement of total haemocyte count,semi granular cells,hyaline cells count,total plasma protein and phenoloxidase by LB7 and LB8 administrations,while superoxide dismutase and catalase increased by LB7 administration.The LB8 and LB9 administration significantly elevated protease-,amylase-and alkaline phosphatase activities.Some bacterial assessments were carried out,and the results displayed no effect on autochthonous intestinal bacteria counts.Moreover,as expected the autochthonous LAB levels were significantly elevated in all probiotic treatments,and the highest levels were revealed in treatments LB8 and LB9.However,permanent colonization was not evaluated.
4.8.Lactobacillus sporogenes
Seenivasan,Radhakrishnan,Shanthi,Muralisankar,and Saravana Bhavan(2014)conducted a study with fresh water river prawn fedLb.sporogenesfor 90 days,and revealed significantly improved growth performance,total protein,total free amino acid,total carbohydrate,and total lipid content;as well as feeding rate,absorption rate,conversion rate,and excretory rate.
4.9.Mixture of LAB
Phianphak et al.(1999)revealed that a mixture ofLactobacillus,includingLb.acidophilus,Lb.bulgaricus,Lb.casei,Lb.caseisubsp.toleransandLb.jenseniioriginally isolated from the GI tract of chicken improved the growth and survival rates of juvenile tiger shrimp exposed toV.harveyiwhen fed the lactobacilli strains for 100 days.A multi-strain probiotic,PrimaLac©(Lb.acidophilus,Lb.casei,E.faeciumandB.bifidium)was fed at 0,0.25,0.5 and 1 g kg-1to Pacific white shrimp for 60 days(Miandare,Yarahmadi,& Abbasian,2016),and generally,there was significant enhancement of weight gain,SGR,body,body crude protein,FCR,protease,amylase,lipase and penaeidin gene expression,in contrast to the decrease of body crude lipid and body moisture.Other studies have also evaluate the combination of several probiotics in Pacific white shrimp diets,such asE.faecium,andLb.pentosus(Sha et al.,2016a;2016b),or combination ofLb.pentosus,Lac.fermentum,B.subtilis,andS.cerevisiae(Wang,Hu,Chiu,& Liu,2019),and these studies revealed significantly enhanced disease resistance againstV.parahaemolyticus.
4.10.Lactococcus garvie ae
Lac.garvieaeat inclusion level of 107CFU g-1was fed to tiger shrimp and challenged withV.harveyiandV.parahaemolyticus(Swain et al.,2009).ForV.harveyi,40% survival was revealed,while approximately 15% was noticed in theV.parahaemolyticusstudy.
4.11.Lactococcus lactis
Maeda et al.(2014)conducted a 7 day feeding trial and a subsequent 24 h challenge trial to determine the effects ofLac.lactison the health status,and disease resistance of kuruma shrimp(Marsupenaeus japonicus).There was significant enhancement of up regulation of lysozyme gene expression in intestine and hepatopancreas,and resistance againstV.penaeicida.However,anti-lipopolysaccharide factor,superoxide dismutase,prophenoloxidase and toll-like receptor in intestine was not enhanced by probiotic administration.Adel et al.(2017a)revealed that administration of 107and 108CFU g-1ofLac.lactisto Pacific white shrimp for 8 weeks enhanced growth rate,survival,body protein level,and the digestive enzymes(cellulase,lipase,amylase and protease).Furthermore,intestinal bacterial levels ofLactobacillusandBacilluswere significantly enhanced by probiotic administration,in contrast toVibriocounts which decreased.In addition,probiotic administration improved disease resistance againstV.anguillarum.Host-derived feeding ofLac.lactissupplemented at a final concentration of 2-4 x 108CFU g-1feed to Pacific white shrimp for 16 days and challenged withV.parahaemolyticuswas investigated by Chomwong et al.(2018).The results revealed significant enhancement of haemolymph PO activity,relative mRNA expression ofLvproPO1,LvproPO2,temporary colonization of theLactococcusin the GI tract,and resistance againstV.parahaemolyticus.
4.12.Pediococcus acidilactici
P.acidilacticishowed an effect on antioxidant defence and oxidative stress of Pacific white shrimp when challenged withVibrio nigripulchritudo.Probiotic administration was effective on the antioxidant defences,superoxide dismutase,catalase,glutathione peroxidase,total antioxidant status,glutathione's,and induced tissue damage,andP.acidilacticiwas efficient in maintaining the antioxidant defence levels for a longer period than the control and uninfected groups(Castex,Lemaire,Wabete,& Chim,2010).This findings suggests that probiotic bacteria besides enhancing the immune defences also maintain the defence levels in the shrimp offering a prolonged protection.When discussing commercial probiotic product,it's worth to mention thatP.acidilacticiMA 18/5M was one of the first officially commercial probiotic product approved for use in fish aquaculture in Europe(Feed Additive Magazine,September 28,2012).However,since then are 29 commercial products available on the marked for shellfish and finfish(Cordero et al.,2014).
4.13.Streptococcus cremoris
In the study of Ajitha et al.(2004),the authors also fed the Indian white shrimp with as single dose(5×106CFU g-1)ofS.cremorisfor 4 weeks,and at the end of feeding trial was shrimp intra-muscular injected with 0.1 mL of an inoculum of 3×109V.alginolyticusper mL,and a mortality rate of 20%was revealed.This rate was lower than that of shrimp fedLb.bulgaricus-57(40%)and shrimp fed the control diet(80%).
4.14.Streptococcus phocae
In an early study,Swain et al.(2009)revealed that administration ofS.phocaeto tiger shrimp improved specific growth rate and resistance againstV.harveyi.In addition,it is of interest to notice thatS.phocaeexhibited higher resistance,94% survival,compared to the other probiotics used,E.faecium(84%),Lac.garvieae(40%)and a commercial probiotic(68%).Pattukumar et al.(2013)evaluated the effect of commercialS.phocaeto tiger shrimp for 30 days and challenged withV.parahaemolyticus,and revealed significant increase in total hemocyte counts,phenol oxidase-,phagocyte activities,respiratory burst and resistance againstV.parahaemolyticus.
4.15.Synbiotic studies
Synbiotic,combination of pro-and prebiotics,studies are well known in finfish aquaculture(Ringø& Song,2016),but is less investigated in shellfish aquaculture.However,in a recent study,the effect of a synbiotic diet,galactooligosaccharide andLb.plantarumwas administrated to Pacific white shrimp for 60 days and challenged withV.alginolyticus(Huynh,Cheng,Chi,Chiu,& Liu,2018).There was significant enhancement of immune parameters such as phenoloxidase activity,respiratory burst,phagocytic activity,prophenoloxidase I,serine proteinase and peroxinectin and survival when the shrimp were challenged withV.alginolyticus.
5.Bacillus sp
Species within genusBacillusare Gram-positive,catalase-positive,endosporing,aerobic or facultative anaerobes,characterized by their rod-shaped morphology(between 2.5 and 10μm),and is classified in the Phylum Firmicutes,Class Bacilli,and Order Bacillales.The genus comprises of approximately 200 bacterial species,and are almost ubiquitous in nature and they have been isolated from compost,extreme environments such as high pH conditions,at high temperature,high salt concentrations,in aquatic environments as well as in the gastrointestinal(GI)tract of aquatic animals(Soltani et al.,2019).Bacillusexhibit quite diverse physiological properties such as the ability to produce cellulase,phytase,tannase,chitinase,xylanase,protease and lipase(Ghosh,Ray,& Ringø,2019;Ray,Ghosh,& Ringø,2012),antimicrobial substances(Abriouel,Franz,Ben Omar,& Gálvez,2011;Stein,2005)as well as modulation of antioxidant enzymes,stress mitigation and prevent tissue damage(Kuebutornye et al.,2019).In addition,dietary supplementation of bacilli may improve digestive enzyme activities(Soltani et al,2019).It is widely accepted,that the level of digestive enzyme activity is a useful comparative indicator of the host's food utilization rate,digestive capacity,and growth performance(Ueberschär,1995;Suzer et al.,2008).
