Thai Perilla frutescens fruit oil alleviates carbon tetrachloride-induced hepatotoxicities in rats
Narisara Paradee, Duangta Kanjanapothi, Tawat Taesotikul, Sarawut Kongkarnka, Adchara Prommaban, Pimpisid Koonyosying, Somdet Srichairatanakool✉
1Department of Biochemistry, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
2Faculty of Pharmacy, Payap University (Maekhao Campus), Chiang Mai, Thailand
3Department of Pathology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
ABSTRACT Objective: To study the effect of perilla fruit oil against carbon tetrachloride (CCl4)-inducedliverdamagein rats.Methods:Perilla fruit oilwas analyzed in terms of fatty acids,tocopherols and tocotrienols using chromatography. Sub-chronic toxicity of perilla fruit oil was investigated in rats for 90 d followed by a 28 d recovery period. Hematological, biochemical and pathological parameters were determined. To evaluate hepatoprotection, rats were divided into five groups and orally administered with Tween 80 for 10 d; Tween 80, silymarin, perilla fruit oil (0.1 mL/200 g) and perilla fruit oil (1 mL/200 g) for 10 d together with subcutaneous injection of CCl4 (2 mL/200 g) on days 9 and 10. Liver enzymes and pathological parameters were determined.Results: Perilla fruit oil contained α-linolenic acid (56.55% of total fatty acid), β-tocopherol (49.50 mg/kg) and γ-tocotrienol (43.65 mg/kg). Rats showed significant changes in the percentage of monocytes and platelet indices following perilla fruit oil consumption for 90 d; in the percentage of neutrophils and lymphocytes, and RBC indices in the recovery period when compared with the deionized water group. Total protein and creatinine levels were increased while alkaline phosphatase and aspartate aminotransferase levels were decreased (P < 0.05). Organ weight index and pathological indicators did not change signifciantly. The liver of CCl4-induced rats showed remarkable centrilobular fatty changes, which was ameliorated by perilla fruit oil pretreatment. Aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels were decreased (P< 0.05) in rats given perilla fruit oil.Conclusions: Perilla fruit oil is rich in α-linolenic acid, β-tocopherol and γ-tocotrienol and improves blood biomarker levels and protects against CCl4-induced hepatotoxicity. Further studies are required before supporting its use for the treatment of hepatitis.
KEYWORDS: Perilla frutescens; Perilla oil; Carbon tetrachloride;Fatty change; Liver injury
1. Introduction
Perilla frutescens (P. frutescens) (L.) Britton fruits (or seeds) have served as a functional food in a number of East Asian countries including Thailand. Perilla leaf extract is abundant with luteolin,apigenin and rosmarinic acid and displays significant antiinflammatory effects by decreasing tumor necrotic factor-alpha,interleukin-17A and -10 in mice with dextran sulfate sodiuminduced colitis[1]. Chromatographic analysis of perilla fruit oil revealed large amounts of ω3-polyunsaturated fatty acid and α-linolenic acid together with small amounts of some other fatty acids, phenolic compounds and flavonoids[2,3]. Recently, we have found that the ethyl acetate and ethanol extracts of perilla fruit, which contain flavones, apigenin, luteolin and chrysoeriol,exhibit antioxidant, free-radical scavenging and hepatic antilipid peroxidation activities[4]. Perilla ketone, an essential oil of P.frutescens, has been previously identified as a potent lung toxic agent in animals[5]. Currently, perilla fruit oil has been reported to be nontoxic in rats (20 g/kg/d) after 14-d feeding periods, and in dogs (3 g/kg/d) after 90-d feeding periods. Additionally, adverse effects in dogs were found at doses of 6-12 g/kg/d, but these effects disappeared after withdrawal[6].
Among liver diseases, nonalcoholic steatohepatitis and nonalcoholic fatty liver disease are highly prevalent in developed countries, while treatments utilizing carbon tetrachloride (CCl4) and paracetamol may act directly upon liver cells resulting in liver toxicity. Hepatotoxicity can be managed through the use of drugs and various natural products (e.g. crude extracts and plant-based oils)[7-12]. Perilla fruit oil has been reported to exhibit hypocholesterolemic, antiatherosclerotic and anti-nonalcoholic steatohepatitis effects in high fat diet-fed animals[13-17]. Furthermore, perilla fruit oil significantly decreased serum levels of triglyceride, total cholesterol and lowdensity lipoprotein-cholesterol in hypercholesterolemia patients[18].Corn oil, a by-product of corn meal production and of the starchmaking industry, is categorized as one of the richest sources of ω3-polyunsaturated fatty acid linoleic acid, β-sitosterols and γ-tocopherols[19]. Clinical studies have identified a correlation between corn oil and certain health benefits[20,21]. However, the perilla benefits for ameliorating metabolic disorders such as oxidative liver damage and nonalcoholic steatohepatitis, and the protective effects of perilla oil against chemical-induced liver inflammation, have been rarely investigated. In this study, we aimed to determine the nutraceutical effects of perilla fruit oil compared with corn oil, and to evaluate its hepatoprotection against CCl4-induced liver injuries in rats.
2. Materials and methods
2.1. Chemicals and reagents
All solvents and reagents used in this experiment (e.g. n-pentane,n-hexane, isopropanol, ethyl acetate and acetic acid) were of the highest purity and of HPLC grade. Pentobarbital sodium was prescribed by a pharmacist and obtained from a drug store located in Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. Boron trifluoride (BF3),carbon tetrachloride (99.9% pure), silymarin and reference standards(e.g. α-tocopherol, α-tocotrienol, β-tocopherol, γ-tocopherol,β-tocotrienol, γ-tocotrienol, δ-tocopherol and δ-tocotrienol) were purchased from Sigma-Aldrich Chemicals Company (St. Louis, MO,USA).
