Syringic acid improves oxidative stress and mitochondrial biogenesis in the liver of streptozotocin-induced diabetic rats
Zahra Sabahi, Mohammad Javad Khoshnoud, Bahman Khalvati, Seyedeh-Sara Hashemi, Zahra Ghasempour Farsani, Hoda Mogholi Gerashi, Marzieh Rashedinia✉
1Medicinal Plants Processing Research Center, Shiraz University of Medical Sciences, Shiraz, Fars, Iran
2Department of Pharmacology and Toxicology, School of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
3Medicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
4Burn and Wound Healing Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
ABSTRACT Objective: To determine the effects of syringic acid on hepatic damage in diabetic rats.Methods: Diabetes was induced by streptozotocin. Diabetic rats were given syringic acid at doses of 25, 50 and 100 mg/kg by oral gavage for 6 weeks. Syringic acid effects on the liver were evaluated by examination of plasma biochemical parameters, and pathological study. In addition, biomarkers of lipid peroxidation and antioxidant status of liver tissues were assessed. Real time-PCR was performed to investigate the mRNA expression levels of mitochondrial biogenesis indices in different groups.Results: Syringic acid significantly attenuated the increase in most of plasma biochemical parameters in diabetic rats.Moreover, syringic acid treatment increased the catalase activity while it reduced the superoxide dismutase activity and hepatic malondialdehyde level in diabetic rats. There was no difference between the glutathione content of the treated and untreated groups.These findings were supported by alleviation of histopathological damages in the syringic acid-treated groups compared to the untreated diabetic group. Syringic acid also significantly upregulated the hepatic mRNA expression of PGC-1α, NRF-1, and NRF-2 and increased the mtDNA/nDNA ratio in diabetic rats.Conclusions: Syringic acid can be considered as a suitable candidate against hepatic complications since it can reduce oxidative damages in diabetic cases. Furthermore, it has the potential of targeting hepatic mitochondria in diabetes.
KEYWORDS: Syringic acid; Diabetic; Liver; Oxidative stress;Mitochondrial biogenesis; Rats
1. Introduction
Diabetes mellitus (DM) is a metabolic disease with inadequate insulin secretion and/or inefficient cellular response to insulin[1,2].These pathological conditions can lead to irregular carbohydrates,protein, and lipid metabolism[3]. The kidneys, eyes, nerves, liver,blood vessels and heart are the organs that suffer the most from abnormal hyperglycemia[1]. An increase in the incidence of diabetes is significant with a worldwide prevalence of 9%, which is predicted to rise to 53.1 million by 2025[4]. Hyperglycemia induces oxidative stress by raising the reactive oxygen species (ROS) level, which is produced due to excess mitochondrial activity and stimulation of the NF-κB signaling pathway in the phagocytes. Moreover, advanced glycation end products can cause the promotion of oxidative stress[5].
Oxidative stress plays a critical role in diabetic complications[5],which is due to its side effects on vital biomacromolecules such as proteins, lipids, and DNA. These cellular damages might lead to cell death via apoptosis and necrosis[6]. Subsequently, the efficiency of antioxidants in reducing the risk of diabetic complications is considerable and still remains as the most important challenge[7,8].
The liver plays a central role in carbohydrate metabolism and the maintenance of normal glucose levels. In diabetic conditions, chronic hyperglycemia, insulin resistance, and reduced peripheral glucose uptake lead to an increase in hepatic glucose output level as well as lipogenesis[9].
There is a strong relationship between diabetes and alteration in mitochondrial function. Diminished mitochondrial density, ATP production, and reduced mitochondrial mRNA levels are other complications in diabetes and insulin resistance[10]. Mitochondrial biogenesis is a process affected by hyperglycemia and insulin resistance. Thus, in the latest treatment strategies, mitochondrial biogenesis is considered[11].
Herbal metabolites are considered as a source of natural products with different structures and biological effects. These components have broad applications in the treatment of diabetes[12]. Syringic acid (SYR) is a derivative of hydroxybenzoic acid with 4-hydroxy-3,5-dimethoxybenzoic acid. It is present in different herbs including Lentinula edodes[13], cereal grains[13], Herba dendrobii[14], Radix isatidis[15]and the leaves of Alpinia calcarata Roscoe[16]. SYR is known as a free radical scavenging agent and a potent antioxidant.Previous reports revealed that SYR has a hepatoprotective effect on different animal models[17].