According to Elshaghabee,Rokana,Gulhane,Sharma,and Panwar(2017)there are 17 probiotic supplements containingBacillusavailable in the global market.GenusBacillushave been widely used as environmental probiotics(Moriarty,1998)and dietary probiotics(Hong,Duc,& Cutting,2005)in aquaculture due to their production of antimicrobial substances,enzymes provide and their ability to colonize the digestive tract and to contribute to nutrition of the host(Soltani et al.,2019).In addition,Bacillushas simple nutritional requirements,fast metabolic rate,ease to isolate and preserve,and these advantages make them to be one of the most studied probiotics in aquaculture.
In their review devoted toBacillusin fish and shellfish aquaculture,Soltani et al.(2019)presented information on their potential ofBacillusas promising probiotics on growth performance,their effect on the immune system and disease resistance against pathogens.In order to avoid duplication,studies reviewed in the aforementioned review are only briefly addressed in the text and Table 2 in the present paper.
5.1.Bacillus sp
Giant tiger prawn(Penaeus monodon).Earlier studies withBacillussuggested thatBacillusS11,a saprophytic strain,was able to enhance resistance of giant tiger prawn when challenged withV.harveyi(Rengpipat et al., 1998a; Rengpipat, Rukpratanporn,Piyatiratitivorakul,& Menasveta,1998b),and in a later study,feeding live bacteria seems to be an effective treatment for improving the growth in pond condition of giant tiger prawn as Rengpipat et al.(2000)revealed thatBacillusS11 stimulate the immune response by activating phenoloxidase,phagocytosis and antimicrobial activity in the haemolymph of giant fresh water river prawn to improve resistance againstV.harveyi.When giant tiger shrimp were fed withBacillusS11 for 100 days,improved growth and higher resistance was seen after challenging withV.harveyi(Rengpipat,Tunyanun,Fast,Piyatiratitivorakul,&Menasveta,2003).In order to monitoringBacillusS11 as probiotics in giant tiger prawn,Rengpipat,Wongtangprasert,and Palaga(2009)used green fluorescent protein,and revealed the bacterium in the intestine after 9 weeks of feeding.
Dalmin,Kathiresan,and Purushothaman(2001)demonstrated that the application of an indigenousBacillusspp.in the rearing water of giant tiger prawn was able to maintain optimum transparency and low organic carbon of the pond,as well as a decrease inVibriocounts in the water column.Matias,Yusoff,Shariff,and Azhari(2002)compared the efficacy of two commercial probiotic products on the water quality of commercial giant tiger prawn grow-out ponds,and relatively a lower concentration in total ammonia N,nitrate,COD and biological oxygen demand was seen in the early culture phase of ponds treated with a mixture ofBacillussp.andSaccharomycessp.than in ponds treated with a mixture ofBacillussp.,Nitrosomonassp.andNitrobactersp.Additionally,no significant difference was recorded in values of salinity,ammonia,hydrogen sulphide or pH,while dissolved oxygen and transparency values were slightly increased.
Pacific white shrimp(Litopenaeus vannamei).Pacific white shrimp is one of the most important species used in aquaculture,and some studies have addressed the use of probioticBacillussp.in Pacific white shrimp aquaculture.Feeding Pacific white shrimp with dietaryBacillusdeceased the total viable counts of bacteria and theVibriocount in the shrimp intestine(Li,Tan,&Mai,2009),whileBacillusOJ enhances the immune response(phenoloxidase,phagocytosis,etc.)and provides protection against white spot syndrome virus(WSSV)in the same species(Li et al.,2009;Tseng et al.,2009).
Chai,Boonthai and Vuthiphandchai(2016)investigated the effects ofBacillusbacteria isolated from the intestine of a healthy,wild shrimps on the growth of Pacific white shrimp and showed that probiotics reduced shrimp culture risks from stressful conditions and improved growth total weight,specific growth rate,FCR,digestion and nutrient absorption.
Chai,Song,Chen,Xu,and Huang(2016)carried out a 30-day feeding trial and a subsequent 20-day anti-virus infection trial to evaluate the effects ofBacillusPC465,at inclusion level of 107,and 109CFU g-1,on growth,health status,and disease resistance of Pacific white shrimp.Probiotic administration significantly increased the weight gain and survival of the shrimp,but the effect of 109CFU g-1on the growth rate was higher than that of 107CFU g-1.Compared to the control group,activities of amylase and protease in the shrimp midgut significantly enhanced by probiotic administration.Histological evaluations by scanning electron microscopy revealed improvements by probiotic treatment.The probiotic feeding significantly enhanced the transcription of penaeidin 3a,peroxinectin,C-type lectin 3,and thioredoxin in the shrimp hemocytes.DGGE analysis of the mid-gut microbiota was modulated by probiotic treatment-fed group than in the control group,and not surprisingly the proportion ofBacillusin the probiotic treatment increased.Moreover,probiotic administration provided protection against viral infection.Bachruddin,Sholichah,Istiqomah,and Supriyanto(2018)reported that addition of probiotics,includingBacillusspp.,into the culture water of Pacific white shrimp significantly improved weight gain,total length and feed conversion rate of shrimp species.
Giant freshwater prawn(Macrobrachium rosenbergii).Growth and survival of giant freshwater prawn was enhanced when prawns were fedBacillussp.,isolated from the same animal species;either orally or as an immersion route(Rahiman et al.,2010).In addition,an improvement in some immune variables;total hemocyte count,phenoloxidase and respiratory burst activities of giant freshwater prawn,suggesting probiotic species-specific effect in this prawn species.
Kuruma shrimp(Marsupenaeus japonicus).Dong et al.(2014)evaluated the role ofBacillusas protective agent and immunomodulator in kuruma shrimp juveniles against temperature stress.The study revealed thatBacillusimproved growth,minimized damage caused by free radicals generated from insufficient oxygen metabolism and enhanced the immune response of kuruma shrimp during the high temperature farming period.
Chinese white shrimp(Fenneropenaeus chinensis).Chai et al.(2016)showed that aBacillusprobiotic strain PC465,isolated from the gut ofChinese white shrimp,enhanced immune parameters including ProPO,peroxinectin,penaeidin,thioredoxin,lectins,haemocyanin and crustin,and provided protection against white spot syndrome virus infection in Pacific white shrimp.
Mud crab(Scylla paramamosain).In mud crab,dietary administration ofBacillusspp.isolated from the crab's intestine significantly enhanced survival rate,respiratory burst activity,immune related genes expression,and resistance toVibrio parahaemolyticus(Wu et al.,2014).
Pacific oyster(Crassostrea gigas).A significant decrease in hemocytes mortality and increase resistance towardV.harveyiwere observed in Pacific oyster fed a mixture of threeBacillussp.isolated from Tunisian hypersaline(Fdhila et al.,2017).
5.2.Bacillus aquimaris
Ngo et al.(2016)selected eight pigmentedBacillusstrains from Pacific white shrimp based on the production of heat-stable spores,carotenoids and their free-radical scavenging activity.One of these strains,Bacillus aquimarisSH6 a red-orange pigmented strain displayed the highest abundance in the gut of Pacific white shrimp,and by feeding Pacific white shrimp a diet containing SH6 spores at>3×106CFU g-1for 4 weeks improved health benefits,coloration,growth and immunity were noticed.Compared with dietary astaxanthin administration,dietary SH6 spores are more beneficial for shrimp weight gain and immunity.Furthermore,as a 300-fold higher concentration of SH6 spores than conventional probiotic concentrations was not toxic to the shrimp,it was concluded that SH6 spores can be a potential supplement as a carotenoid-producing probiotic strain for Pacific white shrimp.
