Identification and histopathological and pathogenicity analysis of Aeromonas salmonicida salmonicida from goldfish(Carassius auratus)in North China
Shibo Jin,Songzhe Fu,Ruijun Li,Huifeng Dng,Dongxu Go,Shigen Ye,Zhiqing Jing
a Agriculture and Rural Affairs Ministry Key Laboratory of Mariculture & Stock Enhancement in North China's Sea,Dalian Key Laboratory of Marine Animal Disease Control and Prevention,College of Fisheries and Life Science,Dalian Ocean University,Dalian,116023,China
b College of Marine Technology and Environment,Dalian Ocean University,Dalian,116023,China
Keywords:
ABSTRACT
1.Introduction
Aeromonas salmonicida is a Gram-negative brevibacteria that belongs to the genus Aeromonas(family Aeromonadaceae).These bacteria are widespread in the environment,especially in freshwater,and are pathogens of humans and animals(Tewari R et al.,2014).Recent studies have shown that A.salmonicida causes serious furunculosis and septicemia in many aquatic species(Dallairedufresne,Tanaka,Trudel,& Lafaille,2014)such as Chinese freshwater fish(largemouth bronze gudgeon and northern whitefish)(Long et al.,2016),Salmo salar(Inglis,Frerichs,Millar,&Richards,2010,Yi et al.,2016),Oncorhynchus mykiss(Bektas,Ayik,& Yanik,2007),Psetta maxima(Lago,Nieto,& Farto,2012),Esox lucius(Wiklund,2010),Petromyzon marinus(Diamanka,Loch,Cipriano,& Winters,2014),Carassius carassius(Wistbacka et al.,2009),and Sebastes schlegelii(Han et al.,2011).Until now,there have no reported cases of this pathogen in goldfish(Carassius auratus)in China.
In July 2017,massive mortality of goldfish occurred in an aquaculture farm in Anshan,Liaoning Province,North China.A predominant bacterial strain was isolated from the diseased goldfish.The results of the present study indicate that the pathogen isolated was A.salmonicida salmonicida.Identification of the strain of the isolated pathogen was based on physiological,biochemical,molecular and multilocus sequence typing(MLST)and the drug susceptibility of the strain,its pathogenicity and the histopathology of infected tissues was established.The results of the present study provide the basis for future studies of A.salmonicida salmonicida disease control and prevention epidemiology.
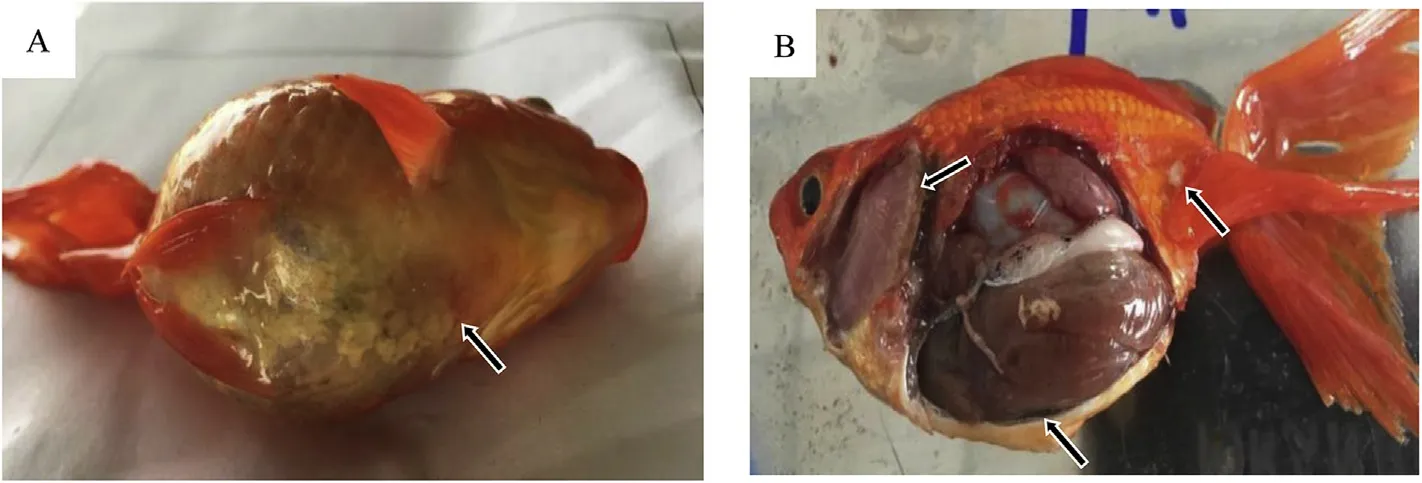
Fig.1.Naturally infected goldfish.A:The body surface has abundant mucus,there is fish jaw congestion,and abdominal bleeding;B:Anemia of gill filaments,skin fester,and a high amount of abdominal ascites.
