Effect of protease inhibitor from Agaricus bisporus on glucose uptake and oxidative stress in 3T3-L1 adipocytes
Reena Vishvakarma, Abha Mishra✉
School of Biochemical Engineering, Indian Institute of Technology (Banaras Hindu University), Varanasi, Uttar Pradesh-221005, India
ABSTRACT Objective: To explore the effect of the protease inhibitor from Agaricus bisporus (J.E. Lange) Imbach (AbPI) on glucose uptake and oxidative stress in 3T3-L1 adipocytes.Methods: Adipocytes were differentiated and stained with Oil-Red-O staining to confirm adipogenesis. The toxic/protective effect of AbPI on the adipocytes was determined by MTT assay,intracellular reactive oxygen species generation through flow cytometry, and morphologically through confocal microscopy using propidium iodide, 4,6-diamino-2-phenylindol dihydrochloride, and 2',7'-dichlorofluorescein diacetate dyes. The uptake of fluorescent glucose analog, 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose by adipocytes was also studied through confocal microscopy.Results: MTT assay showed that the cell survival rate was(28.00±3.00)%, (92.33±2.60)%, and (71.34±2.10)% in the presence of 2 mM H2O2, AbPI alone, and AbPI and H2O2 both, respectively,in comparison to the control. Oil-Red-O staining indicated that AbPI enhanced adipogenesis. AbPI stimulated the glucose uptake by adipocytes similar to the drug rosiglitazone, and showed insulinsensitizing effect in the presence of insulin, but failed to stimulate the uptake in the absence of insulin. Intracellular reactive oxygen species generation was reduced in differentiating adipocytes upon AbPI treatment. Confocal microscopy showed that the damaged cell population rose to 3.50%, 117.84%, and 261.50% in the presence of AbPI alone, AbPI with H2O2, and H2O2 alone, respectively.Conclusions: The protease inhibitor enhances glucose uptake by adipocytes and exhibits a cytoprotective effect on them.
KEYWORDS: Protease inhibitor; Agaricus bisporus; 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose; Oxidative stress; Hydrogen peroxide; 3T3-L1 adipocytes
1. Introduction
Oxidative stress is the excessive generation of reactive oxygen species (ROS) such as superoxide (·O2-), hydroxyl radical (·OH),hydrogen peroxide (H2O2), and singlet oxygen (O2) species/ reactive nitrogen species within a cell, and is unable to be subdued by the cellular antioxidant defence mechanism[1]. Cellular respiration is the major source of ROS generation but external stimuli such as heat stress or oxidative stress are also stimulants of ROS generation. A balance between the generation and elimination of ROS is crucial for cell survival. ROS are important in intracellular signaling pathway but increased intracellular concentration of ROS leads to severe oxidative damage, which is detrimental to the integrity of the cell, and ultimately causing programmed cell death such as apoptosis[2]. Oxidative stress can be corrected by antioxidant enzymes such as superoxide dismutase, glutathione peroxidase (GSH-Px), glutathione reductase and catalase, and non-enzymatic compounds such as glutathione,vitamin A, C and E, coenzyme Q10, carotenoids, bioflavonoids,minerals such as copper, zinc, manganese and selenium, folic acid,albumin, vitamin B1, B2, B6 and B12[3].
Inflammation and oxidative stress in adipose tissue may lead to obesity which can further develop disorders such as diabetes, cardiac disorders, and cancer, hence they can be used as a model to study oxidative stress, antioxidant mechanisms, diabetes mellitus, and heart diseases[4]. Adipocytes are cells that store energy in the form of fats as lipid droplets, majorly composed of triglycerides. The adipocytes are a potential tool to study glucose uptake within the cells, the role of insulin and other potent anti-diabetic drugs in maintaining the glucose homeostasis. 3T3-L1 is a mouse fibroblast cell whose differentiation into adipocytes is quite extensively studied to understand adipogenesis[5].
Adipogenesis is a complex process, tightly regulated by transcriptional factors such as peroxisome proliferator-activated receptor-gamma(PPARγ), a member of the nuclear receptor superfamily of ligandinducible transcription factors which maintains homeostasis and insulin sensitivity and expression of adipokines such as adiponectin, an insulinsensitizing hormone known to have anti-diabetic properties[6].
For diabetes studies involving glucose uptake by cells, adipocytes fare better than other cells, as adipocytes upon differentiation become more responsive to insulin. The enhanced insulin responsiveness increases the glucose transporter-4 (GLUT4) mobility from the intracellular region (present in vesicles) towards the plasma membrane. GLUT4 is responsible for glucose transportation through facilitative diffusion and is the major glucose transporter in adipocytes, heart, and skeletal muscle, solely responsible for the glucose uptake from the extracellular environment. Hence, even at lower insulin concentrations, high glucose uptake can be achieved using mature adipocytes. In mature adipocytes,GLUT4 is highly expressed due to insulin, stimulating glucose uptake and reducing the glucose level in blood. The downregulation of GLUT4 leads to insulin resistance, while over-expression enhances glucose tolerance in adipocytes. Hence, GLUT4 plays a crucial role in body glucose homeostasis[7].