5.3.Bacillus aryabhattai
Tepaamorndech et al.(2019)demonstrated that the supplementation ofB.aryabhattaito Pacific white shrimp reduced the abundance ofVibriopopulations and modulated the bacterial community in the shrimp GI tract.Further,an enhancement in the innate immunity,antioxidant activity and disease resistance toV.harveyiwas noticed,suggesting thatB.aryabhattaiisolated from shrimp environment(sediment)as a useful probiotic in shrimp aquaculture.
5.4.Bacillus cereus
To my knowledge,few studies have usedB.cereusas probiotic in shrimp aquaculture.Bath administration ofB.cereus biovar toyoi(105CFU mL-1)added daily,significantly decreased survival of giant tiger prawn in a larval-culture system(Guo et al.,2009).Yang et al.(2015)isolated 27 protease-producing bacterial strains from intestine of marine-growth Pacific white shrimp,among which a high-yield protease-producing strain,B.cereuswas identified.
A number of studies have shown that species ofBacillusisolated from similar environment where they will be applied,can boost the cellular and humoral components of innate immunity in shrimp species.For example,B.cereusenhanced various immunological variables including phenoloxidase,lysozyme,respiratory burst,bactericidal activity in giant tiger prawn(Chandran et al.,2014).Furthermore,enhancement of immune status was confirmed by higher survival rates in treated shrimp after being challenged withV.harveyi.Later,Dou et al.(2016)isolated aB.cereusstrain from Pacific white shrimp able to produce extracellular protease,amylase and lipase,and protected Pacific white shrimp againstVibrio anguillaruminfection.In a recent study,Vidal,da Cruz Pessôa,dos Santos,Mendes,and Mendes(2018)revealed that aB.cereusoriginally isolated from the intestine of juvenile Pacific white shrimp,and displayed that probiotic feeding significantly improved weight of Pacific white shrimp,but resistance againstV.parahaemolyticuswas not significantly improved.As the shrimp were only fed the probiotic bacteria for 7 days prior to bath challenge of the pathogen,one can speculate that the probiotic feeding time was too short,and this suggestion has to be confirmed in further studies.
5.5.Bacillus coagulans
Firstly,B.coagulanscan promote intestinal digestion,asB.coagulansstrains produce various enzymes that facilitate excretion and digestion.Secondly,B.coagulanscan regulate host symbiotic microbiota and inhibit the growth of pathogenic bacteria.Lastly,due to its ability to normalize both the quantitative parameters of the immune system and immune cells'functional activity,B.coagulanscan significantly benefit the host immune system.Due to the evidence supporting various probiotic effects ofB.coagulans,many different strains ofB.coagulanshave been used in the management and alleviation of several human diseases.Therefore,direct supplementation or prebiotic modulation ofB.coagulansmay be an attractive preventive and/or therapeutic avenue for human diseases.
Several beneficial effects ofB.coagulanshave been reported,and due to its excellent stability,B.coagulanshas been widely used in medicine,food and chemical industry.Recent studies have shown thatB.coagulanshas therapeutic effects on intestinal diseases,such as acute diarrhea,irritable bowel syndrome,antibiotic-related diarrhea,constipation and colitis via modulation of the microbiota composition,host immunity and metabolism(Mu & Cong,2019).B.coagulansare known to improve digestive health by posing antagonistic effects on pathogens(Wang,Fu,&Lin,2012).Additionally,toxicological experiments and a large number of clinical observations have showed thatB.coagulansis safe,and has no effect of mutagenicity,teratogenicity or genotoxicity.B.coagulansat inclusion level of 107CFU g-1feed was administrated to Pacific white shrimp for 50 days(Wang et al.,2012).There appeared significant(P<0.005)enhancement of final weight,daily weight gain,survival rate,and protease-,amylase-and lipase activities by probiotic feeding.However,no significant difference was noticed in muscle composition of moisture,crude protein and ash.
In the study of Zhou,Wang,and Li(2009),the effect ofB.coagulansSC8168,as water additive was evaluated in larval Pacific white shrimp,and the results revealed that supplementation significantly increase survival rate and digestive enzyme activities(protease,amylase and lipase).In a recent study by Amoah et al.(2019),Pacific white shrimp were fed four different inclusion levels,0,106,107and 108CFU g-1ofB.coagulansfor 56 days.The results revealed that dietary supplementation modulate the gut microbiota as the abundance of opportunistic pathogenic bacteria such asVibrio,TenacibaculumandPhotobacteriumsignificantly decreased with increasing probiotic inclusion.Furthermore,probiotic supplementation significantly improved growth performance as well as immune-and antioxidant response[triglyceride,lysozyme,acid phosphatase,glutathione peroxidase,and malondialdehyde],digestive enzyme activities(lipase,amylase and trypsin),and intestinal morphology.In addition,a challenge test revealed enhanced resistance againstV.parahaemolyticusat inclusion level of 108CFU g-1,and based on their results the authors concluded thatB.coagulansat supplementation level of 108CFU g-1,is a promising probiotic in Pacific white shrimp culture.
5.6.Bacillus flexus
Cai et al.(2019)conducted a 30-day feeding trial and a subsequent challenge test usingV.harveyito determine the effects ofB.flexus,isolated from pond water/sediment,and revealed enhanced growth performance,immune related enzymes(alkaline phosphatase,peroxidase and lysozyme),digestive enzymes(protease and lipase),stress tolerance and disease resistance.
5.7.Bacillus fusiformis
Bath administration ofB.fusiformis(105CFU mL-1)added either daily or every 2nd day,increased survival in the larval-culture system of Pacific white shrimp(Guo et al.,2009).
5.8.Bacillus licheniformis
B.licheniformisis another efficienthost-associated probioticin aquaculture.A strain ofB.licheniformiswith the ability to secrete extracellular macromolecule-digesting enzymes,isolated from the surface sediment of shrimp pond was added to shrimp tanks at concentrations of 103,104and 105CFU mL-1for 40 days(Li et al.,2007b).
DietaryB.licheniformissupplementation has revealed to improve resistance towardsV.harveyiin Pacific white shrimp by inhibiting the growth of intestinalVibrio,and increasing the expression of genes related to disease resistance(Zhang et al.,2016).Similar results were revealed by Cai et al.(2019)conducted a 30-day feeding trial and a subsequent challenge test usingV.harveyito determine the effects ofB.licheniformis flexus,and revealed enhanced growth performance,immune related enzymes(alkaline phosphatase and lysozyme),digestive enzymes(protease and lipase),stress tolerance and disease resistance towardsV.harveyi.
5.9.Bacillus pumilus
Bacillus pumilusis ahost-associated probioticof recently interest in aquaculture.Use ofB.pumilusas a known nitrogen removal bacterium together with periphytic algae in a biofloc system;reduced total ammonia and nitrite as demonstrated elsewhere(Banerjee,Khatoon,Shariff,& Yusoff,2010),whereB.pumiluswas added to the rearing water of giant tiger prawn.Furthermore,no significant change were revealed in pH,salinity,dissolved oxygen and temperature.In vitrobioremediation assessment of three indigenousBacillusspecies;B.pumilus,B.licheniformisandB.subtilis,isolated from marine water and soil samples showed that these bacteria were able to reduce total ammonia nitrogen,in tank water of giant tiger prawn(Devaraja,Banerjee,Sariff,& Khatoon,2013),and thus recommendedBacillusas a bioremediator for giant tiger prawn culture systems.In situbioremediation has also been widely applied in aquaculture through bio-augmentation using indigenous or exogenous probiotics,which ameliorate water quality(Wang,Xu,&Xia,2005).Based on their results by inclusion ofB.subtilisat three doses,106,107and 108CFU g-1,Zhao et al.(2019)revealed that administration ofB.pumilusat dose of 108CFU kg-1improved growth,immunity and digestive enzyme activities of giant freshwater shrimps.
5.10.Bacillus stratosphericus
Dietary administration ofB.stratosphericusto abalone(Haliotis diversicolor)for 180 days and challenged withV.harveyi(Zhao,Ling,Zhang,Ke,& Hong,2018),revealed improved nutrient and health status,but no significant disease resistance was noticed towardsV.harveyi.