2.2. Supply of perilla fruit oil and corn oil
Thai perilla (P. frutescens) fruits were harvested from fields located in Wienghang District, Chiang Mai Province, Thailand. They were botanically examined by Dr. C. Leon, Royal Botanic Gardens Kew, and compared botanically and chemically with authentic P.frutescens fruits from the Royal Botanic Gardens Kew Economic Botany Collections (voucher reference EBC 81840 TCMK 411).The fruits were pressed mechanically at ambient temperatures to release oil at a yield of 27% (w/w). Perilla fruit oil was analyzed in terms of composition. Additionally, its toxicity was tested and its hepatotoxicity in rats was studied. Corn oil (Mazola®, Best Food Inc.) was purchased from a local grocery store in Chiang Mai Province, Thailand. The nutritional facts of the corn oil (14 g serving size) are as follows: total fat (14 g), saturated fat (2 g), trans-fat (0 g),monounsaturated fat (4 g), polyunsaturated fat (8 g) rich in linoleic acid (58%-62% of total fatty acid), vitamin E (10%), β-sitosterols(63%-70%) and γ-tocopherols (68%-89%).
2.3. Analysis of fatty acids
Fatty acids of perilla fruit oil were derivatized with boron trifluoride in methanol (BF3-methanol) solution at 100 ℃ for 15 min to produce fatty acid methyl ester. The fatty acid methyl ester was then cooled down and deionized water and n-pentane were added.Afterwards, the fatty acid methyl ester in the n-pentane layer was dried under nitrogen gas flow, reconstituted in n-hexane (100 μL)and subjected to gas-chromatography-mass spectrometry (GC-MS)analysis using an Agilent 7890AGC coupled to an Agilent 5975C MS[22]. The analysis was performed on a 30 m × 0.25 mm ID ×0.25 μm ZB-WAX column (Phenomenex, UK) using a temperature program of 70-250 ℃ at a rate of 3 ℃/min. The flow rate of the helium gas carrier was administered (split 1:10) at 220 ℃ and at 1.0 mL/min. Elutes were detected using an MS machine that was fitted with an electrospray ionization source and operated at 70 eV with a source temperature of 180 ℃. The mass spectra were recorded in a m/z range of 38-600. Compounds were identified by comparing m/z values with those of published data. The percentage of compositions was calculated by integrating peaks in total ion chromatograms.
2.4. Analysis of tocopherols and tocotrienols
Perilla fruit oil was extracted in n-hexane and analyzed using the normal-phase high-performance liquid chromatography/fluorescence detection (HPLC/FLD) method that had been established by Huang and colleagues[23]. In the analysis, n-hexane extract was injected into the HPLC machine, then eluted via a mobile phase consisting of n-hexane, isopropanol, ethyl acetate and acetic acid (976, 8, 8 and 8 mL, respectively) at flow rates varying from 0.7 to 1.5 mL/min.The fluorescence signal was detected at λexcitation290 nm and λemission330 nm. Tocopherols and tocotrienols were identified by comparing the relevant figures with the authentic standards of α-tocopherol,α-tocotrienol, β-tocopherol, γ-tocopherol, β-tocotrienol, γ-tocotrienol,δ-tocopherol and δ-tocotrienol on file. The concentrations of these substances were determined by referencing the standard curves.
2.5. Animal care
Male and female Wistar rats with body weights in a range of 180-200 g were purchased from the National Laboratory Animal Center,Mahidol University (Salaya Campus), Bangkok, Thailand. The rats were housed in individual cages with free access to feed and water and kept under the following conditions; controlled temperatures(20-22 ℃), humidity (50±10)% and light (12-h day/night cycle). The rats were acclimatized for one week prior to the experiment. The rats were fed a nutritionally-balanced commercial diet (No. C.P. 082,Perfect Companion Group Co. Ltd., Thailand) comprised of crude protein 24%, fat 4.5%, fiber 5%, minerals (Ca 1.0%, Na 0.20%, K 1.17%, Mg 0.23%, Mn 171 ppm, Cu 22 ppm, Zn 100 ppm, Fe 180 ppm, Se 0.1 ppm) and vitamins (A 20 000 IU/kg, D 4 000 IU/kg, E 100 mg/kg, B15 mg/kg, B220 mg/kg, B620 mg/kg, B120.036 mg/kg,niacin 100 mg/kg, folic acid 6 mg/kg, biotin 0.4 mg/kg, pantothenic acid 60 mg/kg).
2.6. Evaluation of health indicators in rats
Rats were randomly divided into five groups (10 females and 10 males in each group) and received different treatments using gavage needles. Perilla fruit oil and corn oil at the doses of 1 mL/200 g were given orally to each group of rats daily for 90 d, while the control group received deionized water. In order to assess reversibility effect,perilla fruit oil at the dose of 1 mL/200 g was given once daily to the fourth group of rats for 90 d and kept for another 28 d post treatment, while the fifth group received deionized water. Signs of toxicity and mortality were monitored daily, while the body weights were recorded weekly throughout the study. On day 91st and 118th(satellite group), the rats were anaesthetized by intraperitoneal (ip.)injection of pentobarbital sodium (40 mg/kg) after overnight fasting.Blood was collected from the tail veins of the rats and preserved in tubes with and without an EDTA anticoagulant for the purposes of biochemical and hematological analysis, respectively. Vital organs including the brain, heart, liver, lungs, kidneys, adrenal glands,spleen and reproductive organs (ovaries and uterus of females, testes of males) of all experimental rats were dissected and weighed. The weight index or the relative organ weights were determined using a ratio of organ weight to 100 g body weight. The tissues were preserved in 10% paraformaldehyde for pathological examination.