The glucose uptake can be tracked using a non-invasive, simple, and safe method by 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-d-glucose (2-NBDG), a fluorescent glucose analog, as it is also transported within cells by GLUT4, the same transporter involved in intracellular glucose transport[8].
PPARγ hyper-activation leads to diseases such as obesity, while inhibition causes an anti-adipogenic phenomenon. PPARγ agonists such as thiazolidinediones (TZDs) are insulin-sensitizing drugs and are helpful in type 2 diabetes treatment. TZDs effectively control diabetes but have several undesirable effects on the body such as weight gain and cardiac problems[9]. Recent studies have focused on discovering new agonists of PPARγ with less adverse effects as of TZDs, from natural compounds from plants and microorganisms.
Mushrooms are one such microorganism used for human consumption with benefits of lowered risk of cancer, heart diseases, and diabetes.Mushrooms have a high protein content, hence are a source of bioactive proteins that have therapeutic properties[10,11]. Agaricus bisporus (A.bisporus) (white button mushroom) is a common edible mushroom with therapeutic properties against high blood glucose and cholesterol levels[12,13]. Protease inhibitors inhibit protease enzymes involved in the various cellular process and signaling pathways. Protease inhibitors are common in most of the living organisms and provide a defense mechanism to the host. The use of protease inhibitors in therapeutics is evident in cancer, muscular dystrophy, inflammation, rheumatic arthritis, diabetics, disseminated sclerosis and other diseases[14]. A protease inhibitor extracted from the mushroom A. bisporus, which is effective against serine proteases[15]was utilized to check its therapeutic potential, through a preliminary in-vitro study, as an anti-diabetic agent by studying its ability on the uptake of fluorescent glucose analog 2-NBDG and as an antioxidant against hydrogen peroxide oxidative stress in the 3T3-L1 adipocytes.
2. Materials and methods
2.1. Chemicals
Hydrogen peroxide (H2O2), 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 2’,7’-dichlorofluorescein diacetate (DCFDA), propidium iodide (PI), 4,6-diamino-2-phenylindol dihydrochloride (DAPI), dimethylsulfoxide (DMSO),Dulbecco’s modified eagle medium (DMEM), fetal bovine serum(FBS), trypsin-EDTA, penicillin, streptomycin, insulin, were purchased from HiMedia, India. All chemicals and reagents used were of analytical grade.
2.2. Sample preparation
3T3-L1 mouse fibroblast cells were purchased from the National Centre for Cell Science Pune, India. The purified fraction of the protease inhibitor obtained from A. bisporus was lyophilized at-44 ℃, dissolved in 0.1% DMSO and was maintained at -20 ℃ for further experiments. The protease inhibitor was proteinaceous and purified through ammonium sulphate precipitation, ion-exchange chromatography[15](results not included), and gel filtration chromatography. The characterized protease inhibitor was found to exhibit uncompetitive inhibition against trypsin protease enzyme,with a molecular weight of 24.6 kDa (227 amino acid peptide),maximum activity at pH 7.0 and temperature 50 ℃. The purified protease inhibitor was studied for antioxidant potential, DPPH, and ABTS·+scavenging activity comparable to ascorbic acid[16](results not included).
2.3. 3T3-L1 adipocyte culture and maintenance
DMEM was used for the growth and maintenance of the 3T3-L1 mouse fibroblast cells in a 25 cm2tissue culture flasks[17]at 37 ℃in a humidified chamber supplemented with 5% CO2. DMEM consisted of 5.5 g/L glucose, L-glutamine and 1 mM sodium pyruvate and was supplemented with 3.7 g/L sodium bicarbonate,100 U/mL penicillin, 100 μg/mL streptomycin, and 10% (v/v) FBS.The pH of the medium was adjusted to 7.4 using 1 mol/L HCl and 1 mol/L NaOH buffers and sterilized using a 0.22-μm filter. The cells were grown up to 70%-90% confluency. Sub-culturing of the cells was done when they attained 90% confluency after 2-3 days of growth and were maintained for further experiments between fourth and sixth passages.