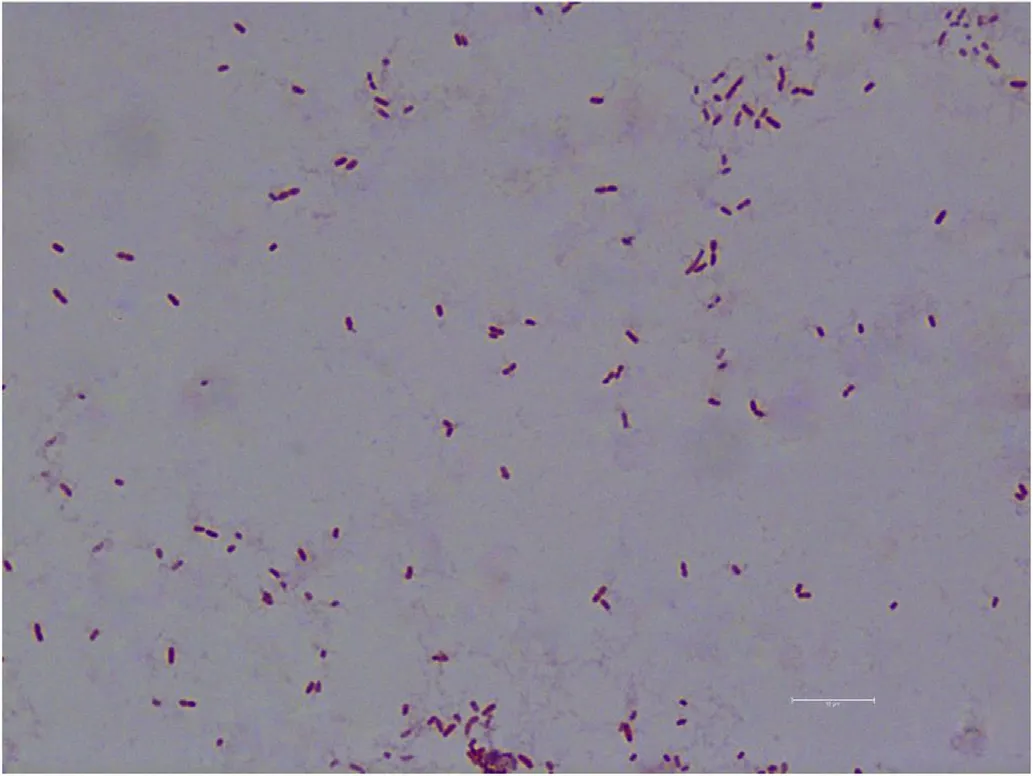
Fig.2.Gram-staining of AS.17(×1000).