2.7. Effect of perilla fruit oil on CCl4-induced liver damage
Hepatotoxicity in rats was induced with CCl4using the method established by Bhoopat and colleagues[24]. Silymarin is a flavonolignan that is purified from milk thistle fruit (Silybum marianum Linn.)and is widely used as a hepatoprotective agent through anti-viral,antioxidant, anti-inflammatory and immune-modulatory actions of the liver and immune cells[25,26]. Rats (n = 8 each) were randomly divided into five groups and fed once a day for 10 d with 10% Tween 80 (1.0 mL/200 g/d), silymarin (20 mg/200 g/d) and perilla fruit oil(0.1 and 1.0 mL/200 g/d) using gavage needles. Additionally, CCl4(2.0 mL/200 g/d) was given subcutaneously (sc.) to the experimental group on days 9 and 10, while none was given to the control group.The rats were fed a diet of normal commercial chow, and with free access to deionized water and body weight was recorded every week throughout the course of the study. On day 11, all rats were anesthetized by intraperitoneal (ip.) injection of pentobarbital sodium (40 mg/kg), and heart blood was collected for analysis of the biochemical parameters. The serum was analyzed for biochemical parameters. Livers were excised immediately afterward, weighed and fixed in 10% neutralized formalin for pathological examination.
2.8. Analysis of hematological parameters
EDTA blood was analyzed in terms of complete blood count using an Automate Blood Cell Counter according to the manufacturer’s instructions at the Investigation Laboratory, Animal Hospital,Faculty of Veterinary Medicine, Chiang Mai University, Chiang Mai,Thailand[27].
2.9. Analysis of biochemical parameters
Serum was separated from clotted blood samples by centrifugation at 3 000 g for 10 min and kept frozen at -20 ℃ until analysis.Fasting blood glucose (FBG), blood urea nitrogen, creatinine, total protein, albumin, aspartate aminotransaminase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were measured using a Randox Daytona Chemistry Analyzer (Randox Laboratories Limited, Antrim, United Kingdom) according to the manufacturer’s instructions[28].
2.10. Histopathological examination
Fixed tissues were embedded in a paraffin block, cut into slides using a microtome, deparaffinized, and stained with hematoxylin and eosin (H ffamp; E) dye reagent. The stained tissue section was examined and photographed by an expert pathologist at the Department of Pathology, Faculty of Medicine, Chiang Mai University.
2.11. Ethical information
The study protocol of all animal experiments was approved by the Animal Ethical Committee of Medical Faculty, Chiang Mai University, Thailand (Protocol Number 4/2558). The studies followed the guidelines for experimental liver research: thioacetamide model in mice and rats[29].
2.12. Statistical analysis
Data are expressed as mean ± standard deviations (SD). Statistical significance was analyzed using one-way analysis of variance(ANOVA) with post-hoc Tukey-Kramer, for which P < 0.05 was considered significant.
3. Results
3.1. Composition of lipids in perilla fruit oil
3.1.1. Fatty acids
GC-MS analysis revealed the profile and amounts of fatty acids in perilla fruit oil (Supplementary Figure 1 and Table 1). The results indicated the presence of palmitic acid (7.49 % of total fatty acids),stearic acid (1.91% of total fatty acids), arachidic acid (0.09% of total fatty acids), oleic acid (13.16% of total fatty acids), linoleic acid (19.95% of total fatty acids) and α-linolenic acid (56.55% of total fatty acids). In addition, HPLC/MS analysis demonstrated that phenolic compounds were present in perilla fruit oil (data not shown).
3.1.2. Tocopherol and tocotrienol content
A HPLC profile and concentrations of tocopherols and tocotrienols existing in perilla fruit oil are shown in Supplementary Figure 2 and Table 2. In stoichiometry, β-tocopherol (49.50 mg/kg) was found to be the most abundant. Additionally, α-tocopherol (25.05 mg/kg) and δ-tocopherol (12.78 mg/kg) were moderately detected,while γ-tocopherol was not detectable. In addition, the recorded amounts of γ-tocotrienol and β-tocotrienol were 43.65 and 1.00 mg/kg, respectively, whereas α-tocotrienol and δ-tocotrienol were not detected.
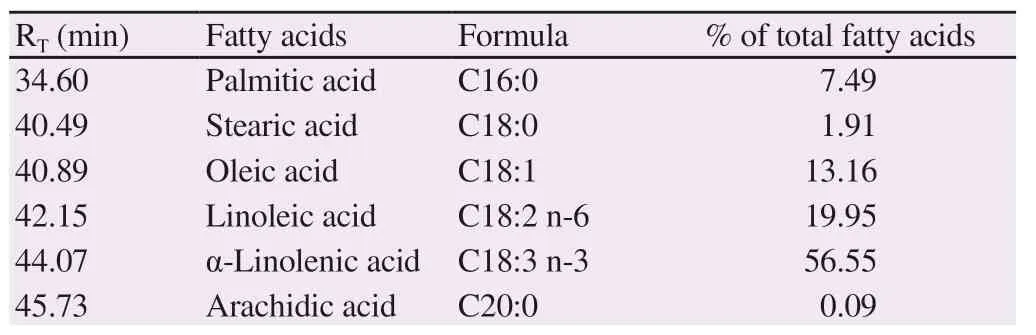
Table 1. Fatty acid composition of perilla fruit oil.