2.4. Cell viability measurement
Cytotoxicity of the protease inhibitor from the different fractions of A. bisporus (AbPI) [crude extract, ion exchange chromatography fraction (IEX), and gel filtration chromatography fraction (GF)], the least toxic concentration of AbPI, and effect of AbPI in the presence and absence of H2O2on the adipocytes were determined through MTT assay[18]. The cells were seeded in a 96 well plate (1×104cells/well) and incubated in a humidified chamber containing 5%CO2for 24 h at 37 ℃. The 24 hours incubation was to facilitate the adherence of the cells. The media in each well were replaced with the fresh media and with different fractions of AbPI (crude extract,IEX, and GF); varying concentrations from 5-100 μg/mL of the least toxic AbPI fraction (except in control); and finally, 2 mM H2O2, 10 μg/mL AbPI alone, and 2 mM H2O2with 10 μg/mL AbPI and again incubated at 37 ℃ in a humidified chamber containing 5% CO2for 24 h. The control and media containing the AbPI were replaced with 20 μL of MTT (in PBS) and incubated in dark for 2 h followed by 100 μL of DMSO (100%) with incubation for 30 min with gentle shaking. Absorbance was taken at 590 nm using Synergy HI Microplate reader (Biotek). The blank consisted of only the media.Survival rate (%) = [(Asample-Ablank) / (Ac-Ablank)]×100
2.5. 3T3-L1 pre-adipocyte differentiation
3T3-L1 pre-adipocytes were differentiated in mature adipocytes.The procedure followed was in accordance with the method given by Alonso-Castro et al[19]. 3T3-L1 pre-adipocytes (4×104cells/well) were seeded in a 12-well plate and incubated to reach 90%confluency. The pre-adipocytes were maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, at 37 ℃ with 5% CO2in a humidified chamber. When the cells reached 70% confluency on the second day of incubation(Day 0), the media was replaced with differentiation initiation medium (DMEM, 10% FBS, 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone and 10 μg/mL insulin) for 2 d. On day 2, the media was replaced with differentiation progression medium or induction medium (DMEM, 10% FBS and 10 μg/mL insulin) for 2 d. On day 6, the media was replaced with maintenance medium (DMEM, 10%FBS) to obtain fully differentiated adipocytes. The maintenance medium was replaced after two days till day 10 to attain completely differentiated adipocytes. To study the effect of the protease inhibitor on adipogenesis, the cells were treated with 10 μg/mL of the inhibitor during the differentiation process. Untreated cells were taken as control. Also, a negative control was prepared by supplementing the pre-adipocytes with the basal medium (DMEM, 10% FBS) without insulin and with/without 10 μg/mL of the protease inhibitor.
2.6. Oil-Red-O staining
The differentiation of the pre-adipocytes into adipocytes,which involves adipogenesis was confirmed through Oil-Red-O staining where the dye stained the lipid droplets produced during differentiation[20]. The mature 3T3-L1 adipocytes were grown in a 12-well plate at 37 ℃ in a humidified atmosphere supplied with 5% CO2for 24 h. The cells were then washed with 1×PBS twice.The cells were fixed in 10% formalin (in PBS) for 1 h at 37 ℃ in a humidified chamber with 5% CO2.The cells were washed thrice with 1×PBS and incubated with 60% isopropanol for 10 min.The isopropanol was discarded and the cells were allowed to dry completely to stain with 1 mL of 0.3% Oil-Red-O solution. The cells with the stain were incubated for 30 min. The stain was removed and cells were washed twice with MilliQ water. The cells were air-dried and visualized under Eclipse TS100 Nikon inverted microscope at 40×magnification. The absorbance of the cells was determined at 540 nm to quantify the lipid content.
2.7. 2-NBDG uptake by 3T3-L1 adipocytes
The modified procedure of Alonso-Castro et al[19]was followed for the determination of fluorescent glucose uptake by 3T3-L1 adipocytes. The 10-day old cells (mature adipocytes) were grown in 12-well plate (1×104cells/well) in DMEM (without FBS and glucose, and with 100 U/mL penicillin, 100 μg/mL streptomycin)for 48 h at 37 ℃. The adipocytes were incubated along with 80 μM of the fluorescent glucose analog, 2-NBDG in the medium[21]. AbPI from A. bisporus (1.25-10 μg/mL), rosiglitazone (1.25-10 μg/mL), a TZDs family drug, which enhances the insulin sensitivity through activation of PPARγ, and insulin (1.25-10 μg/mL) were added to the adipocytes in DMEM and 2-NBDG, to study their individual effect on the glucose uptake by the 3T3-L1 adipocytes. AbPI at 10 μg/mL and the drug rosiglitazone (10 μg/mL) in presence of 10 μg/mL insulin were also studied to understand the synergistic effect of the inhibitor and insulin as well as rosiglitazone and insulin on the glucose uptake by the 3T3-L1 adipocytes. The samples were incubated for 2 h at 37 ℃. The uptake reaction was stopped by washing the cells twice with ice-cold 1×PBS. A control consisted of the adipocytes in DMEM and 2-NBDG. Rosiglitazone and insulintreated adipocytes were taken as the positive and negative control,respectively. The fluorescence intensity of the cells was determined using the Synergy HI Microplate reader (Biotek) at an emission wavelength of 540 nm.
Confocal microscopy was performed to visualize the uptake of 2-NBDG through fluorescence emitted by the glucose incorporated into the adipocytes. A total of 20 μL of the cell suspensions of the samples treated with AbPI (10 μg/mL), rosiglitazone (10 μg/mL), insulin (10 μg/mL), AbPI with insulin (10 μg/mL each),rosiglitazone with insulin (10 μg/mL) and control were placed on glass slides and air-dried. The adipocytes were visualized at 40×magnification to study the 2-NBDG uptake. Rosiglitazone and insulin-treated adipocytes were taken as the positive and negative control, respectively. Cells in DMEM were taken as control. The fluorescence intensity [corrected total cell fluorescence (CTCF)] of the images acquired was determined using ImageJ software using the following formula:
CTCF= Integrated density-(Area under study×Mean fluorescence of the background)
The background was the region selected just beside the cell emitting fluorescence[22]. Graphs were plotted using GraphPad Prism version 8.3.0 (538).
2.8. Oxidative stress studies
Intracellular ROS generation during the pre-adipocyte differentiation was determined using DCFDA which is a fluorogenic dye and could determine the ROS activity in the intracellular environment. When DCFDA/H2DCFDA enters into the cell, it is cleaved by intracellular esterases into its non-fluorescent H2DCFanion (low fluorescence emitting compound) form. The H2DCFanion is then oxidized into highly fluorescent oxidized state 2’,7’-dichlorodihydrofluorescein (DCF) by ROS[23].
3T3-L1 pre-adipocytes upon 90% confluency (4×104cells/well) were seeded in a 12-well plate along with the differentiation induction medium and protease inhibitor (10 μg/mL) for 2 d followed by differentiation progression medium. After 5 d, the differentiated adipocytes were treated with 10 μL (5 μg/mL) of DCFDA for 30 min. The cells were again washed with 1×PBS and treated with 500 μL of 1×Trypsin-EDTA at 37 ℃ for 2 min,to detach the cells. Trypsin-EDTA action was terminated using icecold 1×PBS. Cells were subsequently resuspended in 1 mL of cold fresh DMEM (5.5 g/L D-glucose, L-glutamine, 100 U/mL penicillin,100 μg/mL streptomycin and 10% FBS). Untreated adipocytes were taken as control. The experiment was performed in triplicate.The samples were prepared in the dark and analyzed using the BD FACSCaliburTMflow cytometer using Cell Quest software. A total of 5000 single-cell events were taken for each analysis with an excitation and emission wavelength of 488 and 569 nm, respectively.A histogram plot was generated to quantify the cell population for each sample[24].
The effect of the protease inhibitor on the H2O2induced stress in 3T3-L1 adipocytes was studied through confocal microscopy[25]using three fluorescent dyes to understand the effect of the oxidative stress on mature 3T3-L1 adipocytes: PI (red fluorescence), DAPI(blue fluorescence) and DCFDA (green fluorescence).
PI is a fluorescent intercalating agent to visualize the nuclei of dead cells as it is unable to cross the live cell membrane. So, PI helps differentiate necrotic, apoptotic, live and healthy cells[26]. The other fluorescent dye used to study the damaged nuclei is DAPI which binds to the adenine-thymine rich regions in the nucleic acid(DNA). It stains both live and dead cells, though it passes through the cell membrane less efficiently in live cells[27]. Live cells can be quantified using DCFDA to determine the ROS activity in the intracellular environment[23] after inducing stress conditions.
To observe the 3T3-L1 adipocytes under oxidative stress in presence of the fluorescent dyes, the 10-day old cells (mature adipocytes)were grown in 12-well plate (1×104cells/well) (each well with 12 mm round coverslip) in DMEM and 10% FBS, glucose, 100 U/mL penicillin, and 100 μg/mL streptomycin for 48 h at 37 ℃. The 3T3-L1 adipocytes (500 μL) were washed with 1×PBS twice and observed under an inverted microscope. The adipocytes were then incubated with the 2 mM H2O2(500 μL), 2 mM H2O2and 10 μg/mL AbPI (250 μL each), 10 μg/mL AbPI (500 μL) and MilliQ water (control) (500 μL) at 37 ℃ for 1 h. The cells were again washed with 1×PBS,visualized under an inverted microscope and treated with 10 μL (5 μg/mL) of the respective dye (PI, DCFDA, and DAPI) and allowed to air dry.
The fixed slides were visualized under the confocal microscope(Carl Zeiss LSM 780) at 40×magnification to study the degraded nuclei using a blue filter (DAPI), apoptotic nuclei using a red filter (PI) and the live cells using the green filter (DCFDA).The fluorescence intensity (CTCF) of the images acquired was determined using ImageJ software as mentioned in section 2.7.Graphs were plotted using GraphPad Prism version 8.3.0 (538).
2.9. Statistical analysis
All experiments were performed in triplicates and expressed as mean±SD. The data was analyzed statistically by one way ANOVA followed by Tukey’s multiple comparisons test for post hoc analysis with the level of statistical significance at P<0.05.
3. Results
3.1. Cell viability measurement
The cytotoxicity of AbPI was determined through MTT assay using crude and purified AbPI fractions (ion exchange and gel filtration chromatography). The gel filtration chromatography fraction of the inhibitor was used for further studies on the adipocytes as it was found to be the least toxic of all the fractions. There was no cytotoxic effect of the protease inhibitor on the cells as the cell survival rate,which was comparable to the control (Figure 1A). It was observed that up to 10 μg/mL concentration of the inhibitor, there was no toxic effect on the cells. With the increase in the concentration of the inhibitor, the cell viability decreased exponentially indicating that at higher concentrations, the AbPI was toxic to the 3T3-L1 cells.From this result, the AbPI concentration for further experiments was taken as 10 μg/mL (Figure 1B). The cell survival rate (%) in the presence of the AbPI was (92.33±2.60)% (Figure 1C). This inferred that the inhibitor was not toxic to the cells. The viability of the cells was decreased in the presence of 2 mM H2O2to (28.00±3.00)%, but in the presence of AbPI, the cell survival rate of the stressed cells was enhanced to (71.34±2.10)%, which implied that the protease inhibitor, AbPI from A. bisporus had a protective effect against the H2O2induced stress (Figure 1C).