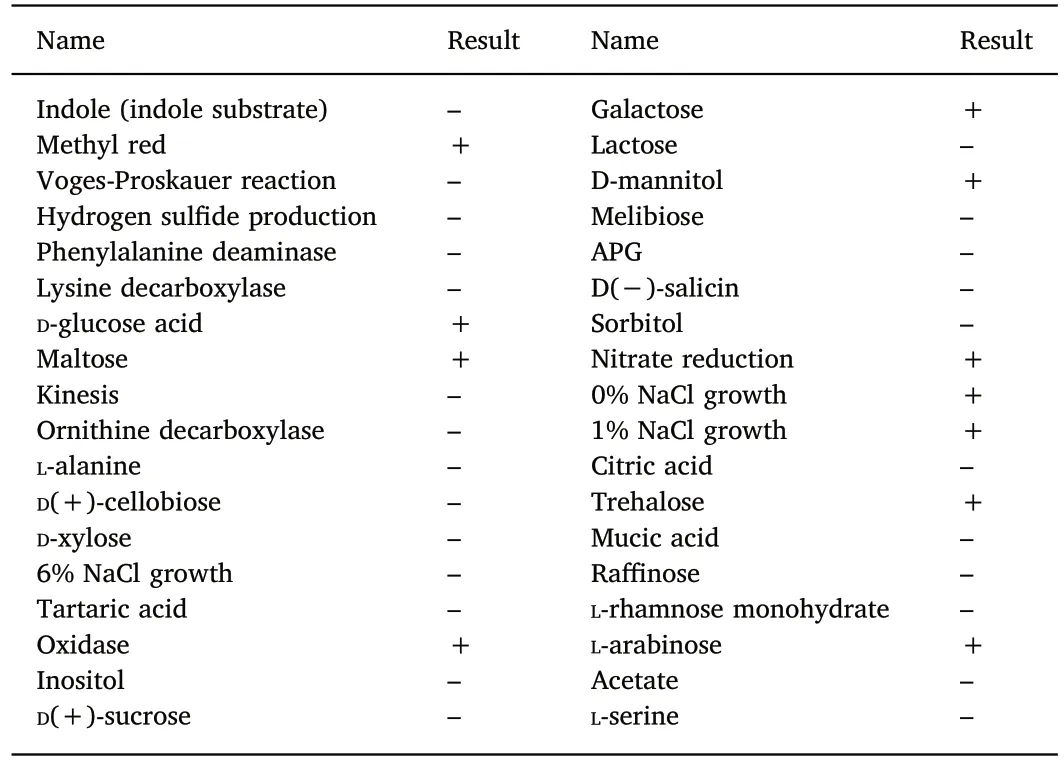
Table 1Physiological and biochemical characterization of isolates.
2.Materials and methods
2.1.Isolation of the pathogen
Bacteria from the liver,spleen,kidney,heart,and muscle of diseased fish(Fig.1)were inoculated on nutrient agar(NA)plates and incubated at 28℃ for 24 h.Predominant bacterial colonies were selected and grown on NA plates to obtain a pure culture.Colony morphology,size,shape,and color were analyzed using standard methods.
2.2.Identification of pathogen
To fulfill Koch's first and second postulate(the pathogen is present in all cases of the disease and can be isolated from the diseased host)(Grimes,2006),we employed 16S rDNA sequence analysis to verify if the isolated strains belonged to the same species.
The strains were stained with Gram stain following the protocol of the Gram staining kit(Solarbio,China)and preparations were examined with a microscope.Bacterial morphology was observed by light microscopy.The isolates were inoculated in bacterial physiological and biochemical reaction tubes(Hangzhou Tianhe Microbial Reagent Co.,Ltd).Bacterial identification was based on the manual of identification of common bacterial systems and physiological and biochemical criteria(Brenner,Krieg,& Staley,2011,pp.89-100).
Five strains were inoculated into NA broth and cultured at 28℃ for 18 h in a shaker at 220 rpm;the cells were then collected by centrifugation.Total bacterial DNA was extracted using the TIANamp Bacteria DNA Kit(Tiangen Biotech,Beijing,China).The DNA extracted from the bacterial isolates were used for PCR amplification of 16S rRNA genes with the universal primers 27F(5′-AGAGTTTGATCCTGGCT CAG-3′)and 1492R(5′-GATACCTTGTTACGACTT-3′)(Weisburg,Barns,Pelletier,& Lane,1991).PCR reaction conditions were as follows:denaturation at 94℃ for 5 min;and then 30 cycles of denaturation at 94℃ for 30 s,annealing at 55℃ for 30 s,extension at 72℃ for 1 min 30s,plus a final extension step at 72℃ for 10 min.The 16S rRNA PCR products were purified and sequenced and bacterial identity assigned using BLAST analysis in GenBank(https://blast.ncbi.nlm.nih.gov/Blast.cgi)and phylogenetic tree was constructed with Neiboring-Joining method using MEGA5.0 software(Tamura et al.,2011).
2.3.MLST typing
MLST was conducted as described by Maiden et al.(2013)to determine if the isolates belong to the same sequence type(ST).Briefly,PCR was conducted using previously described PCR primers for dnaE,dtdS,gyrB,pntA,pyrC,recA,and tnaA(Beaz-Hidalgo et al.,2013).The PCR products were analyzed by 1% agarose gel electrophoresis and visualized using an ultraviolet(UV)transilluminator.The amplified PCR products were isolated and sequenced using an ABI Prism 3700 DNA analyzer by Hetaibio(Beijing,China).Sequences were concatenated and uploaded to the MLST 1.8 server of the Center for Genomic Epidemiology for MLST typing(https://cge.cbs.dtu.dk//services/MLST/)(Larsen et al.,2012).