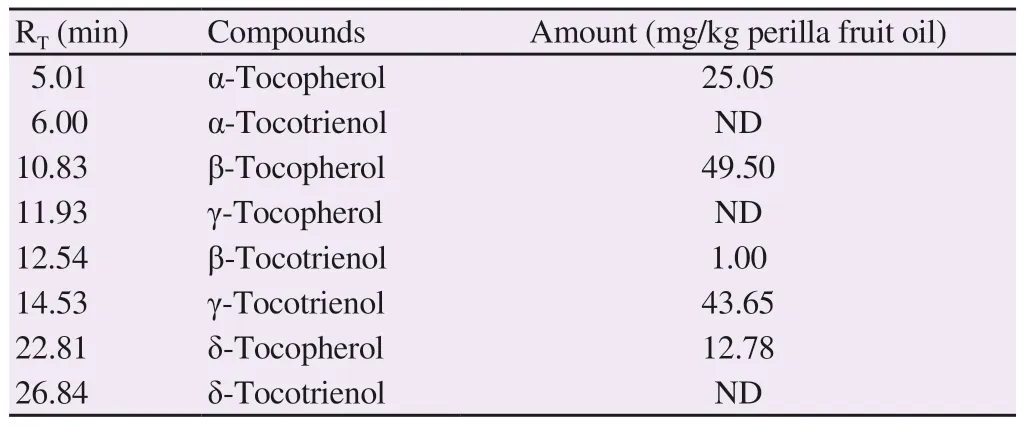
Table 2. Amounts of tocopherols and tocotrienols in perilla fruit oil.
3.2. Effect of perilla fruit oil on health indicators
3.2.1. Body weight
Oral administration of perilla fruit oil and corn oil which was given at 1 mL/200 g BW/d for 90 d (standard study) and a period of 28 d post-treatment (satellite study) did not induce mortality or reveal any clinical signs of toxicity in male and female rats. The body weight in all rat groups was increased in both the standard and satellite studies, while no differences were observed among both genders (Supplementary Figure 3). The body weights of corn oil and perilla fruit oil treated groups were not significantly different when compared with those of the deionized water group.
3.2.2. Hematological parameters
As shown in Table 3, perilla fruit oil increased the percentages of differential monocytes in male rats when compared with those of the deionized water group (P < 0.05). RBC levels of all treatment groups fell in normal ranges with no significant difference. Delayed increases in RBC numbers, Hb and Hct were observed in female rats fed with perilla fruit oil in a satellite study. Notably, slight decreases in platelet numbers and plateletcrit values, and increases of mean platelet volume (MPV) and platelet distribution width (PDW) values(P < 0.05) were found in male rats of the perilla fruit oil group when compared with the group given deionized water.
3.2.3. Blood biochemical parameters
As shown in Table 4, FBG levels in all groups fell within a normal range and were not significantly different, while the group treated with perilla fruit oil in the satellite study decreased FBG levels.Furthermore, perilla fruit oil significantly reduced ALP activity levels in both male and female rats and the AST activity levels in only female rats when compared with the deionized water group.In contrast, corn oil significantly increased ALP activity levels in female rats. Moreover, levels of total protein and globulin were significantly increased in the group treated with perilla fruit oil,whereas these levels were decreased in male and female rats together with reduced albumin levels in the corn oil group. Perilla fruit oil did not change blood urea nitrogen levels significantly, whereas it increased serum creatinine levels compared to the deionized water group, suggesting that it could possibly be harmful to kidneys.
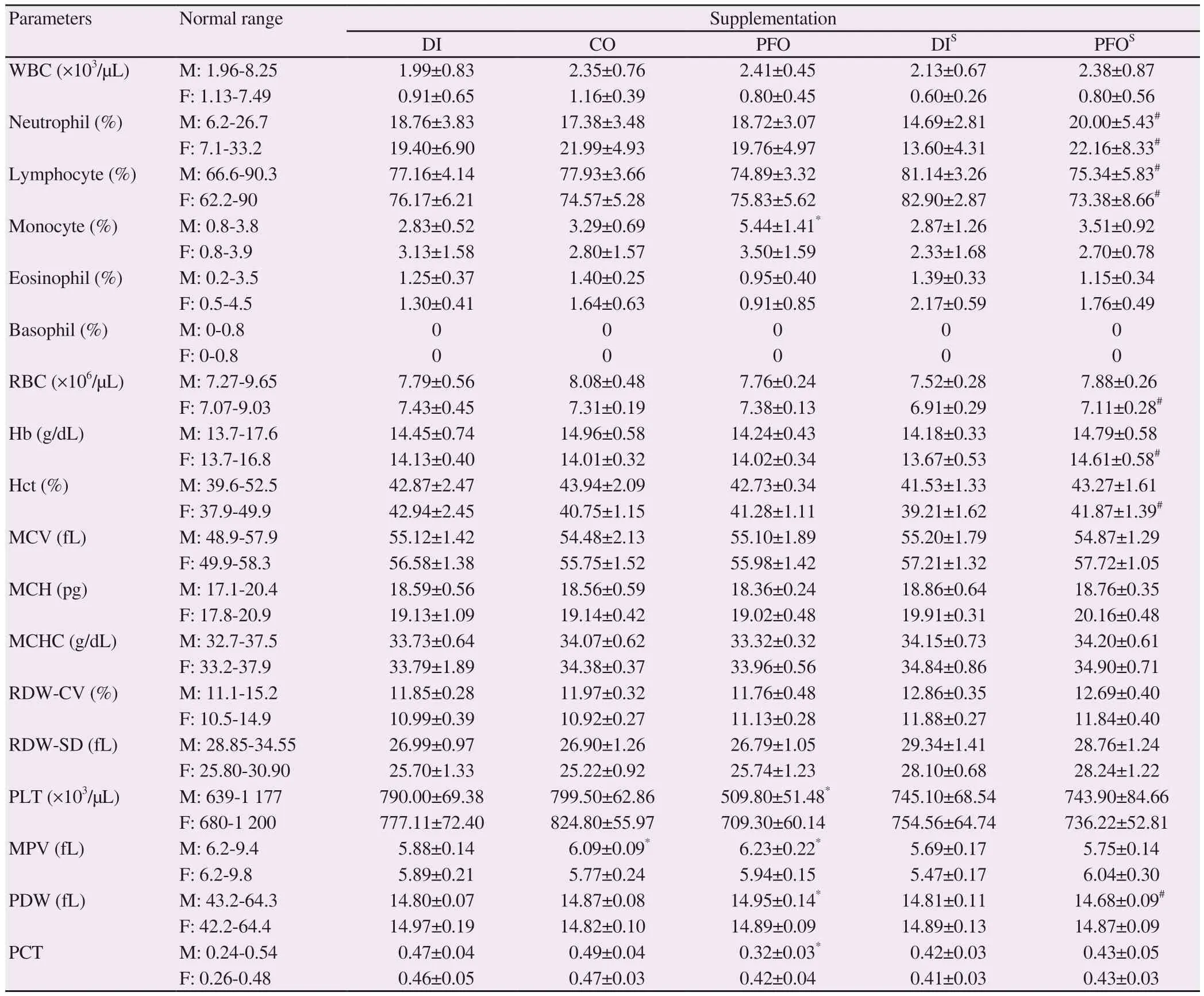
Table 3. Effects of perilla fruit oil on hematological parameters of rats (mean±SD).