The aim of this study was to determine the potential of SYR in the management of hyperglycemia and oxidative damages of the hepatic tissue in the streptozotocin (STZ)-induced diabetic rat model. Moreover,the effect of SYR treatment on biochemical factors, antioxidant activities including catalase (CAT) and superoxide dismutase (SOD)activities and glutathione (GSH) and malondialdehyde (MDA) level was also determined. Pathological state and mRNA expression levels of mitochondrial biogenesis indices were also examined. The results provide important information on the effectiveness of SYR as a therapeutic agent for the treatment of liver complications in diabetes cases.
2. Materials and methods
2.1. Chemicals
All the chemicals used in this experiment were purchased from Sigma-Aldrich (St. Louis, USA), Merck Company (Darmstadt,Germany) commercially available and were of the highest grade .
2.2. Animal care and use
Sixty male Sprague-Dawley rats (220-240 g weight), 10-12 week-old were purchased from the Center of Comparative and Experimental Medicine of Shiraz University of Medical Sciences,Shiraz, Iran, and housed in cages at a temperature of (25±3) ℃ and 12 h light/dark cycle. All the rats had access to water and food ad libitum.
DM was experimentally induced in animals using 60 mg/kg of STZ(freshly dissolved in 0.1 mol/L, pH 4, citrate buffer) intraperitoneally after fasting for 12 h[18]. Non-diabetic animals in the control group received an intraperitoneal injection of an equivalent volume of citrate buffer. One week after the STZ treatment, the development of diabetes in the experimental rats was confirmed by measuring the levels of glucose in the blood taken from the tail vein using glucometer (Gluco Lab, Korea). The rats with a blood glucose level of more than 300 mg/dL were considered diabetic and were included in the study[1].
2.3. Study design
The animals were randomly divided into six groups (n=10) as follows:Group Ⅰ: non-diabetic control, Group Ⅱ: non-diabetic SYR (100 mg/kg), Group Ⅲ: diabetes control (STZ), Group Ⅳ: STZ +SYR (25 mg/kg), Group Ⅴ: STZ+SYR (50 mg/kg), Group Ⅵ: STZ+SYR (100 mg/kg). SYR was administered daily via intragastric gavage for a period of six weeks. Only a higher dose of SYR 100 mg/kg was performed to verify the non-toxic effects of SYR on non-diabetic rats. Body weight and death numbers were recorded every day, and body weight gain was calculated as follows: Body weight gain =final body weight-initial body weight. Due to mortality during the study [4 in the diabetic group and 3 in STZ + SYR (25 mg/kg)], we sacrificed an equal number of animals in each group (n=6).
After 6 weeks of treatment, the animals were fasted overnight and then anesthetized intraperitoneally with ketamine-xylazine 100/10 mg/kg. The subjects were sacrificed by cervical decapitation (six animals in each group). For further analysis, blood samples were collected. Their livers were removed and washed in ice-cold saline;separated tissue fragments were weighed and homogenized in cold phosphate buffer (PBS, 0.1 mol/L, pH = 7.4) for biochemical analysis, RNA and DNA extraction. Homogeneous tissues were aliquoted and then frozen at -70 ℃ until use.
2.4. Evaluation of plasma biochemical parameters
Blood was collected from cervical dislocation in EDTA-coated tubes and their plasma was separated by centrifugation at 3 000 rpm for 10 min. Plasma glucose, glutamic-oxaloacetic transaminase(SGOT), glutamic-pyruvic transaminase (SGPT), low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol(HDL), and triglycerides (TG) were measured using commercial Pars Azmoon kits (Tehran, Iran) by MindrayBS-200®auto analyzer.
2.5. Evaluation of oxidant/antioxidant status
The level of liver lipid peroxidation was assessed by thiobarbituric acid reactive substances (TBARS) test that measures MDA. The reaction mixture consisted of 500 μL of 10% liver homogenate,250 μL of HCl (0.5 mol/L) and 500 μL of 0.6% thiobarbituric acid,which were vortexed and then heated in boiling water for 45 min.After cooling and centrifugation (6 000 rpm, 10 min), the absorbance of supernatants was read at 540 nm using BioTek, ELX800 ELISA Reader, USA. MDA level was reported as nmol/mg protein[19].