3.2. 3T3-L1 pre-adipocyte differentiation and Oil-Red-O staining
The differentiated adipocytes showed the presence of red lipid droplets via Oil-Red-O staining (Figure 2). Lipid accumulation in the differentiated adipocytes treated with the protease inhibitor was greater in comparison to the untreated differentiated adipocytes. This indicated enhancement in adipogenesis by protease inhibitor. The negative control cultures indicated no adipogenesis in the absence of insulin, demonstrating that the protease inhibitor alone did not affect adipogenesis.
3.3. 2-NBDG uptake by 3T3-L1 adipocytes
The fluorescence intensity for 2-NBDG uptake by the 3T3-L1 adipocytes treated with AbPI, rosiglitazone, insulin, AbPI with insulin and rosiglitazone with insulin, were enhanced by 1.01, 8.03,11.26, 16.73, and 19.47-folds in comparison to the control (at a concentration of 10 μg/mL each) (Figure 3). This indicated that AbPI alone had no significant effect on increasing the uptake of 2-NBDG,but in the presence of insulin, its efficiency increased by 16.73 folds.Rosiglitazone with insulin enhanced the uptake of 2-NBDG in the adipocytes in the presence of insulin, exhibiting insulin sensitizing property.
Rosiglitazone alone also stimulated the uptake of glucose by 3T3-L1 adipocytes as an insulin mimic, while the uptake was enhanced significantly in the adipocytes treated with rosiglitazone and insulin together by 2.42-folds (Figure 3) in comparison to rosiglitazone alone.
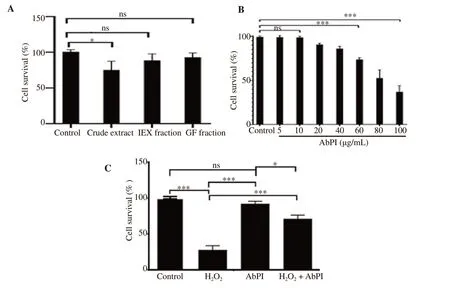
Figure 1. MTT assay result. A: Cytotoxicity of the AbPI from different fractions of Agaricus bisporus [crude extract, ion exchange chromatography fraction(IEX), and gel filtration chromatography fraction (GF)]; B: the least toxic concentration of AbPI; C: effect of AbPI in the presence and absence of H2O2 on the adipocytes. AbPI: the protease inhibitor from Agaricus bisporus. Data are expressed as mean±SD, n=3, ns: P>0.05, *P<0.05, ***P<0.001.
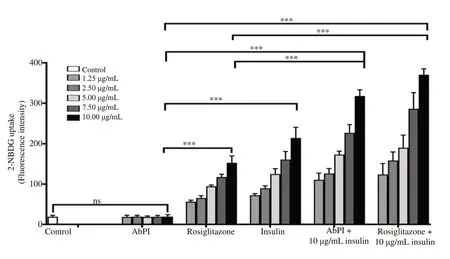
Figure 3. Effect of AbPI (1.25-10 μg/mL), rosiglitazone (1.25-10 μg/mL), insulin (1.25-10 μg/mL), AbPI +insulin (10 μg/mL each) and rosiglitazone+insulin (10 μg/mL each) on the uptake of 2-NBDG. AbPI: the protease inhibitor from Agaricus bisporus. 2-NBDG: 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose. Data are expressed as mean±SD, n=3, ns: P>0.05, ***P<0.001.
In Figure 4, confocal micrographs confirmed the fluorescent intensities obtained in Figure 3 by indicating the visual change in the fluorescence intensity (CTCF) generated by the differential uptake of 2-NBDG by 3T3-L1 adipocytes. The CTCF values increased by 1.81, 6.61, 9.90, 12.81, and 14.21-folds when the adipocytes were treated by AbPI, rosiglitazone, insulin, AbPI with insulin and rosiglitazone with insulin, respectively. AbPI stimulated the uptake of 2-NBDG in 3T3-L1 adipocytes in the presence of insulin (insulinsensitizing agent); but AbPI alone was not able to accelerate the uptake rate in comparison to the drug rosiglitazone, which acted as insulin mimic (in the absence of insulin) as well as insulinsensitizing agent (in the presence of insulin).
It was observed that the protease inhibitor stimulated the adipogenesis process indicating that it sensitized insulin in the differentiating medium and enhanced the glucose uptake. The same insulin-sensitizing effect of the inhibitor was evident during the 2-NBDG uptake by the adipocytes in the presence of the protease inhibitor and insulin.