3.2.4. Pathological examination
Herein, the absolute and relative weights of the brain, liver, heart,lungs, kidneys, adrenal glands, spleen, and reproductive organs of both male and female rats were recorded during the treatment period(Figure 1). When compared with the deionized water group, perilla fruit oil consumption in both the standard and satellite studies had no discernable effect or revealed any significant alterations in weights of investigated internal organs, except for the kidney weights which were decreased significantly by perilla fruit oil and corn oil treatments. However, weight index of all the organs were clearly not influenced by the perilla fruit oil and corn oil treatments. Moreover,there were no notable abnormalities observed in the histopathological examinations of the main internal organs. Yet, there was evidence of mild-degree hemosiderin pigment-laden macrophages in the spleens of rats in all groups.
3.3. Hepatoprotective effect of perilla fruit oil
3.3.1. Clinical signs and changes of body and organ weight Body weight increased in the rats of all groups that were pretreated with 10% Tween 80, silymarin (20 mg/200 g) and perilla fruit oil (0.1 mL and 1 mL/200 g) for 10 d. Body weights were not significantly different following subcutaneous injections of CCl4for 2 d (Supplementary Figure 4). Clinical signs and adverse effects were not found throughout the 10-day study. Liver weight and weight index of the rats treated with CCl4were found to increase when compared with the Tween control group. The rats treated with silymarin showed a slight increase in liver weight compared with the Tween group with hepatotoxicity induced by CCl4; however, there was not any significant difference in weight index among these study groups (Table 5).
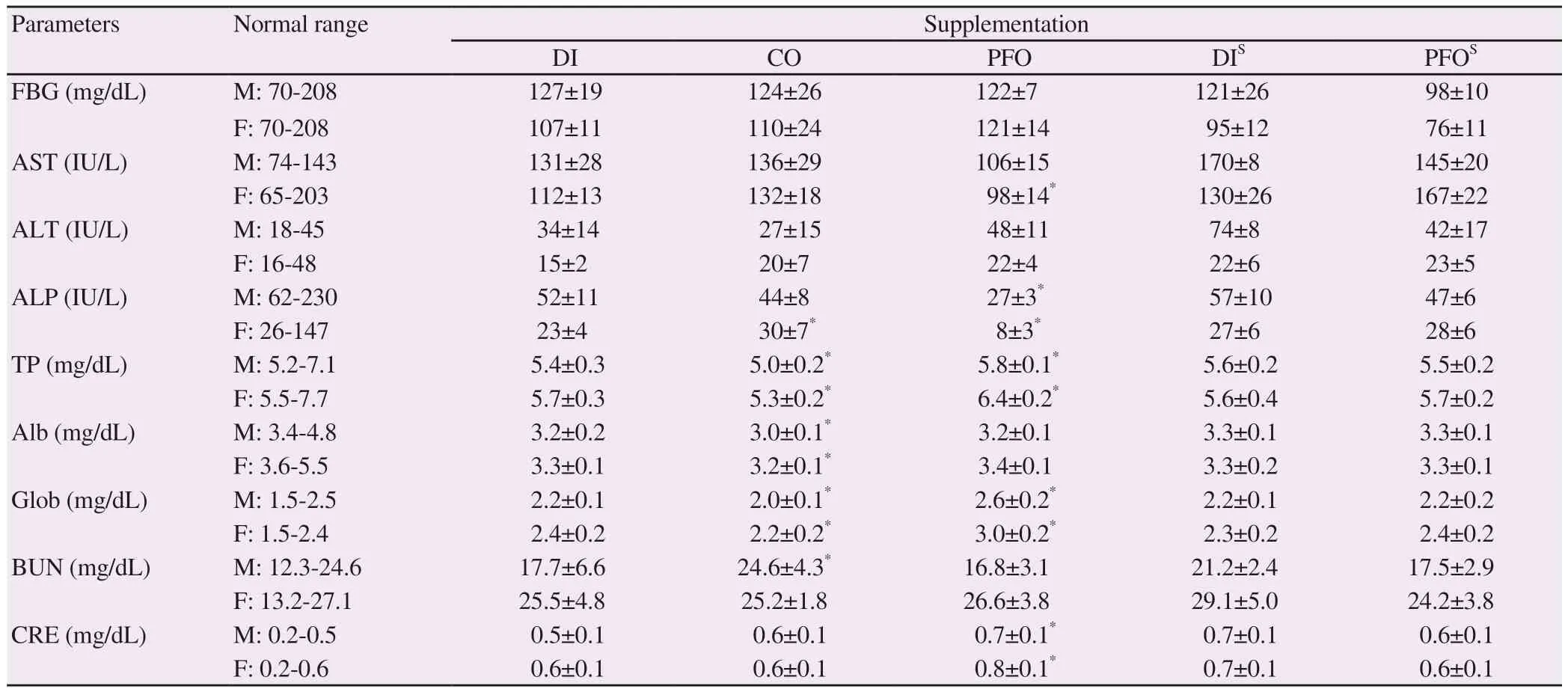
Table 4. Effects of perilla fruit oil on biochemical parameters in blood (mean±SD) of rats.