5.11.Bacillus subtilis
B.subtilisis one of the most studied species within genusBacillus.It has been also shown thatBacillusfeeding increased the survival of shrimp species against bacterial and viral pathogens through immune modification.For example,bath treatment of giant tiger prawn with cell-free extracts ofB.subtilisBT23 significantly reduced mortality level after shrimp being challenged withV.harveyi(Vaseeharan &Ramasamy,2003),while Keysami,Saad,Sijam,Daud,and Alimon(2007)studied by usingB.subtilis,on larval growth and development rate of fresh water river prawn in Malaysian hatchery.B.subtilisE20,isolated from fermented soybeans,has been reported to improve the resistance of Pacific white shrimp exposed toVibrio alginolyticusthrough stimulation of immune responses variables such as lysozyme and prophenoloxidase I and II,survival and stress tolerance to water temperature,salinity and nitrite-N in Pacific white shrimp(Liu,Banerjee,Sariff,& Khatoon,2010).Liu,Chiu,Shiu,Cheng,and Liu(2010)supplemented Pacific white shrimp diets with two doses(108CFU and 109CFU L-1)of differentB.subtilisE20 for 14 days,and at the end of feeding trial shrimp were exposed to stress test;suddenly exposed to fresh water and 60‰ salt water.The results recorded that postlarvae had significantly lower cumulate mortality in the probiotic treatments.Analysis of immune-related gene expressions showed that the expression of prophenoloxidase I,prophenoloxidase II,and lysozyme of larvae significantly increased after being reared in probioticcontaining water.However,no significant difference in serine proteinase or glutathione peroxidase gene expressions was revealed.Based on their results,the authors suggested that 109CFU L-1of B.subtilis E20 should be added to the rearing water for shrimp larva breeding.
In a study by Olmos et al.(2011),improved growth performance and better stress tolerance to ammonia oxygen deficiency was revealed in juvenile Pacific white shrimp orally fed withB.subtiliscompared to both control shrimps and those fed withBacillus megaterium,suggesting that probiotic species selection is an important factor.
Zokaeifar et al.(2012;2014)demonstrated that administration ofB.subtilisat two different doses,105and 108CFU g-1fed to Pacific white shrimp increased the activity of protease and amylase digestive enzyme and subsequently improved the growth(final weight and weight gain)of shrimp juveniles.However,food conversion ratio(FCR)was unaffected by probiotic feeding.Expression of immune-related genes was significantly up-regulated.After eight weeks of probiotic feeding was shrimp challenged withV.harveyi,and the results revealed significant protection.Administration ofB.subtilisfed at 5×109CFU,5×1010CFU and 5×1011CFU kg-1to Pacific white shrimp for 8 weeks and challenged withV.harveyi(Liu et al.,2014).There was significant enhancement of phagocytic rate,lysozyme,superoxide dismutase phenoloxidase and antibacterial activity and disease resistance againstV.harveyi,while body crude lipid decreased significantly.Application of an indigenous probioticB.subtilisonce a week into a Pacific white shrimp culture enhanced water quality by decreasing pH,nitrite,water transparency and soluble reactive phosphorus,and increasing COD and Chlorophyll a(Wu et al.,2016).Probiotic administration also affected the bacterial community of culture water,with greater impact in the early and middle phases,than in the late phase of shrimp culture.The greater impact early in culture can probably be attributed to faster initial colonization and multiplication of the probiotic through peptone addition in the water column.Recently,Interaminense et al.(2018)reported thatB.subtilisadministration enhanced Pacific white shrimp growth and reducedVibriocounts in hepatopancreas.However,Vibriocounts in intestine and faeces increased during probiotic administration.This study also investigated the effect of probiotic feeding ofShewanella algae,and clearly revealed difference by the probiotic administrations.
5.12.Bacillus thuringiensis
Dou et al.(2016)isolated aB.thuringiensisstrain from Pacific white shrimp able to produce extracellular protease,amylase and lipase,and protected Pacific white shrimp againstVibrio anguillaruminfection.
5.13.Bacillus vireti
Hindu,Chandrasekaran,Mukherjee,and Thomas(2018)conducted a 2 week feeding trial of freshwater prawn and a subsequent 15 day infection trial to determine the effects ofB.viretion antioxidant defense enzymes activities and disease resistance againstPseudomonas aeruginosa.The results revealed considerably higher antioxidant defense enzymes activities,superoxide dismutase,catalase and GSH in muscle,hepatopancreas and gills of prawns infected by the pathogen by probiotic administration,leading to improved resistance againstP.aeruginosa.
5.14.Mixture of Bacillus
Use of a commercial probiotic product,Sanolife MIC,mixture of some bacilli strains;B.subtilisandB.licheniformis,administrated in hatcheries of giant tiger prawn and Pacific white shrimp,improved water quality and reduced the density ofVibriobacteria in the water column(Decamp,Moriarty,& Lavens,2008).Other species within genusBacillus;BacillusOJ,Bacillus amyloliquifaciens,andBacillus aerophilus,isolated from GI tracts of Pacific white shrimp,yellow fin bream(Acanthopagrus latus),and rohu significantly enhanced growth performance,immune response,and disease resistance of Pacific white shrimp(Li et al.,2009).
Rahiman et al.(2010)reported lower values of ammonia,nitrite and pH in giant fresh water prawn culture ponds treated withBacillussp.during 60 days cultivation,while no change was observed in dissolved oxygen compared to control ponds.Similarly,Lara-Anguiano et al.(2013)demonstrated that the use of molasses as a fertilizer increasedBacillusspp.density in the water column of Pacific white shrimp.Nimrat,Suksawat,Boonthai,and Vuthiphandchai(2012)revealed lower levels of pH,ammonia and nitrite in treated Pacific white shrimp pondsvs.controls,when two mixtures ofBacillusspecies(first mixture-B.thuringiensis,B.megaterium,B.polymyxa,B.licheniformis,B.subtilis,and second mixture-B.subtilis,B.polymyxa,B.megaterium,B.circulans,B.pumilus)were used in the form of microencapsulated probiotics.Zokaeifar et al.(2014)reported that application of aBacillusspp.mixture into the culture water of Pacific white shrimp revealed significant improvement in water quality parameters(reduction in ammonia,nitrite and nitrate ions)and increased growth performance,digestive enzyme activity,immune response and disease resistance againstV.harveyi.In other studies,administration ofBacillusspp.(e.g.B.subtilis,B.licheniformis)to Pacific white shrimp culture water,enhanced the activity of immune parameters including prophenoloxidase(ProPO),peroxinectin,lipopolysaccharide andβ-1,3-glucan-binding protein,lysozyme and serine protein(Madani,Adorian,Ghafari Farsani,& Hoseinifar,2018;Zokaeifar et al.,2014,2012).Moreover,the probiotic bacteria also enhanced disease resistance in Pacific white shrimp juveniles against pathogenicV.harveyi(Zokaeifar et al.,2012,2014).Recently,Cai et al.(2019)revealed enhanced weight gain and SGR of Pacific white shrimp and improved water quality,stimulated innate immune enzyme activities,digestive enzyme activities,stress tolerance and disease resistance toV.harveyiin shrimp after feedingB.licheniformisandB.flexuseither in single or in a combined form for 21 days at 28 ℃.