3.4. Oxidative stress studies
Flow cytometry study was performed to determine the intracellular ROS production using DCFDA fluorescent dye during pre-adipocyte differentiation and to understand the effect of the protease inhibitor from A. bisporus on ROS generation. It was observed through the histograms that the protease inhibitor reduced the amount of ROS in comparison to the control (Figure 5).
Through confocal microscopy, the population of the damaged and dying cells was visualized using PI and DAPI, whereas the ROS containing live cell population was quantified using DCFDA.The viable cell population decreased on treatment with H2O2and increased when treated with 2 mM H2O2in the presence of AbPI.
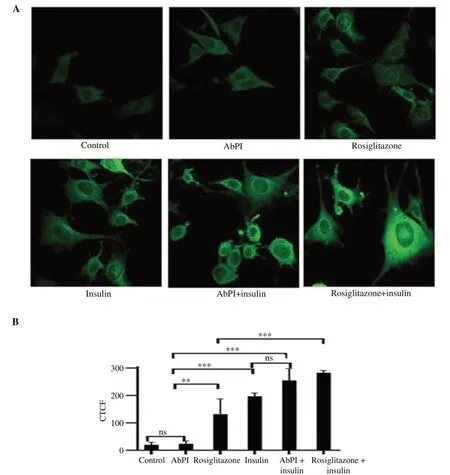
Figure 4. Confocal micrographs of 3T3-L1 adipocytes (A) (40× magnification) and the intensity of corrected total cell fluorescence (B). Cells treated with rosiglitazone and insulin were taken as positive and negative control, respectively. AbPI: the protease inhibitor from Agaricus bisporus; CTCF: corrected total cell fluorescence. Data are expressed as mean±SD, n=3, ns: P>0.05, **P<0.01, ***P<0.001.
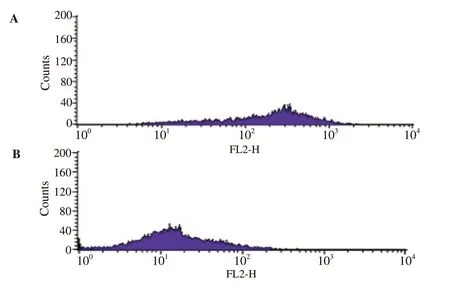
Figure 5. Reactive oxygen species determination using flow cytometry analysis. A: Control (3T3-L1 adipocytes incubated with DCFDA); B: 3T3-L1 adipocytes treated with 10 μg/mL AbPI. AbPI: the protease inhibitor from Agaricus bisporus. DCFDA: 2',7'-dichlorofluorescein diacetate.
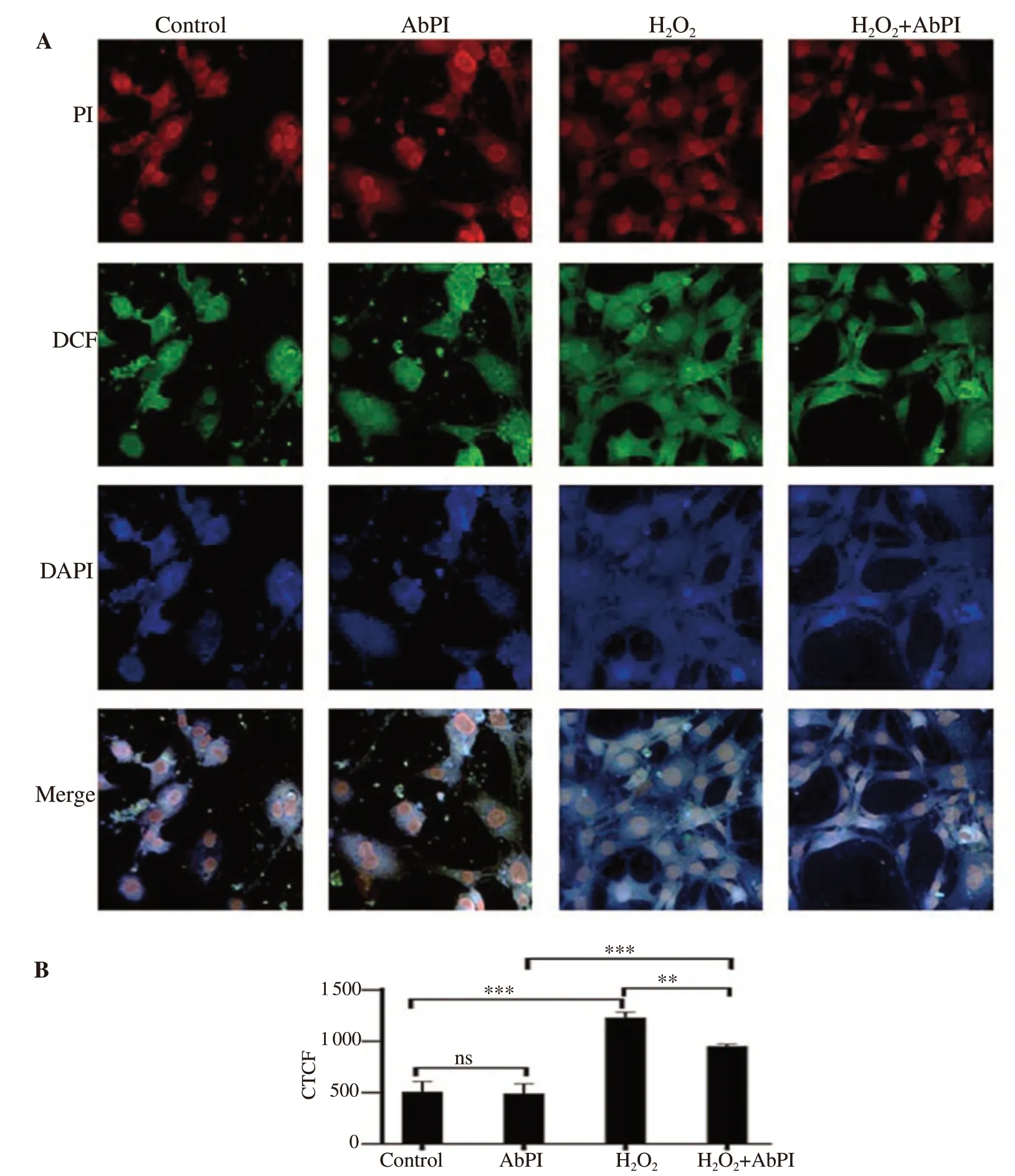
Figure 6. Confocal microscopy of 3T3-L1 adipocytes with and without the presence of H2O2 (A) (40× magnification) and the intensity of corrected total cell fluorescence (B). AbPI: the protease inhibitor from Agaricus bisporus; CTCF: corrected total cell fluorescence; DCF: 2',7'-dichlorofluorescein; PI: propidium iodide; DAPI: 4,6-diamino-2-phenylindol dihydrochloride. Data are expressed as mean±SD, n=3, ns: P>0.05, **P<0.01, ***P<0.001.
The stress generated by 2 mM H2O2in 3T3-L1 adipocytes led to apoptotic conditions (Figure 6). For all the three dyes, the control and 10 μg/mL AbPI treated (Figure 6A) samples also exhibited few fluorescent adipocytes, demonstrating more normal and undamaged cells (PI and DAPI) and fewer ROS in the viable cells (DCFDA).This showed that AbPI alone was non-toxic to the adipocytes.The enhanced fluorescence, in the case of the stressed cells, was indicative of the events leading to the apoptotic condition of the cells (Figure 6A). In the presence of AbPI from A. bisporus, the H2O2treated cells exhibited