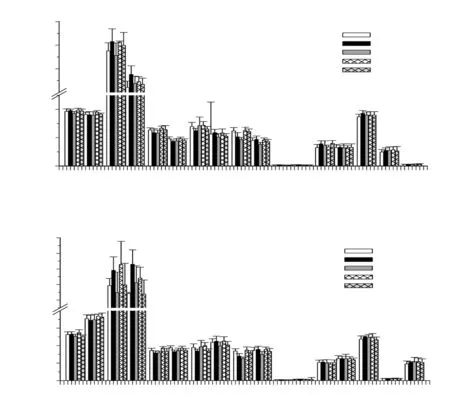
Figure 1. Wet weight (A) and weight index (B) of rat organs (10 M, 10 F) fed with deionized water, corn oil and perilla fruit oil (1 mL/200 g/d) for 90 d and then observed without treatment for further 28 d (satellite study). Data are expressed as mean±SD. *P < 0.05 when compared with the deionized water group.CO = corn oil, DI = deionized water, F = female, M = male, PFO = perilla fruit oil, DIS = deionized water (satellite study), PFOS = perilla fruit oil (satellite study).
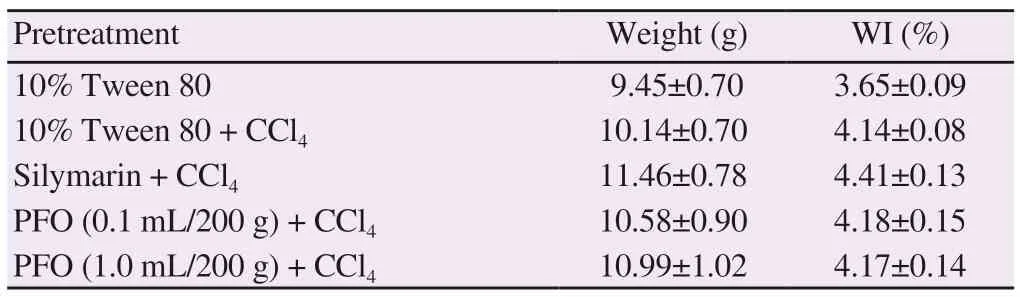
Table 5. Wet weight and weight index of livers of rats (n = 8).
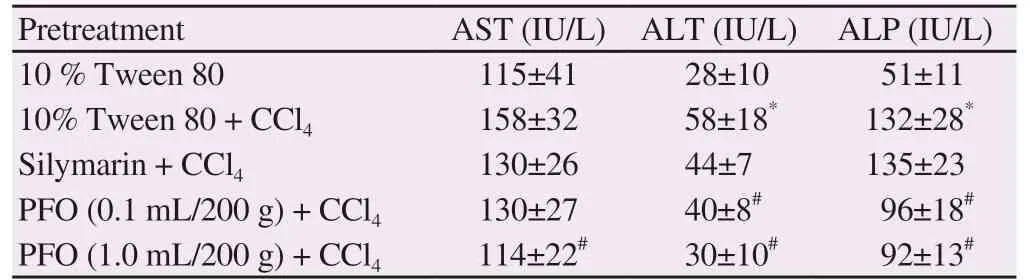
Table 6. Levels of serum aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase activities of rats.
3.3.2. Liver enzyme activity
As shown in Table 6, CCl4treatment significantly increased the serum levels of ALT and ALP compared to the specimens that did not receive treatment (Tween alone), and thus resulted in liver damage.Pretreatment of silymarin, which is known as a hepatoprotective drug, reversed CCl4-induced increase of AST and ALT levels except for ALP. Pretreatment with high-dose of perilla fruit oil (1.0 mL/200 g) significantly restored the levels of AST, ALT and ALP compared with the CCl4group. Interestingly, low-dose perilla fruit oil treatment(0.1 mL/200 g) also decreased the levels of AST, ALT and ALP. The data implied that perilla oil had a hepatoprotective effect on CCl4-induced liver damage in rats, and high doses of perilla oil were found to be more effective.
3.3.3. Histopathological examination
Photographs of H ffamp; E-stained liver tissues showed no inflammation and cell necrosis in all study groups. However, the liver tissues showed moderate to severe degree microvesicular-type centrilobular fatty changes (3/8 and 5/8 rats, respectively) in the CCl4-treated control group, while no pathological change was observed in the Tween control group. Surprisingly, the degree of fatty changes was remarkably lower in the silymarin (20 mg/200 g) and perilla fruit oil (0.1 and 1.0 mL/200 g) groups than in the CCl4control group (Figure 2).