In most probiotic shellfish studies,evaluations have concentrated on the effect to promote the growth of cultures organisms,enhancement of the immune system and on control and outcompete the pathogens.However,less information is available on the probiotic effect on the gut microbiota.This is of importance to evaluate,as the gut microbiota provide multitude biological functions including growth,metabolisms,development and immunity.Vargas-Albores et al.(2017)evaluated the probiotic administration ofBacillusand Gamma-proteobacteria on the gut microbiota of Pacific white shellfish.Alpha diversity values(Shannon-index)were higher,2.03 for shrimp reared in the probioticbased system,compared to 1.64 for shrimp held in the traditional system.The taxonomic profile of the shrimp microbiome revealed that some bacteria were common,but probiotic administration had a significant effect on the bacterial profile,as bacteria belonging to Proteobacteria(Methylomonas methanica,Pseudomonas stutzeri,andPseudoxaanthomonas suwonensis),Firmicutes(B.subtilisandGeobacillusthermoleovorans),Bacteroides(Sphingobacterium ingobacterium),Deinococcus-Thermus(Oceanithermus profundus)and Tenericutes(Mycoplasma synoviae)were detected in shrimp cultured with the probiotic mixture.
5.15.Bacillus and poly-β-hydroxybutyrate(PHB)
In addition to the beneficial role of probiotics on shrimp growth,there are also reports suggesting that application of poly-β-hydroxybutyrate(PHB),a bacterial storage compound deposited intracellular in cytoplasm a cellular energy and carbon reserve by various bacterial species,have been reported as a biocontrol agent for crustacean culture and revealed promising control of vibriosis(Borah,Thakur,& Nigam,2002;Defoirdt,Boon,N.,Sorgeloos,Verstraete,& Bossier,2007;Jiang et al.,2008;Sui,Cai,Sun,Wille,& Bossier,2012).Two previous studies,displayed that differentBacillusspecies from different sources accumulated high amount of under optimized conditions(Kaynar &Beyatli,2009;Singh,Patel,& Kalia,2009).Defoirdt,Boon,Sorgeloos,Verstraete,and Bossier(2007)displayedB.thuringiensiscarry PHB,stimulated both the specific and non-specific immune mechanism in aquatic animals.Laranja et al.(2014)highlighted the beneficial role of PHB-accumulatingBacillusspp.on immunity and survival of shrimp species against pathogenic microorganisms.In a later study by the same research group,Laranja et al.(2017)reported thatArtemialarvae enriched withBacillussp.JL47 with PHB increased the relative expression of innate immune genes for ProPO,transglutaminase(Tgase)and heat shock protein 70(Hsp70)after challenge withV.campbellii.
Information about the use of other probiotics,Aeromonas,Alteromonas,Arthrobacter,Bifidobacterium,Clostridium,Paenibacillus,Phaeobacter,Pseudoalteromonas,Pseudomonas,Rhodosporidium,Roseobacter,Streptomyces,Vibrioand other potential probiotics used in shellfish aquaculture are presented in Table 3.
6.Aeromonas
GenusAeromonasis Gram-negative,facultative anaerobic,rodshaped bacteria,and are mainly associated with diseases.In shellfish aquaculture less information is available onAeromonasas probiotics.However,in an early study,Gibson,Woodworth,and George(1998)tested the probiotic ability ofAeromonas mediastrain A199 revealing antagonistic activity against several pathogens at 104CFU mL-1for 5 days on Pacific oyster(Crassostrea gigas)challenged withVibrio tubiashii,and revealed a significant effect on survival after 5 days.
7.Alteromonas
GenusAlteromonasbelongs to family Alteromonadaceae is commonly isolated from sea water,either in the open ocean or in the coast.They are Gram-negative and are curved rods with a single polar flagellum.Some information is available onAlteromonasas probiotics in shellfish aquaculture.Douillet and Langdon(1994)revealed that strain CA2,probably anAlteromonas,enhanced survival and growth of Pacific oyster(Crassostrea gigas)when administered in water.In two studies testing potential probiotics,Kesarcodi,Miner,Nicolas and Robert(2010)revealed thatAlteromonas macleodii0444 controlled ofVibrio splendidusinfection in Greenshell mussel(Perna canaliculus).Later,Kesarcodi,Kaspar,Lategan and Gibson(2012)displayed that strain 0444 also protected scallop(Pecten maximus)and flat oyster(Ostrea edulis)larvae againstV.coralliilyticus,V.splendidusandV.pectenicidainfections.
8.Arthrobacter
Arthrobacter,a genus of Gram-positive bacteria,have no spores and capsule,utilize a wide and diverse range of organic substances and displayed ability to produce antimicrobial compounds.Li et al.(2006)revealed thatArthrobacterXE-7 isolated from the culture water of Chinese white shrimp(Penaeus chinensis)and supplemented at a dose of 106CFU mL-1toP.chinensisin a 14 days study,and reported that the bacterium is non-pathogenic to the shrimp larvae,and increased total numbers of culturable bacteria in water;mostly related to the probiotic supplementation.In addition,NH3-N and NO3-N in the culture water was reduced.With regard to survival,administration did not affect survival.A 63-d feeding experiment determined the effects of dietary administration ofArthrobacterXE-7 on immune responses and resistance againstV.parahaemolyticusin the Pacific white shrimp(Li et al.,2008).Probiotic supplementation increased total hemocyte counts,percentage phagocytosis,respiratory burst activity,and serum phenoloxidase activity.With increasing dietary supplementation ofArthrobacterXE-7,shrimp mortality decreased in the challenge study.Vibriocounts in intestine of shrimp fed the probiotic bacterium was generally lower than that in the control shrimp.Xia,Zhu,and Zhang(2014)conducted a 25-day feeding trial usingArthrobactersp.CW9 isolated from guts of Pacific white shrimp,and revealed significantly higher survival rates,mean shrimp weights,phenoloxidase activity,phagocytic activity and clearance efficiency of Pacific white shrimp compared to control shrimp.Xue,Liang,He,and Wen(2016)reported significant improved survival of Pacific white shrimp administrated withArthrobacter enclensisisolated from the water column of Pacific white shrimp at a dose of 109CFU g-1in a 4 day study.In order to fully to conclude the probiotic effect ofA.enclensis;the study should lasted more than 4 days,and should evaluated immunostimulatory properties and challenge study.
9.Bifidobacterium
Bifidobacterium,called the“good bacteria”,is the first bacteria to colonize the intestinal tract in infants as they pass through the birth canal.A number of health benefits have been claimed for probiotic bacteria such asBifidobacteriumspp.because of the potential health benefits.Bifidobacteria are not as acid tolerant asLb.acidophilus;the growth of the latter organisms ceases below 4.0,while the growth of theBifidobacteriumspp.is retarded below pH 5.0(Shah,1997).However,these organisms are less incorporated into diets or rearing water in finfish and shellfish aquaculture.In a 95 days study,peptidoglycan(PG)derived fromBifidobacterium thermophilumenhanced the phagocytic activity of granulocytes and increased resistance ofP.japonicuswhen challenging toVibrio penaeicida(Itami et al.,1998),but PG administration had no effect on body weight.
10.Clostridium butyricum
C.butyricumcommonly reported in soil and intestines of healthy animals and humans is a butyric-acid producer and Gram-positive bacterium,and some strains have been used as probiotics to enhance growth and immune response in animals,humans as well as finfish(e.g.Han et al.,1984;Kuroiwa,Kobari,&Iwanaga,1990;Nayak,2010;Pan et al.,2008).In shellfish aquaculture some information exists on the use ofC.butyricumas probiotics.Duan et al.(2017)conducted a 56-day feeding trial to determine the effects ofC.butyricumon growth performance,health status,and resistance to ammonia stress in Pacific white shrimp,revealed increased growth performance,intestinal amylase and protease activity in the C.butyricum groups,while lipase activity was only affected in two groups.Total antioxidant capacity content,lysozyme activity,and the relative expression level of Toll and immune deficiency gene increased in probiotic groups.After exposion of ammonia stress,intestine immune biochemical parameters and genes(HSP70,Toll and Imd)expression level increased by C.butyricum feeding.Furthermore,probiotic administration increased the intestine epithelium height.Li,Tian,and Dong(2019a)fedC.butyricum,inclusion levels of 107-1012CFU kg-1,to Pacific white shrimp for 42 days and challenged withV.parahaemolyticus.There was significant enhancement of specific growth rate,feed conversion ratio,intestinal villi height and intestinal wall thickness.Activities of alkaline phosphatase,acid phosphatase,lysozyme and total nitric oxide synthase in serum.However,probiotic administration did not affect superoxide dismutase activity.Respiratory burst activity of haemolymph was significantly higher at administration of 108-1012CFU kg-1.Moreover,inclusion of 1011and 1012CFU kg-1revealed significant higher survival when Pacific white shrimp were challenged withV.parahaemolyticus.Li,Tian,Zhao,Jiang,and Dong(2019b)revealed thatC.butyricumincreased growth performance,improved intestinal histology in mid intestine,immune gene expression,and disease resistance againstV.parahaemolyticus.