The liver GSH contents were assessed using the Ellman reagent[5,5’-dithiobis-(2-nitrobenzoic acid) (DTNB)]. Equal amounts of tissue homogenates were mixed with 10% w/v trichloroacetic acid.After centrifugation, 500 μL of the supernatants were mixed with reaction buffer containing 3 mL of phosphate buffer (0.3 mol/L, pH 8.9) and 500 μL of DTNB (0.01 mol/L). The absorbance of yellow color was read at 412 nm using an automated plate reader[20].
The CAT activity was determined according to the method described previously by Goth with a slight modification[21]. Briefly,60 μL of the tissue homogenate supernatant was incubated in 300 μL substrate (130 mmol/mL hydrogen peroxide in 60 mmol PBS,pH 7.4) for 3 min. The reaction was stopped by adding 300 μL of 32.4 mmol ammonium molybdate; the absorbance was measured at 405 nm against the blank. The enzyme activity was calculated and expressed as U/L. The SOD activity in the liver tissue was measured using a commercial kit (Nasdox, Navand Salamat, Iran) following the manufacturer’s instruction, based on the Pyrogallol autoxidation method.
2.6. Evaluation of PGC-1α, TFAM, NRF-1, NRF-2 and mtDNA/nDNA ratio by quantitative real-time polymerase chain reaction (qRT-PCR)
Homogeneous hepatic tissues were subjected to RNA extraction.Total RNA was isolated by Pars Tous kit (Mashhad, Iran) according to the manufacturer’s protocol, and cDNA was then synthesized using reverse transcription reaction (Easy cDNA Synthesis Kit;Pars Tous, Iran). The cDNA was amplified using real-time PCR(RealQ Plus 2× Master Mix Green Low ROXTM, Ampliqon)with a thermocycler and SYBR green detection system (Applied Biosystems, USA). The sequences of primers used in real-time PCR were as follows[22-24]:
PGC-1α, forward: 5’-CGGGATGGCAACTTCAGTAAT-3’, reverse:5’-AAGAGCAAGAAGGCGACACA-3’;
TFAM, forward: 5’-GAAAGCACAAATCAAGAGGAG-3’, reverse:5’ CTGCTTTTCATCATGAGACAG-3’;
NRF-1, forward: 5’-GGGGAACAGAACAGGAAACA-3’, reverse:5’-CCGTAATGCACGGCTAAGTT-3’;
NRF-2, forward: 5’-GGGGAACAGAACAGGAAACA-3’, reverse:5’-CCGTAATGCACGGCTAAGTT-3’;18S, forward: 5’-CGAACGTCTGCCCTATCAACTT-3’, reverse: 5’-CTTGGATGTGGTAGCCGTTTCT-3’. 18S rRNA was used as an internal control for normalization.
Total DNA was obtained from the liver tissue by the tissue extraction DNA kit (Pars Tous, Iran). Real-time quantitative PCR was performed to assess the mitochondrial DNA copy number. The ratio of mtDNA(RNR2)/nDNA (GAPDH) was used as an estimation of the number of mitochondrial tissue. mtDNA-encoded RNR2 gene primer sequence was: forward, 5’-AGCTATTAATGGTTCGTTTGT-3’; reverse, 5’-AGGAGGCTCCATTTCTCTTGT-3’. Nuclear-encoded GAPDH gene primer sequence was: forward, 5’-GGAAAGACAGGTGTTTTGCA-3’;reverse, 5’-AGGTCAGAGTGAGCAGGACA-3’.
2.7. Histopathological investigations
For the histopathological evaluation, liver samples were fixed in 10% formalin and embedded in paraffin. The sections (5-μm thickness) were stained with hematoxylin and eosin (Hffamp;E)techniques. They were observed with an Olympus BX51 microscope at a magnification of 200× for evaluation of histopathological parameters.