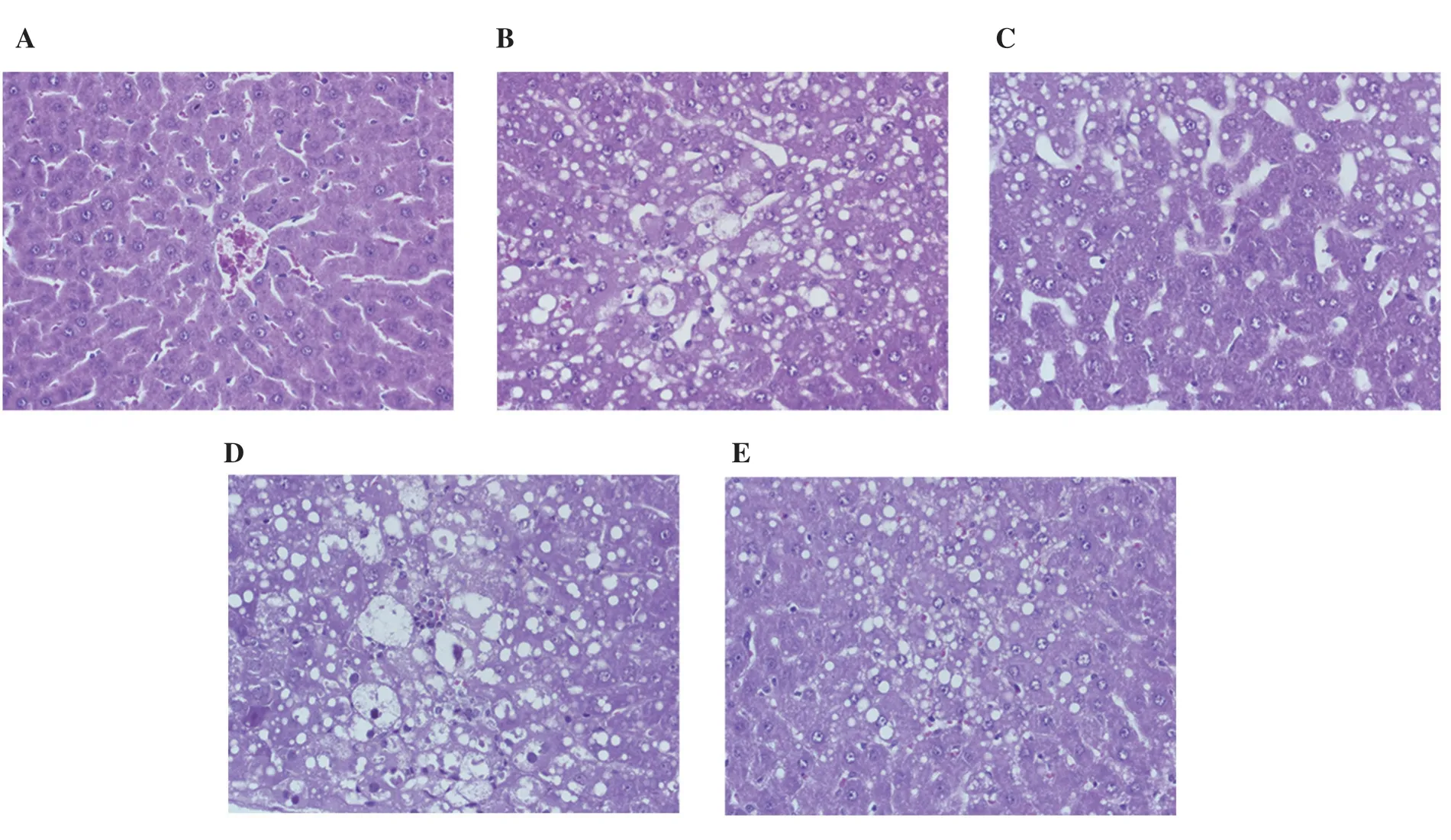
Figure 2. Representative liver histology of rats given different treatments for 10 d and after induction of hepatotoxicity with carbon tetrachloride (2 mL/200 g)on days 9-10. Liver sections were stained with hematoxylin-eosin (original magnification ×40). The result showed no pathological change in 10% Tween 80 group (A) while moderate centrilobular necrosis with macrovesicular fatty change was noted in 10% Tween 80 + CCl4 group (B). Mild centrilobular necrosis was found in sylimarin + CCl4 group (C), massive necrosis was present in PFO (0.1 mL/200 g) + CCl4 group (D), and mild hepatic necrosis was identified in PFO (1 mL/200 g) + CCl4 group (E). CCl4 = carbon tetrachloride and PFO = perilla fruit oil.
4. Discussion
This study reports on the chemical constituents and nutraceutical properties of the oil of the P. frutescens fruit (known as Nga keemon in northern Thai language). GC-MS analysis revealed that the major fatty acid of perilla oil were α-linolenic acid, linoleic acid and oleic acid. The ratio of ω6 to ω3 fatty acids in perilla oil was found to be 2.8, which was within the healthy ratio range of 1-4[30,31].Herein, the method used in Thailand for analysis of the fatty acid composition in perilla fruit is similar to the methods that are commonly used for Korean and Chinese seeds[32-34]. Our findings revealed that the dominant contents of α-linolenic acid, α-tocopherol,β-tocopherol, γ-tocotrienol and δ-tocopherol in perilla seeds, which are in accordance with the findings of two previous studies[35,36],could act as lipophilic antioxidant compounds and contribute to scavenging membrane lipid hydroperoxides.