11.Neptunomonas
GenusNeptunomonasbelong to family Oceanospirillaceae(Garrity,Bell,& Lilburn,2005)withinGammaproteobacteria,and are Gram-negative,facultative aerobic,oxidase and catalase positive,and they can use several carbohydrates,sugar alcohols,organic acids,and some PAH as sole carbon and energy sources.Kesarcodi-Watson,Miner,Nicolas and Robert(2010)demonstrated thatNeptunomonas0536 was capable to control infection caused byV.splendidusin Greenshell mussel(P.canaliculus).
12.Paenibacillus
Previously,Paenibacillusspecies were included in genusBacillusdue to their common morphological and physiological characteristics with the type speciesB.subtilis.However,in 1993,Paenibacilluswas reassigned as a new genus based on 16S rRNA gene sequences.GenusPaenibacillushas been isolated from humans,animals,and plants,but their use as probiotics in shellfish aquaculture is less investigated.Ravi,Musthafa,Jegathammbal,Kathiresan,and Pandian(2007)isolatedPaenibacillusfrom marine sediment and reported antagonistic activities againstVibriostrains.Based on their results,the author used thePaenibacillusstrain in a challenge study,and revealed improved disease resistance of giant tiger prawn againstV.harveyi.This results was surprising as giant tiger prawn was only treated for probiotic administration for one day prior to challenge.
13.Phaeobacter
Phaeobacterbelongs to theRoseobacterclade ofα-Proteobacteria.Phaeobacter gallaeciensisimprove the disease resistance of scallop larvae againstV.coralliilyticusandV.splendidus,and also protected flat oyster larvae againstV.coralliilyticusandV.pectenicida,and Pacific oyster larvae againstV.coralliilyticus,but no protection was noticed againstV.pectenicida(Kesarcodi-Watson,Kaspar,Lategan,& Gibson,2012).P.daeponensisis seldom used as probiotic in shellfish aquaculture,but recently Zhao et al.(2018)conducted a 180-day feeding trial to determine the effects ofP.daeponensison growth and health status of abalone,and disease resistance againstV.harveyi.The results revealed significant enhancement in shell length,wet weight,glutathione peroxidase-,catalase,alkaline phosphatase-,acid phosphatase-and superoxide dismutase activities,and disease resistance againstV.harveyiand modulation of the gut microbiota.
14.Pseudoalteromonas
GenusPseudoalteromonas,Gram-negative bacteria,previously members of genusAlteromonasis well-known to produce a wide variety of biologically active secondary metabolites(Bowman,2007).In an early study,Longeon et al.(2004)isolated aPseudoalteromonassp.X153 with high similarity toPseudoalteromonas piscicidafrom marine environment and displayed that the crude culture was highly active against human pathogens as well asVibriostrains.The probiotic strain was further tested in a challenge experiment with scallop(Pecten maximus),and the results revealed improved disease resistance againstVibriosp.PseudoalteromonasCDM8 and CDA22 isolated from hindgut of Pacific white shrimp was fed to Pacific white shrimp for 28 days and challenged withVibrio parahaemolyticus(Wang et al.,2018),and significant enhancement of disease resistance was noticed.Moreover,viableVibriocounts in hindgut was revealed.Sorieul et al.(2018)revealed thatPseudoalteromonassp.NC201 isolated from marine environment,enhanced survival by hyposaline stress of Pacific shrimp,and disease resistance againstV.nigripulchritudo,but the relative gene expression of lysozyme and penaeidin evaluated 24 h post infection was lower in probiotic fed shrimp.
15.Pseudomonas
GenusPseudomonasis Gram-negative bacteria belonging to the family Pseudomonadaceae and containing nearly 200 species.The genus has a great metabolic diversity,inhibiting a wide range of pathogenic bacteria(Ninawe&Selvin,2009),and are able to colonize a wide range of niches,including the GI tract of finfish and shellfish.Alvandi et al.(2004)isolated some Gram-negative bacteria(Pseudomonassp.PM11 andVibrio fluvialisPM17)from the gut of farm-reared shrimp,giant tiger prawn,and tested for their effect on the immunity indicators of black tiger shrimp.However,the results did not indicate desirable effect of improvement of the immune system in the shrimp.Administration ofPseudomonas aestumarinaisolated from intestine of adult Pacific white shrimp was fed to Pacific white shrimp for 28 days and challenged withV.parahaemolyticus(Balcázar,Rojas-Luna,& Cunningham,2007),and revealed significant enhancement of final weight,FCR and disease resistance againstV.parahaemolyticus.The application ofPseudomonasprobiotics(P.synxanthaandP.aeruginosa)improved FCR of Western king prawn(Penaeus latisulcatus),but no difference was revealed in SGR and survival(van Hai,Buller,&Fotedar,2009b),but administration of probiotics,enhanced the number of probiotics in intestine of prawn and lower the bacteria numbers in haemolymph.
16.Rhodosporidium
GenusRhodosporidium,yeast cells are globose,ovoid,or elongate and budding is multilateral or polar.The genus form visible carotenoid pigments and the cultures are pink to orange in color.In sexual reproduction it is seen that some species are heterothallic,whereas others are self-fertile(Sampaio,2011,pp.1523-1539).According to Scholz et al.(1999),Rhodosporidiuma carotenoid-rich red yeast,has successfully been used as dietary supplement in aquaculture as it reduces oxidative stress in aquatic animals.A strain ofR.paludigenumobtained from the coastal water at Zhanjiang,China has been included in the diet of Pacific white shrimp due to its high carotenoid content and because the strain exhibits safe and nontoxic growth in the intestinal mucus(Yang,Wu,Jian,& Zhang,2010).Dietary administration of either live(108CFU/g diet)or dryR.paludigenum(1 g/100 g diet)for 42 days to the shrimp significantly increased growth,serum and total antioxidant competence in muscle,and hepatopancreatic superoxide dismutase and glutathione peroxidase activities and decreased the malondialdehyde content in muscle.Dietary dryR.paludigenumsignificantly increased hepatopancreatic protease and lipase activity in shrimp,in contrast to liveR.paludigenumwhich only increased protease activity.Both live and dryR.paludigenumadministration to the diet of shrimp decreased the totalVibriocount in the intestine.
17.Roseobacter
Roseobacters,first described in 1991 with the discovery ofResobacter litoralisandRoseobacter denitrificans,and both were pinkpigmented bacterialchlorophyll strains isolated from marine algae.Roseobacterare thought to promote algal growth by biosynthesizing and secreting antibiotics and growth stimulants(auxins).Ruiz-Ponte,Samain,Sanchez,and Nicolas(1999)isolated aRoseobacter(BS 107)from marine environment and revealed that the bacterium in co-culture withV.anguillarum,was inhibitory toVibrio,with cell extracts of BS107 enhancing the survival of larval scallop(Pecten maximus).Balcázar et al.(2007)revealed significant improvement of survival,final weight,FCR and disease resistance againstV.parahaemolyticusin study whereRoseobacter gallaeciensisisolated from intestine of adult Pacific white shrimp was fed to Pacific white shrimp for 28 days and challenged withV.parahaemolyticus.