2.4.Antimicrobial sensitivity tests
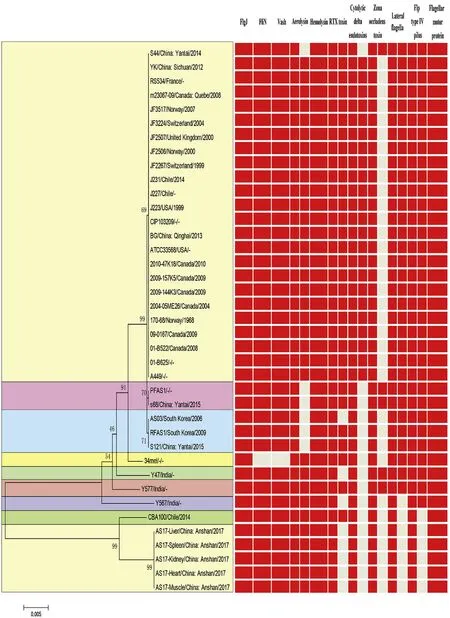
Fig.3.Strain AS.17 MLST typing.Phylogenetic tree was constructed with Neiboring-Joining method using MEGA5.0 software.The unit of the scale bar indicates the evolutionary distance in substitutions per nucleotide.Red:presence;Grey:absence.
Drug susceptibility was tested using the diffusion method(K-B method)(Jorgensen & Turnidge,2015,pp.1253-1273).The AS.17 strain was inoculated into NA broth and incubated at 28℃ overnight with shaking.The bacteria number was adjusted to 1.0×107CFU/mL and aliquots(0.1 mL)were evenly spread on NA plates,and then drug susceptibility papers were placed on the inoculated agar.To determine the susceptibility of the AS.17 strain to 17 common drugs(rifampicin,bacitracin,nalidixic acid,neomycin,lomefloxacin,erythromycin,vancomycin,gentamicin,tobramycin,spectinomycin,kanamycin,penicillin,streptomycin,ofloxacin,ciprofloxacin,tetracycline and compound sulfamethoxazole),the test plates were incubated upside down at 28℃ for 24 h,and then the diameter of the inhibition zone was measured.
2.5.Artificial infection
To fulfill Koch's third postulate,we verified if the strain isolated from dead goldfish caused the same symptoms in healthy,susceptible gold fish.The AS.17 strain was inoculated in NA broth and cultured at 28℃ for 24 h to prepare bacterial suspensions containing 1108,1×107,and 1×106CFU/mL.After one week of acclimation to the experimental circuit,15 healthy goldfish(43.2±0.5 g)were injected intraperitoneally with the bacteria suspensions(0.2 mL/fish);control fish(n=15)received 0.9% saline solution(pH=8.5).Experimental fish were quarantined and kept in a glass aquarium(240 L,0.8 m×0.5 m×0.6 m)after injection.The aquarium water(1/3 of the total)was changed every 2 days.The water temperature was kept at 24-28℃.The diseased and the number of dead fish were observed and recorded daily(8:00 a.m.,each day)for 8 days.
2.6.Histopathological analysis
The liver,spleen,kidney,and intestine were extracted from imminent death fish and control fish.The tissues were fixed with formaldehyde for 24 h at room temperature.Then,samples were dehydrated through a graded ethanol series(50%-100%),cleared in xyleneand embedded in paraffin wax.For staining,sectioned material was dewaxed in xylene,rehydrated through a graded ethanol series(100%-0%)and stained with hematoxylin and eosin.Permanent preparations were obtained by dehydrating(through an ethanol series 50%-100%),cleared in xylene and neutral resin was used to seal tissue sections.Sections were examined with a Leica DM4000 microscope.