2.8. Statistical analysis
The data are presented as mean ± SD. One-way ANOVA with Tukey’s test was used for multiple comparisons amongst groups. The significant level was set as α=0.05. All the calculations were carried out by SPSS 16.0 software. The GraphPad Prism 5.0 software was used for plotting the graphs.
2.9. Ethical statement
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the local Ethics Committee, Shiraz University of Medical Sciences,Shiraz, Iran (Permit; IR.SUMS.REC.1395.S853).
3. Results
3.1. Biochemical parameters
The diabetic group showed significant increases in plasma glucose,SGOT, SGPT and TG and a decrease in HDL (P<0.05) as compared to the control group. Treatment with SYR at 25 and 50 mg/kg did not significantly affect the level of plasma glucose, SGOT and TG activities as compared to the diabetic group, but the diabetic group treated with SYR at 100 mg/kg showed significant reductions in these parameters (P<0.01) (Table 1). Moreover, SYR treatment at 50 mg/kg significantly reduced the SGPT activities (P<0.05) while at 25 mg/kg did not change its activities.
Plasma LDL level showed no significant difference between the diabetic and control groups. Moreover, SYR-treatment (25, 50 and 100 mg/kg) did not significantly affect LDL and HDL levels as compared to diabetic rats (Table 1).
Table 1 also shows the effect of SYR administration on body weight gain. Before the experiment, body weight was not significantly different among groups, but after 6 weeks of treatment, body weight gain was significantly reduced in diabetic rats in comparison with the control group (P<0.001). The body weight gain showed no significant change in the low dose treatment groups (SYR 25 and 50 mg/kg) in comparison to the diabetic group, while treatment of diabetic rats with SYR at 100 mg/kg significantly (P<0.05) increased body weight gain.
3.2. Antioxidant assay analysis
The diabetic group showed significant increases in the levels of MDA and SOD (P<0.05), and a significant decrease in CAT(P<0.001), but no significant difference in GSH in liver tissue ascompared to the control group.
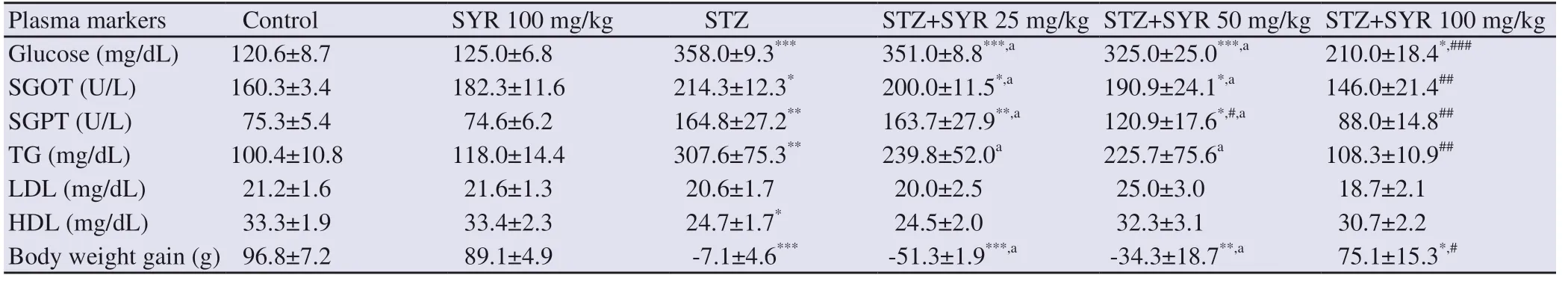
Table 1. Effect of syringic acid on blood glucose level, liver injury markers and body weight of streptozotocin-induced diabetic rats in various experimental groups.