Any deviation of internal organ weights obtained from the control group suggests the existence of pathological changes in the organs and, therefore, helps in determining the cause of death under various pathological conditions. However, absolute organ weights may depend on the seasons and on the ages of the animals in question,while relative organ weights and/or the organ weight index are extremely useful resources. These changes included lower levels of platelets (only in males), AST (only in females), ALP as well as delayed lower FBG values, whereas higher levels of monocyte (only in males), total protein and creatinine were observed when compared with the control group. However, these alterations were within the normal laboratory range[37]and some parameter changes affected either only the male or the female group. Therefore, perilla oil at a dose of 1 mL/200 g body weight caused no toxicity. Interestingly, a longer period of survival was observed in rats fed with α-linolenic acid-rich perilla oil when compared with rats fed with linoleic acidrich corn oil[38]. Yang and colleagues have shown that perilla fruit oil-based lipid emulsion was toxic to dogs by causing cholestatic liver injury and abnormal fat metabolism, while the phytosteroldepleted perilla fruit oil product revealed non-toxicity[39]. Moreover,perilla fruit oil at 20 g/kg/d did not cause acute toxicity or mortality in rats, while the dose of 3 g/kg/d given for 90 d was not found to cause any adverse effects in dogs[6].
We also evaluated the hepatoprotective effects of perilla oil on liver damage induced by CCl4in male Wistar rats. Previous studies demonstrated that CCl4induced severe hepatic damage leading to increased levels of liver enzymes, which can result in liver damage or injury[40,41]. Indeed, perilla oil at 1 mL/200 g body weight had a more potent effect on lowering the levels of these liver enzymes than silymarin. Likely, perilla oil that was abundant with α-linolenic acid and tocopherol may effectively reduce liver damage through antioxidant and anti-inflammatory activities. Notably, perilla fruit oil feeding was non-toxic to rodents at up to 14 d; however, adverse
effects, including changes in hematological and blood biochemical parameters and liver tissue, were observed in dogs fed with perilla fruit oil (6-12 g/kg/d) for 90 d[6]. Furthermore, an oral administration of 15% (w/w) perilla fruit oil-supplemented diet given to Japanese quails reduced levels of plasma cholesterol and lipid-rich intimal thickening lesions in the aorta when compared with corn oiland oleic acid-supplemented groups[42]. Yemitan and Izegbu demonstrated that pretreatment with Zingiber officinale rhizome extract could decrease the serum levels of the liver enzymes in rats challenged by CCl4and paracetamol, suggesting that it has a preventive effect on chemical-induced acute liver injuries[11]. Consistent with silymarin (25 mg/kg, po.), Himoliv (a polyherbal formulation)pretreatment significantly lowered the elevation of the serum levels associated with liver enzymes found in rats diagnosed with CCl4-and paracetamol-induced hepatotoxicity[12]. In addition, acute hepatoprotective effects of chia seeds (Salvia hispanica L.) against CCl4-induced steatohepatitis may be correlated to its high content of α-linolenic acid (ω3-PUFA) and phenolics[43]. Furthermore,mixed palm tocotrienols showed hepatoprotective effects in hypercholesterolemic adults diagnosed with nonalcoholic fatty liver disease[44], while tocopherols and tocotrienols demonstrated protective effect against xenobiotic-induced liver injury in rats[45].Surprisingly, the ethanolic extract of Lepidium sativum seeds that are rich in α-linolenic acid exerted hepatoprotective effects against CCl4-induced acute liver injury in rats, which could possibly be attributed to relevant antioxidant and anti-inflammatory activities[46].
Based on the above results, perilla fruit oil is an excellent source of α-linolenic acid for the synthesis of long-chain polyunsaturated fatty acids, together with tocopherols and tocotrienols which are known to be lipophilic antioxidants. The production of perilla fruits can be acknowledged as an enrichment of agricultural crops due to the premium price that is paid for specialty oils, while the formulation of perilla fruit oil will be of value as a nutraceutical providing health benefits to consumers. Importantly, perilla fruit oil displays inhibitory effects on CCl4-induced acute liver injury by enhancing anti-oxidative and anti-inflammatory activities. Additionally, the findings may have therapeutic value in the treatment or prevention of chemical-induced liver inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Agricultural Research Development Agency (Public Organization) of Thailand (Project Code: CRP 5705020580); and the Research and Researchers for Industries (RRI) Ph.D. Program, Thailand Research Fund, through Miss Narisara Paradee (PHD56I0016); Newton Fund 2016 PhD.Placement was accomplished through Miss Narisara Paradee, Faculty of Medicine Endowment Fund, Chiang Mai University through Dr.Somdet Srichairatanakool (Research ID: 2558 03504 / Study Code BIO 2558 03504); and Tipco BioTech Company, Prachuapkhirikhan,Thailand.
Funding
This research was supported by the Agricultural Research Development Agency (Public Organization) of Thailand (Project Code: CRP 5705020580); and the Research and Researchers for Industries (RRI) Ph.D. Program, Thailand Research Fund, through Miss Narisara Paradee (PHD56I0016); Newton Fund 2016 PhD.
Authors’ contributions
NP prepared P. frutescens fruit oil, conducted the animal experiments and analyzed the data; DK and TT applied for grants,designed and advised on experiments, and participated in the discussion; AP and PK conducted the experiments, measured blood biomarkers, and investigated the macro-pathologic appearance; SK performed and reported on the histopathological examinations; and SS applied for grants, designed experiments, wrote and submitted the manuscript, and responded to all suggested revisions. All authors have read, contributed to and approved this manuscript.
 Asian Pacific Journal of Tropical Biomedicine2020年3期
Asian Pacific Journal of Tropical Biomedicine2020年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for AuthorsAsian Pacific Journal of Tropical Biomedcine
- Mayaro fever: A brief review on the immune profile
- Effect of protease inhibitor from Agaricus bisporus on glucose uptake and oxidative stress in 3T3-L1 adipocytes
- Deoxyelephantopin induces ROS-mediated autophagy and apoptosis in human colorectal cancer in vitro and in vivo
- Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats