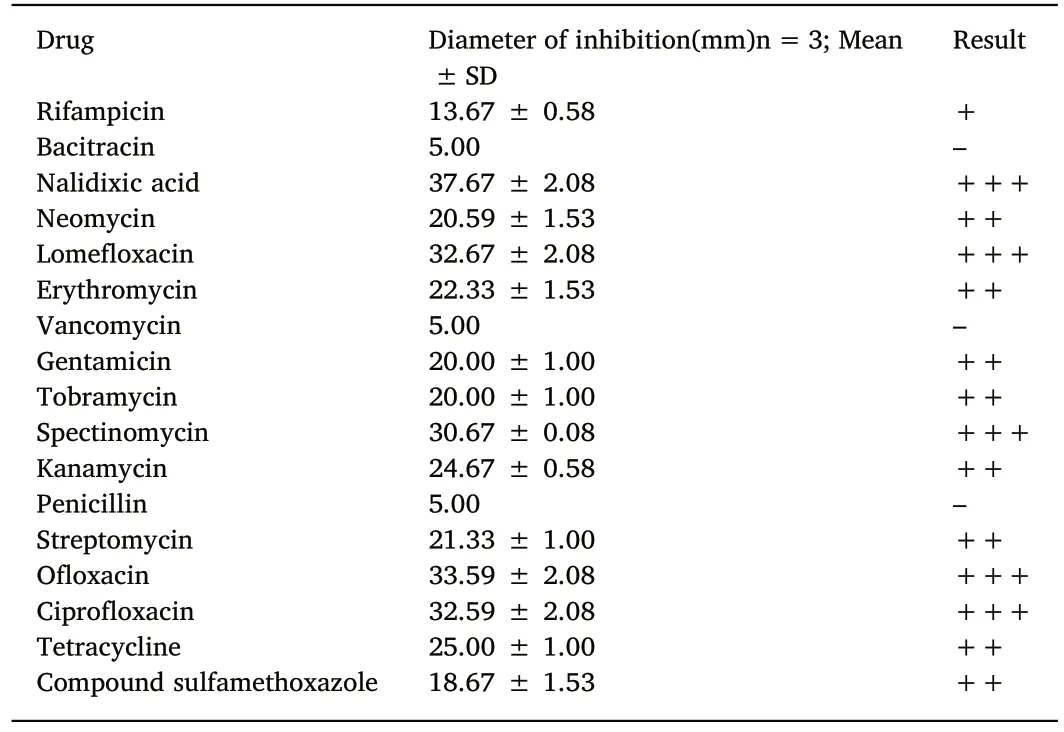
Table 2Sensitivity of the AS.17 strain to 17 kinds of antibiotics.
3.Results
3.1.Morphological and biochemical characteristics
Colonies growing on NA plates were slightly rounded,yellowish,and were approximately 1 mm in diameter.The isolates were not motile.The Gram-stained result were shown in Fig.2.The physical and chemical characteristics of the bacterial isolates were shown in Table 1.Based on 16S rDNA BLAST analysis and the results of physiological and biochemical experiments,the pathogen was identified as Aeromonas salmonicida salmonicida;the Genbank number of the 16S rDNA is MG384968.
3.2.MLST analysis
Strains of Aeromonas salmonicida(34 in total)were retrieved from NCBI and analyzed for their relationship to the pathogens isolated from the diseased C.auratus(Fig.3).The results of the MLST analysis showed that all the isolates from the diseased C.auratus were of the same type as the CBA100 strain(A.salmonicida salmonicida)and their virulence factors shared 100% sequence identity.All the isolates of the bacterial pathogens were named as AS.17.
3.3.Drug sensitivity
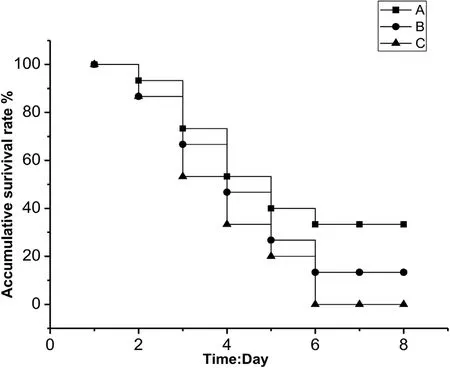
Fig.4.Cumulative survival rate(%)after 8 d of infection with AS.17 strain in Carassius auratus.Control group no deaths were detected at 8 d;A,Bacterial concentration of 1.0×106 CFU/mL;B,Bacterial concentration of 1.0×107 CFU/mL;C,Bacterial concentration of 1.0×108 CFU/mL.
The drug sensitivity tests of the strain AS.17 revealed it was highly sensitive to nalidixic acid,lomefloxacin,spectinomycin,ofloxacin,and ciprofloxacin(Table 2).The AS.17 strain was moderately sensitive to neomycin,erythromycin,gentamicin,tobramycin,kanamycin,streptomycin,and tetracycline(Table 2).The AS.17 strain was resistant to rifampicin,bacitracin,vancomycin,penicillin,and compound sulfamethoxazole(Table 2).
3.4.Artificial infection experiments
Artificial infection experiments showed that AS.17 was strongly virulent in goldfish.All the inoculated fish died after 6 days of infection(Table 3,Fig.4).The pathogenic bacteria were isolated from tissues and organs of the AS.17 inoculated goldfish.The colony morphology and growth characteristics of the strains isolated from the goldfish were the same as the strain AS.17.The symptoms of the diseased fish in the infection experiment was similar to those of a natural infection.In our experiments,the main symptoms were bleeding at the base of the fin,bleeding at the jaw,and a large amount of ascites in the body cavity accompanied by symptoms of enteritis.The analysis of 16S rDNA gene sequence and physiological and biochemical identification showed that the pathogen isolated from the AS.17 inoculated goldfish was A.salmonicida salmonicida(Data not shown).Koch's fourth postulate was fulfilled by re-isolation of A.salmonicida salmonicida from the artificial infection experiments.
3.5.Histopathology
Biopsies were compared microscopically to analyze pathological changes between the healthy and diseased fish from the AS.17 inoculation experiment.Pathological changes in diseased fish were observed in liver,kidney,spleen,and intestine(Fig.5A).shows healthygoldfish liver cells that have a circular arrangement and clear cell boundaries.In the liver from infected fish there was congestion,hepatocyte necrosis,and vacuolization or even dissolution of cells(Fig.5B).
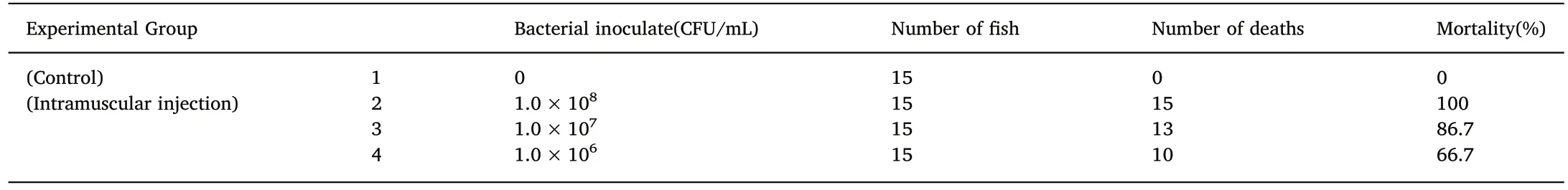
Table 3Mortality of Carassius auratus infected with AS.17 strain.
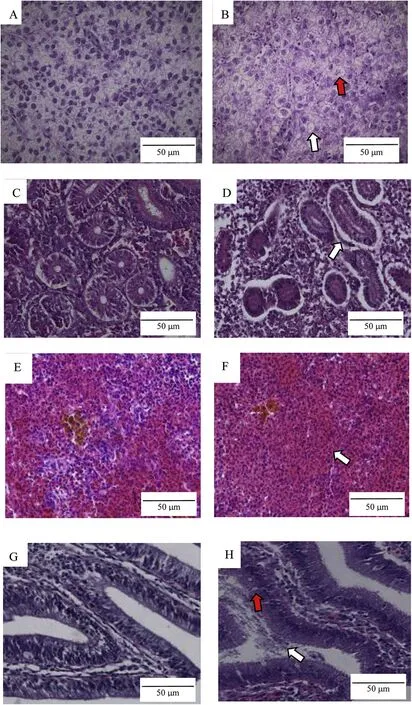
Fig.5.Histopathology of tissue from Carassius auratus infected with A.salmonicida salmonicida.A,liver of healthy goldfish(×400).B,vacuolization of cells(white arrow);dissolution of cells(red arrow);liver of infected goldfish(×400).C,kidney of healthy goldfish(×400).D,basement membrane separation(white arrow);kidney of infected goldfish(×400).E,spleen of healthy goldfish(×400).F,white pulp disappear(white arrow)spleen of infected goldfish(×400).G,intestine of healthy goldfish(×400).H,epithelial cells necrosis(red arrow);intestinal contents(white arrow);intestine of infected goldfish(×400).The scale bar represents 50μm.(For interpretation of the references to color in this figure legend,the reader is referred to the Web version of this article.)
In the kidney of healthy goldfish,the boundary between the epithelial cells in renal tubular cross-sections was clear,the renal tubules were connected with the surrounding tissues,and the organization of the glomeruli and the renal vesicles was orderly(Fig.5C).In the kidney of infected goldfish there was basement membrane separation,and increased cystic space(Fig.5D).