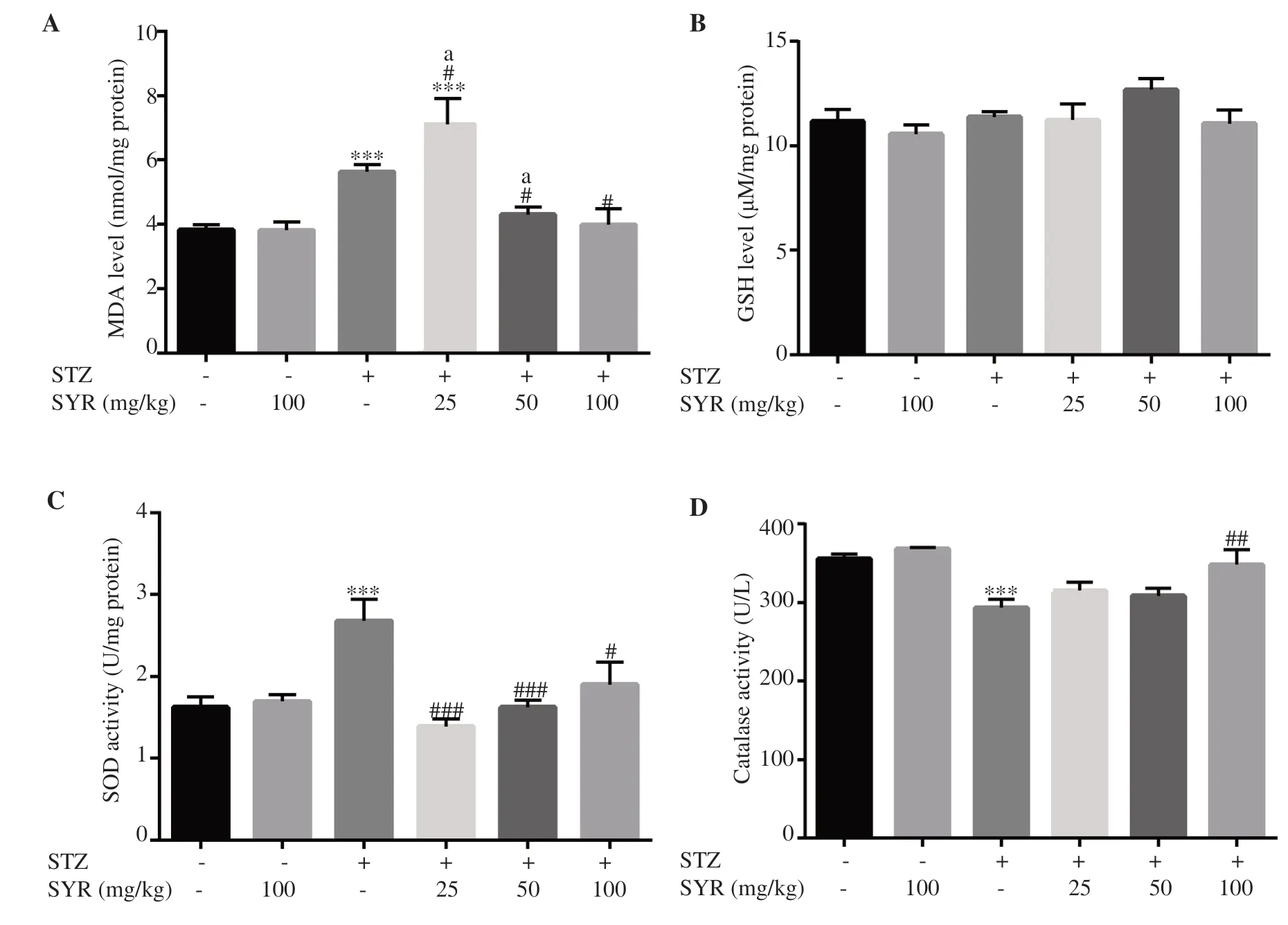
Figure 1. Effect of syringic acid on oxidative stress and antioxidant markers in the liver of streptozotocin (STZ)-induced diabetic rats in various experimental groups. A: thiobarbituric acid reactive substances (TBARS); B: reduced glutathione (GSH); C: superoxide dismutase (SOD); D: catalase.SYR: syringic acid; All values are expressed as mean± SD; n= 6 per group. *P<0.05, ***P<0.001 vs. the control group; #P<0.05, ##P<0.01, ###P<0.001 vs. the diabetic group; a: P<0.05 compared with STZ+SYR 100 mg/kg.
It is worth mentioning that comparing with the control group,the increase in MDA levels of SYR 25 mg/kg group was more significant than the diabetic group (P<0.001 vs. the control group,and P<0.05 vs. the diabetic group), while SYR at 50 and 100 mg/kg significantly reduced the MDA level compared with the diabetic group (P<0.05) (Figure 1A).