less fluorescence indicating more normal and viable cells (PI and DAPI) and less ROS generation in viable cells(DCFDA) (Figure 6A). The damaged cell population was 141.74%,and 87.07% higher, in the presence of H2O2, and AbPI with H2O2,respectively, and decreased by -3.35% in the presence of AbPI alone, in comparison to the control. The inhibitor decreased the toxic effect of H2O2, hence prevented the stress condition (Figure 6B).
4. Discussion
A. bisporus (white button mushroom) constitutes of several polysaccharides, vitamins, antioxidants, minerals and is considered a rich source of proteins[28]. It is reported that A. bisporus could lower the blood glucose level in diabetic mice[12]. To date, protease inhibitors from fungi have not been reported to have anti-diabetic property but in plants, several studies showed that protease inhibitors have a hypoglycaemic effect. Oxidative stress in cells causes ROS formation which may culminate into diabetes. Protease inhibitors derived from plants Allium sativum L., Curcuma longa L., Solanum tuberosum L., Syzygium cumini (L.) Skeels, Glycine max (L.) Merr.,Momordica charantia L., Zingiber officinale Roscoe have shown to possess antioxidant potential[29]. These plants have been known to have the anti-diabetic property which may be attributed to the protease inhibitors. Protease inhibitors from plants have been reported to exhibit a protective effect during the stress conditions induced in plants[30].
Previous studies have suggested that serine proteases have a cytotoxic effect and are responsible for ROS generation and induction of apoptosis in endothelial cells[31]and in cancer development[32].
Several bioactive compounds have been found to be non-toxic upto 10-20 μg/mL concentration and have a toxic effect on the cells on increasing their concentrations[33]. AbPI, itself did not show any cytotoxic effect on 3T3-L1 adipocytes. Also, in the stress-induced cells,AbPI enhanced the cell survival rate. The possible explanation for the increased cell viability would be that the serine proteases generated during the oxidative stress due to H2O2were neutralized by the protease inhibitor. Hence, the damage to the targeted proteins by the proteases in the cells was minimal in the presence of protease inhibitors. Serine protease inhibitors have been reported as non-toxic to the host cells by protecting them from the Toxoplasma gondii protozoan invasion[34]. A safe concentration of AbPI (10 μg/mL), was used to study the oxidative stress and the glucose uptake by 3T3-L1 adipocytes.