18.Streptomyces
GenusStreptomyces(phylum:Actinobacteria)are Gram-positive,high G+C(70%)genome content,soil-living bacteria with characterized branching filamentous morphology,and is an excellent antibiotic producer(Tan,Chan,Lee,& Goh,2016;Tarazona,León,Galindo,Vallejo,& Marguet,2018),and produce extract inhibits biofilm formation(Balasubramanian et al.,2017).The probiotic effects byStreptomycesthrough different mechanism of actions are;antagonistic compound production,anti-biofilm and anti-quorum sensing activity,anti-virulence activity,anti-viral activity,exoenzymes secretion,low pH tolerance and intestinal enzymes resistance etc.(Tan et al.,2016).The potential ofStreptomycesas probiotics in aquaculture has recently been reviewed by Tan et al.(2016),but is to a lesser extent used in shellfish aquaculture(Das,Lyla,& Khan,2006,2010;Aftabuddin,Kashem,Kader,Sikder,&Hakim,2013;Augustine,Jacob,&Philip,2016;García Bernal et al.,2018;García Bernal,Medina Marrero,Campa-Córdova,&Mazón-Suástegui,2017;Liu et al.,2014).
In an early study,Streptomyceswas used as a probiotic in a laboratory culture of giant tiger prawn,and the results revealed better water quality parameters,increased length and weight in terms of growthvs.control fed shrimp(Das,Lyla,&Khan,2006).Later,the same research group(Das,Ward,& Burke,2010)displayed that feed supplemented withStreptomycesstrain CLS-39 increased weight of giant tiger prawn,and the authors suggested thatStreptomycessecreted hydrolytic exoenzymes which improved the amylolytic and proteolytic activity in the shrimp GI tract for more efficient use of the feed.The study further evaluated the efficacy ofStreptomycesto protect the shrimp toV.harveyichallenge.Feed supplemented withStreptomycessp.for 15 days revealed protection of giant tiger prawn in a 12 h challenge withV.harveyi(LD50 at 106.5CFU mL-1).
Aftabuddin et al.(2013)isolated aStreptomyces fradiaefrom mangrove sediment;used the isolate in a study with giant tiger prawn,and revealed that inoculation ofS.fradiaeto the rearing water at a concentration of 106bacteria mL-1rearing water,and 109bacteria g-1feed;decreased CFU and viableVibriocounts per mL rearing water.Furthermore,an improved growth performance was noticed,but best effect was revealed by dietary supplementation.It is also worth to mention that similar effects were noticed when B.megaterium was used.These results showed the probiotic potential ofStreptomyces,but further evaluations including challenge-and immune response studies merits investigations.
Augustine et al.(2016)reported that a marineStreptomyces rubrolavendulaerevealed antagonistic activity towardsV.harveyi,V.alginolyticus,V.parahaemolyticusandV.fluvialisin anin vitroco-culture experiment.In order to confirm thein vitrofindings,the authors displayed in a 28 day study that biogranulesS.rubrolavendulaeM56 resulted in improved survival of giant tiger prawn post-larvae with a reduction of viableVibriosp.in the culture system.
In a study with Pacific white shrimp,García Bernal et al.(2017)studied the effect of two Streptomyces strains,N7 and RL8,supplemented either alone or in combination with Bacillus and Lactobacillus for 30 days and challenged with V.parahaemolyticus.The results revealed no significant effect on growth performance by N7 and RL8 administration.The Streptomyces groups,displayed a significant reduction of Vibrio from hepatopancreas,and the probiotic-fed groups had a higher hemocyte count.The challenge test revealed significantly higher survival rate by RL8 administration,but not by N7.Based on their results,the authors suggested that combination ofStreptomycesand aBacillusmixture,consisting ofB.tequilensis,B.endophyticusC2-2 andB.endophyticusYC3-B2 was the most promising group by improving growth performance,immune response,modulating microbiota and enhanced resistance against V.parahaemolyticus.The same research group,García Bernal et al.(2018)highlighted the potential of Streptomyces strain N7 and RL8 by growth-and histological evaluations of hepatopancreas,and protection against V.parahaemolyticus of Pacific white shrimp.
Even though Tan et al.(2016)discussed limitations ofStreptomycesas probiotics in aquaculture due to the two common semivolatile terpenoid compounds,geosmin and 2-methylisoborneol,the genus seems to have a probiotic potential that merits further investigations.
19.Vibrio
GenusVibriois Gram-negative,with a curved-rod(comma)shape and several species cause fish diseases.They are typically found in salt water,and they are facultative anaerobe and oxidase positive.Vibriosis is among the most common diseases leading to massive mortality of cultured shrimp,fish,and shellfish(Frans et al.,2011;Ina-Salwany et al.,2019).Even through most Vibrios caused diseases,some studies have used Vibrios as probiotic in finfish and shellfish aquaculture.A probiotic administration ofV.alginolyticusoriginal isolated from intestine of adult Pacific white shrimp was fed to Pacific white shrimp for 28 days and challenged withV.parahaemolyticus(Balcázar et al.,2007).There was significant(P<0.001)enhancement of final weight,FCR and disease resistance againstV.parahaemolyticus.
DietaryVibrioNE17 was fed to giant fresh water river prawn for 60 days(Rahiman et al.,2010).There was significant enhancement of SGR,weight gain,survival,water quality parameters,total haemocyte counts,phenoloxidase and respiratory burst.
20.Other potential probiotics
Two studies have revealed enhanced disease resistance againstV.tubiashiiandV.coralliilyticuswhenPhaeobactersp.S4 andPhaeobacter inhibenswas administrated to Eastern oyster(Crassostrea virginica)and bay scallop(Argopecten irradians)by Karim,Zhao,Rowley,Nelson,and Gomez-Chiarri(2013)and Sohn et al.(2016),respectively.Xue et al.(2016)isolated 27 bacteria species from the water column of Pacific white shrimp and tested them by probiotic administration on survival of Pacific white shrimp.Of the tested bacteria,three strains,Phaeobacter gallaeciensis,Arthrobacter enclensisandMicrobacterium aquimarissignificantly enhanced survival of the shrimp.The reason for this may be due to antagonistic activity,degradation of toxic organic compounds and production of bioactive compounds,but this hypothesis merits further investigations.
21.Combination of several potential probiotics
Information on the use of commercial probiotics in shellfish aquaculture is available(de Paiva Maia,Alves-Modesto,Otavio-Brito,Olivera,&Vasconcelos-Gesteira,2013;Kesselring et al.,2019;Madani,Adorian,Ghafari Farsani,& Hoseinifar,2018;Nimrat et al.,2012;Porubcan,1991;Wang et al.,2005).Some probiotic products like Super-biotic,Super Ps,Zymetin,and Mutagen(Soundarapandian,Ramanan,&Dinakaran,2010)were reported to play a vital role in giant tiger prawn post-larvae by maintaining good water quality parameters throughout the culture period.Boonthai et al.(2011)fed a mixture consisting ofB.subtilis,B.megateriumandB.thuringiensis;live sprayed and freeze-dried to giant tiger prawn for 120 days.TheBacillusspecies were isolated from giant tiger prawn intestine and shrimp pond,and the results revealed increasedBacilluscounts in hepatopancreas and intestine by probiotic administration.However,culturable bacteria counts in hepatopancreas,intestine and cultured water were not affected.The decreased counts ofV.parahaemolyticus,V.alginolyticus,V.vulnificusandV.cholera,andA.hydrophila,Shewanella putrefaciensandPhotobacterium damselaeobserved in hepatopancreas and intestine by probiotic feeding,respectively,and might be due to the antagonistic effects and out competition of the pathogenic bacteria;adherence and colonization.
Nimrat et al.(2011)reported that Pacific white shrimp fed freezedried microencapsulated probiotics,fiveBacillusstrains(B.thuringiensis,B.megaterium,B.polymyxa,B.licheniformisandB.subtilis)and two yeast strains(Debaryomyces hanseniiandRhodotorula)isolated from a shrimp pond,presented significantly improved growth performance,and survival rate;as well as increasing the number of additional beneficial microbial probiotics in shrimp larvae and postlarvae as well as bacterial community in culture water.