The SYR treatment groups (25, 50 and 100 mg/kg) induced no significant change in liver GSH level (Figure 1B), while SOD activities were significantly reduced by SYR treatments (P<0.05)(Figure 1C). Moreover, 25 and 50 mg/kg treatment groups showed more significant improvement in SOD than 100 mg/kg. In addition,CAT was significantly increased in the diabetic group treated with 100 mg/kg SYR (P<0.01), while the changes in 25 and 50 mg/kg treatment groups were not significant (Figure 1D).
The levels of MDA, GSH, CAT and SOD in the group treated with SYR 100 mg/kg were comparable to the normal control group. In addition, there was no significant differences in level of GSH, CAT or SOD among STZ+SYR 25 mg/kg, STZ+SYR 50 mg/kg and STZ+SYR 100 mg/kg groups.
3.3. mRNA expression level of mitochondrial biogenesis indice
The levels of αPGC-1α, NRF-1, and TFAM mRNA decreased in the diabetic group in comparison with the control group (P<0.01).Treatment with SYR 100 mg/kg significantly increased the levels of PGC-1α, NRF-1, and NRF-2 mRNA in comparison with the diabetic group (P<0.05) (Figure 2A, 2C, and 2D), but no significant difference was shown in TFAM level (Figure 2B).
As shown in Figure 2E, the mitochondrial copy number was significantly reduced in the diabetic group and SYR 25 mg/kg group as compared with the control (P<0.01). However, treatment with 100 mg/kg SYR significantly increased the mitochondrial copy number,which was comparable to the control group (P<0.01).
3.4. Histopathological results
As shown in Figure 3, in the liver of the STZ-induced diabetic rats, damages to the hepatocytes were observed, consisting of the distended portal vein, infiltration of inflammatory cells, sinusoids dilation, and loss of normal architecture. In the SYR-treated group at 25 and 50 mg/kg, the liver cells degenerated with mild necrosis,which appeared as focal areas with leukocytes infiltration in the portal areas. Severe congestion, hemorrhage hypertrophy of the hepatocytes as well as hepatocyte swelling were observed. However,the administration of SYR 100 mg/kg improved hepatocytes, central vein architecture, and sinusoids.