It has been reported that Syzygium aqueum (Burm.f.) Alston leaf extracts and its bioactive compounds enhanced glucose uptake in 3T3-L1 cells and pre-adipocyte differentiation. Rosiglitazone, when was used to compare with Syzygium aqueum leaf extract and its bioactive compounds for glucose uptake in 3T3-L1 cells, gave similar results[35].ReishiMax, dietary supplement prepared from an edible mushroom Ganoderma lucidum containing triterpenes and polysaccharides was reported to have insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 adipocytes[36]. Similarly,the result has been observed for artepillin C, a PPARγ ligand that stimulated adipocyte differentiation and glucose uptake in 3T3-L1 adipocytes[37].
No study related to other protease inhibitors isolated from fungal sources has been reported similar to the results of AbPI. Hence it can be considered that the protease inhibitor plays a role in activating PPARγ,thus increasing the adipogenesis.
Insulin is known for inducing glucose uptake in mature adipocytes by binding to the receptor proteins in the cells, causing the GLUT4 protein translocation to the cell surface. Hence, the glucose uptake by the cells increases proportionately to the increase in insulin concentration. The stimulation of 2-NBDG uptake in 3T3-L1 adipocytes was studied through confocal microscopy.
The exact mechanism related to the enhancement of uptake of the fluorescent glucose analog 2-NBDG by the 3T3-L1 adipocytes in presence of the protease inhibitor, AbPI, has not been fully understood,but the enhancement of adipogenesis during 3T3-L1 adipocyte differentiation and stimulation of glucose uptake in the presence of insulin by the protease inhibitor indicate its role as a PPARγ agonist,similar to the thiazolidinedione: rosiglitazone or PPARγ up-regulator.Hence, further study at a molecular level is desired to understand the process. PPARγ upon activation could bring about metabolic changes which ultimately cause insulin sensitivity, reduction in blood glucose level. PPARγ is known to affect the expression of several antioxidant genes during oxidative stress conditions such as catalase, and GSHPx[38,39]. This indicates that the up-regulation of PPARγ, by drugs such as TZDs and bioactive compounds, may subdue the effect of oxidative stress during adipogenesis.
The effect of the protease inhibitor on the ROS generated during adipogenesis was studied through flow cytometry and it indicated a decrease in the ROS production. This confirmed that the protease inhibitor was capable of reducing the oxidative stress in the adipocytes.The mature 3T3-L1 adipocytes were treated with 2 mM H2O2to study the effect of the inhibitor on the cells subjected to external stress. H2O2converted to highly reactive free hydroxyl radical which decreased the glutathione level in the internal cellular environment[40]. This led to the sequence of events that ultimately caused morphological changes in the cell membrane, nuclear membrane, fragmentation of the nuclei and finally the shrinkage of the cells. Concentrations at 0.1-2.0 mM could induce oxidative stress leading to DNA damage, considerably in human adipose derived-mesenchymal cells[41]. The stress was due to DNA damage. In the presence of AbPI, the effect of 2 mM H2O2was found to decrease, and the cells retained their normal morphology and were protected from the induced stress.
In recent years, the antioxidative property of some edible and wild mushrooms have been reported. The lyophilized extract of the mushroom Agaricus arvensis (horse mushroom) is known to exhibit antioxidative property against carbon tetrachloride induced oxidative stress in rats through a reduction in erythrocyte fragility and regulation of antioxidant enzymes including glutathione S transferase, GSHPx, catalase, and glutathione reductase. The antioxidant effect was attributed to the phenolic, mineral, and volatile (benzaldehyde,palmitic acid) compounds present in the extract[42,43]. Similarly, a wood-rotting basidiomycete Lentinus tigrinus (Bull.) has been cited as a potential antioxidant source[11]. The ethanolic extracts of wild and cultivated edible mushrooms Pleurotus ostreatus, as well as A.bisporus, have exhibited antioxidant potential[13].An edible mushroom Clavariadelphus truncatus has shown a protective effect against oxidative stress[44].
The protease inhibitor extracted from A. bisporus was found to behave similar to PPARγ agonists, enhancing adipogenesis and lowering blood sugar level by increasing the uptake of glucose by the adipocytes, and also reducing the ROS generation. With further studies linking the action of the protease inhibitor on the targets involved in adipogenesis such as PPARγ and GLUT4, the exact mechanism of action of the inhibitor can be deciphered. Our study shows that A. bisporus can be considered as a potential candidate for oral therapeutic agents in oxidative stress and diabetic studies.
Conflict of interest statement
We declare that there is no conflict of interest.
Authors’ contributions
RV performed the experiments, contributed reagents, materials,analysis tools or data, and wrote the manuscript. AM conceived and designed the experiments, analyzed and interpreted the data.
 Asian Pacific Journal of Tropical Biomedicine2020年3期
Asian Pacific Journal of Tropical Biomedicine2020年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for AuthorsAsian Pacific Journal of Tropical Biomedcine
- Mayaro fever: A brief review on the immune profile
- Deoxyelephantopin induces ROS-mediated autophagy and apoptosis in human colorectal cancer in vitro and in vivo
- Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats
- Thai Perilla frutescens fruit oil alleviates carbon tetrachloride-induced hepatotoxicities in rats