A multiple probiotic administration ofLb.plantarum,Lb.salivariusandLb.rhamnosusisolated from the gut of femaleP.pelagiuswas fed toP.pelagiusfor 14 days(Talpur et al.,2012).There was significant enhancement of growth and protease and amylase activities by multiple probiotics administration.Even though beneficial results were revealed,further studies on the effect on immune system and disease challenge should be evaluated.
de Paiva Maia et al.(2013)appraised the efficacy of commercialBacillusspp.probiotics on bacterial population and phytoplankton concentration in intensive Pacific white shrimp culture with a recirculation system.Probiotics bacteria improved total heterotrophic bacteria count in the sediment and caused a marked increase in the concentration of Pyrrophyta algae,and an improvement in the water and sediment quality.In closed recirculation systems,aeration is important to provide sufficient dissolved oxygen in the production system to maintain probiotic efficiency.
Nimrat et al.(2012)and Madani,Adorian,Ghafari Farsani and Hoseinifar(2018)assessed the effect of commercialBacillusprobiotics on growth performance,bacterial number,feed efficiency and body composition during rearing of Pacific white shrimp,and both studies revealed that administration of probiotics significantly improved length and weight gain,SGR and FCR compared to controls.
A microbial agens product MA containing Bacillaceae,Lactobacillaceae, Leuconostocaceae, Enterococaceae and Streptococcaceae and Rhodobacteraceae was fed Pacific white shrimp for 21 days(Liu et al.,2018).The results showed that MA feeding modulated the shrimp intestinal community,the relative abundance of Actinobacteria,Gammaproteobacteria,Deltaproteobacteria and Planctomycetes.However,probiotic feeding did not improve growth and survival.Azad et al.(2019)investigated the effect of a commercial product containing(B.mesentericus,C.butyricumandEnt.faecalis)at 5 g kg-1to giant fresh water river prawn for 60 days and challenged withVibriospp.andAeromonasspp.The results revealed significantly enhancement of total haemocyte count in the haemolymphs,resistance againstVibriospp.andAeromonasspp.Furthermore,probiotic administration modulate the culturable bacteria in water and intestine,by increasing the beneficial bacteria and declining some harmful bacteria.
A multiple-strain probiotics consisting of Lb.pentosus BD6,Lac.fermentum LW2,B.subtilis E20,Saccharomyces cerevisiaeP13 at inclusion level of 107,108and 109 CFU kg-1diet was fed Pacific white shrimp for 56 days and challenged with V.parahaemolyticus(Wang et al.,2019).There was significant enhancement of growth and health status of shrimp fed the diets containing BD6 and E20 at 109CFU kg-1,in contrast to diets containing P13 and LW2.No significant difference in the carcass composition was revealed among the control and treatments.Moreover,in groups fed the probiotic mixture groups,increased phenoloxidase activity,respiratory bursts,and lysozyme activity of hemocytes were revealed,leading to higher survival after injection with theV.alginolyticus.Based on their results,the authors suggested that the probiotic mixture could adequately provide probiotic efficiency for Pacific white shrimp,and recommended a probiotic mixture diet containing 108CFU kg-1diet.
22.Conclusions
The importance of probiotic administration,their beneficial health effects has been discussed in several reviews,for example Falcinelli et al.(2018)discussed the effect of probiotic appetite control,glucose and lipid metabolisms.Even though there is numerous information available on the use of probiotics in aquaculture,there is no concrete evidence to conclude that probiotics are better than immunostimulants or vaccines,the beneficial effects upon the host and their environment ensure that probiotics will remain one of the most promising approaches used to control diseases and the subsequent environmental modifiers(Newaj-Fyzul & Austin,2015).In shrimp aquaculture,manipulation of microbiota using probiotics have been shown to control or inhibit pathogenic bacteria,improve digestive enzyme activity and growth performance,and enhance immune responses of the host against pathogenic infection or physical stress.
The functionality of gut microbiota,depends on the ability of microorganisms to interact within the GI tract,which benefit the host through influence on inflammation,metabolism,immunity and even behavior(e.g.Neuman,Debelius,Knight,& Koren,2015;Boulange,Neves,Chilloux,Nicholson,&Dumas,2016;Ramírez&Romero,2017).When discussing disease resistance,the importance of a stable microbiota is of importance.In a early study,Collins and Carter(1978)revealed that germfree animals are more susceptible to diseases compared to corresponding conventional animals with a“complete gut microbiota”.Germfree mouse was killed with 10 cells ofSalmonella enteritidiswhereas 106cells were needed to kill conventional mouse with a conventional gut microbiota.Based in this finding,it is crucial to increase our knowledge on probiotics adhering and colonizing the GI tract of shellfish,in the context of improved growth performance and health.
In the recent review of Van Doan et al.(2019a)devoted to“hostassociated probiotics”in aquaculture,the authors revealed benefits of host-associated probiotics to include improved growth performance,feed value,enzymatic contribution to digestion,inhibit adherence,and colonization of pathogenic microorganisms in the GI tract,increase hematological parameters,and immune response.Probiotics should be of animal-species origin.This criterion is based on ecological reasons,and takes into consideration the original habitat of the selected bacteria;part of the indigenous gut microbiota.In an early study,Moriarty(1998)suggested that probiotic cultures could also originate from the general rearing environments;sinceBacillusspp.generally dwell in the sediment from which shrimps feed.Many studies believe that these bacteria have a better chance of out-competing resident bacteria and establishing themselves at a numerically significant level in their new host(e.g.Rengpipat et al.,2003;Alvandi et al.,2004;studies listed in Tables 1-3).In addition,the existence of a dominant bacterial strain in high densities in culture water indicates its ability to grow successfully under the prevailing conditions,and one can expect that this strain will compete efficiently for nutrients with possible harmful strains(Verschuere et al.,2000).The alternative strategy,host-associated probioticswithin shellfish aquaculture has gained attention as numerous studies showed in Tables 1-3,have used probiotics isolated from the host environment or the host,which fit into the definition given by Van Doan et al.(2019a);“bacteria originally isolated from the rearing water or the GI tract of the host to improve growth and health of the host”.However,in shellfish aquaculture,per seit is not clear,whether host-associated probiotics are more effective than probiotics from other origins,and this merits further investigations.
In future studies on probiotics in shellfish aquaculture,bio-floc culture system using probiotics should be investigated on growth performance,immune response,gut microbiome and disease resistance as only some information is available on this topic(Banerjee et al.,2010;Correa et al.,2018;Dash et al.,2018;Kim,Min,Kim,Koo,& Kang,2015;van Doan et al.,2019b).In addition to probiotics may also paraprobiotics(cell wall components;Taverniti & Guglielmetti,2011)serve as an alternative to the use of antibiotics in prevention and treatment of infections caused by pathogens.In this regard it is of interest to notice that both probiotics and paraprobiotics can bind directly pathogenic bacteria,which limits adherence and colonization of the pathogen to gut cells.
 Aquaculture and Fisheries2020年1期
Aquaculture and Fisheries2020年1期
- Aquaculture and Fisheries的其它文章
- Identification and modulation of expression of a TNF receptor superfamily member 25 homologue in grass carp(Ctenopharyngodon idella)
- Identification and histopathological and pathogenicity analysis of Aeromonas salmonicida salmonicida from goldfish(Carassius auratus)in North China
- Comparison of juvenile growth of wild and hatchery-raised olive flounders Paralichthys olivaceus as examined by otolith microstructure
- The effects of on-farm produced feeds on growth,survival,yield and feed cost of juvenile African sharptooth catfish(Clarias gariepinus)
- Biofloc technology(BFT):Adjusting the levels of digestible protein and digestible energy in diets of Nile tilapia juveniles raised in brackish water