The healthy goldfish spleen cells had a circular organization and the cell boundaries and the white pulp was clear(Fig.5E).In spleens from diseased goldfish the cells were vacuolized and some were disintegrating and the white pulp was lost(Fig.5F).
The layers of the intestine in healthy goldfish were orderly(Fig.5G).In infected fish(Fig.5H)there was hyperemia,epithelial cells necrosis,and an increased number of mucus cells and intestinal contents(Fig.5H).
4.Discussion
Research on A.salmonicida from goldfish(C.auratus),one of the most important ornamental fishes around the world(Komiyama et al.,2009),is very limited.In this study,bacterial pathogens were isolated from diseased C.auratus.MLST analysis showed that the bacterial isolates from different diseased organs were of the same type,and clustered in phylogenetic analysis with A.salmonicida salmonicida.We named the bacterial pathogen,AS.17.Artificial infection experiments with AS.17 showed that the infected goldfish had the same symptoms as the naturally diseased C.auratus.In addition,MLST analysis indicated that AS.17 has many virulent factors and most likely strong pathogenicity.This study is the first to report notorious A.salmonicida salmonicida isolates from C.auratus in North China.Further research on the virulence factors and genome of this isolate(AS.17)is ongoing.Moreover,in view of the ease of pathogen transmission and spread by ornamental fish,epidemiological studies of A.salmonicida salmonicida are urgent.
The AS.17 strain was resistant to rifampicin,bacitracin,vancomycin,penicillin,and compound sulfamethoxazole.The notable drug resistance of AS.17 may be due to the extensive local use of antibiotics for bacterial control in aquaculture.In the past decade,there has been an increasing use of probiotics by the China's aquaculture industry,at present more than a hundred companies are producing many types of probiotics for aquaculture,and probably over 50,000 t of commercial probiotic products are sold annually with a market value estimated at 50 million euros(Fečkaninová,Koščová,Mudroňová,Popelka,&Toropilová,2017).However,there is a lack regulations or guidelines for the use of drugs for non-edible aquatic products and ornamental fish.The situation is even worse in the ornamental fish industry,and urgent action is required and emerging bacterial antibiotic resistance and the mechanisms behind it deserve more attention.
The main A.salmonicida histological lesions generalized vascular changes,such us intense vascular congestion,edema,microvascular thrombosis,widespread hemorrhages,epithelial cells necrosis,separation of renal tubules and basilar membrane(Peters,Unger,Brunner,&Kirkpatrick,2003;Remick,2007;Vale et al.,2007).In the present study,after artificial infection of goldfish with the AS.17 strain,histological analyses showed that the spleen and the kidney of infected fish had severe pathological alterations.It is worth noting that our findings were similar to those observed in salmonid species infected with A.salmonicida and other diseases caused by A.hydrophila,atypical A.salmonicida or V.anguillarum(Björnsdóttir,Gudmundsdóttir,Bambir,&Gudmundsdóttir,2005;Burr,Pugovkin,Wahli,Segner,& Frey,2005;Rey,Verján,Ferguson,& Iregui,2009;Treasurer,Birkbeck,Laidler,&Cox,2014).
This study reports for the first time the isolation of A.salmonicida salmonicida from C.auratus in North China.Results of the study will provide a reference and he basis for future studies on A.salmonicida disease control,prevention,and epidemiology.
5.Conclusions
We report A.salmonicida salmonicida(AS.17 strain)isolated from C.auratus in North China.The results will provide the basis for future studies on A.salmonicida salmonicida disease control and prevention epidemiology.
 Aquaculture and Fisheries2020年1期
Aquaculture and Fisheries2020年1期
- Aquaculture and Fisheries的其它文章
- Probiotics in shellfish aquaculture
- Identification and modulation of expression of a TNF receptor superfamily member 25 homologue in grass carp(Ctenopharyngodon idella)
- Comparison of juvenile growth of wild and hatchery-raised olive flounders Paralichthys olivaceus as examined by otolith microstructure
- The effects of on-farm produced feeds on growth,survival,yield and feed cost of juvenile African sharptooth catfish(Clarias gariepinus)
- Biofloc technology(BFT):Adjusting the levels of digestible protein and digestible energy in diets of Nile tilapia juveniles raised in brackish water