4. Discussion
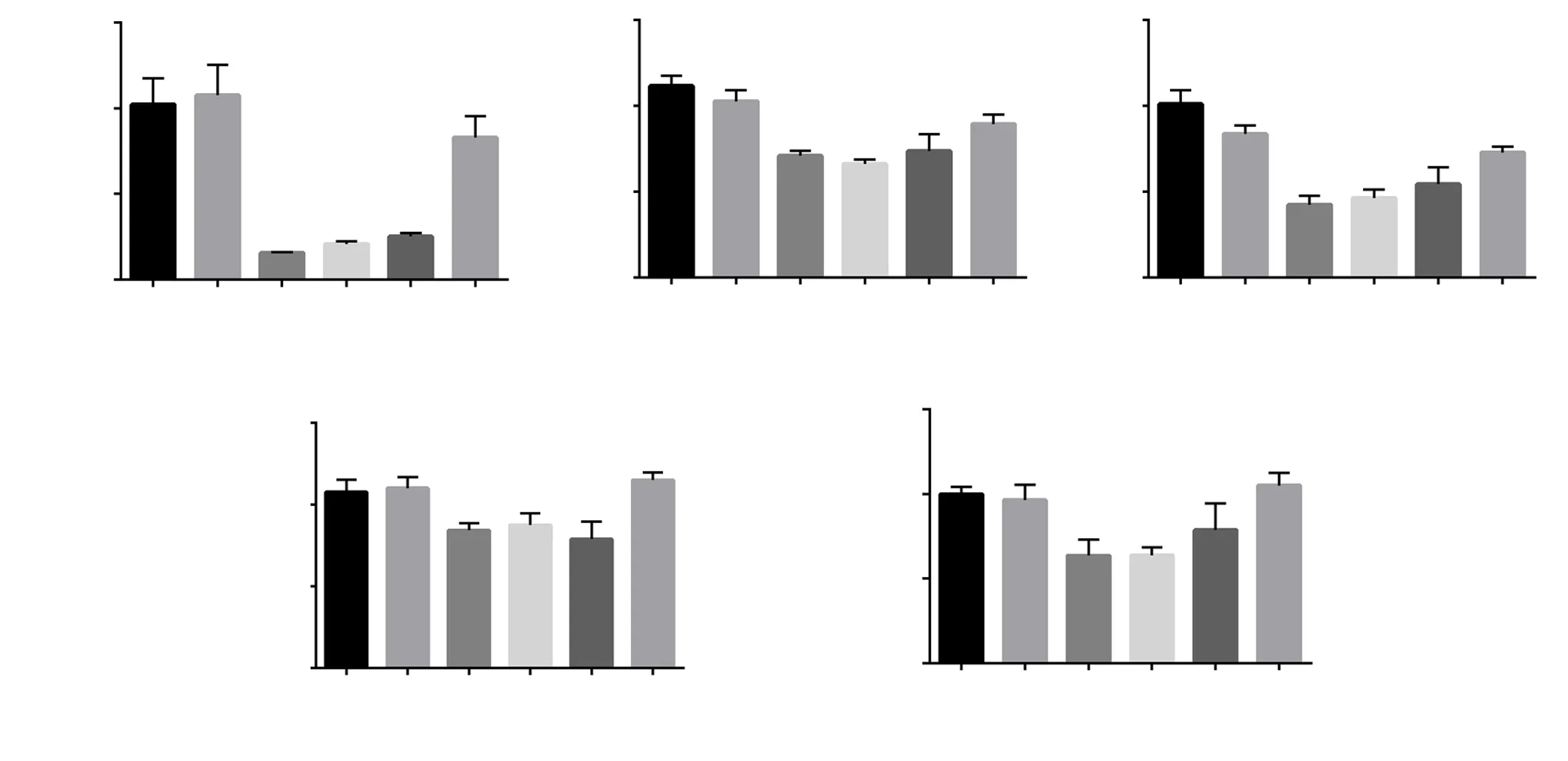
Figure 2. Effect of syringic acid on mitochondrial biogenesis factors in the liver of streptozotocin (STZ)-induced diabetic rats in various experimental groups. The mRNA expression of mitochondrial biogenesis factors was measured by real-time RT-PCR. A: PGC-1α; B: TFAM; C: NRF-1; D: NRF-2; E:mtDNA/nDNA. The relative mitochondrial DNA content was quantified by PCR. SYR: syringic acid; All values are expressed as mean±SD; n=3 per group.*P<0.05, **P<0.01 vs. the control group; #P<0.05, ##P<0.01, ###P<0.001 vs. the diabetic group; a: P<0.05 compared with STZ+SYR 100 mg/kg.
In this study, the STZ injection induced significant hyperglycemia.SYR doses and duration of treatment were chosen to achieve maximum anti-hyperglycemic activity and non-toxic effects according to previous researches[12,25]. Six-weeks treatment can confirm SYR beneficial effects[25,26]. Biochemical analysis of different groups in this study showed that the levels of glucose were increased in the diabetic control group. Administration of SYR 100 mg/kg showed the anti-hyperglycaemic potency of this natural compound via improving glucose levels, which is the aim of controlling diabetes. Muthukumaran et al.[12]showed that SYR could regenerate β-cells, restore insulin sensitivity, and increase the consumption of glucose via peripheral tissues to elevate its release from the pancreas, then to increase the insulin level. An additional mechanism is involved in reducing the power of phenolic acid in carbohydrate metabolism, which includes the inhibition of α-glucosidase, α-amylase and aldose reductase activities[27], and elevation of glucokinase activity[28]. Besides SYR,other phenolic acids introduce anti-hyperglycemic agents, such as chlorogenic[29], gallic acid[30], and ferulic acid[31]. In addition, it was proposed that phenolic acids regulate postprandial glycemia and reduce glucose intolerance by facilitating the insulin response as well as stimulating the glucose-dependent insulinotropic polypeptide release and glucagon-like peptide-1[27].
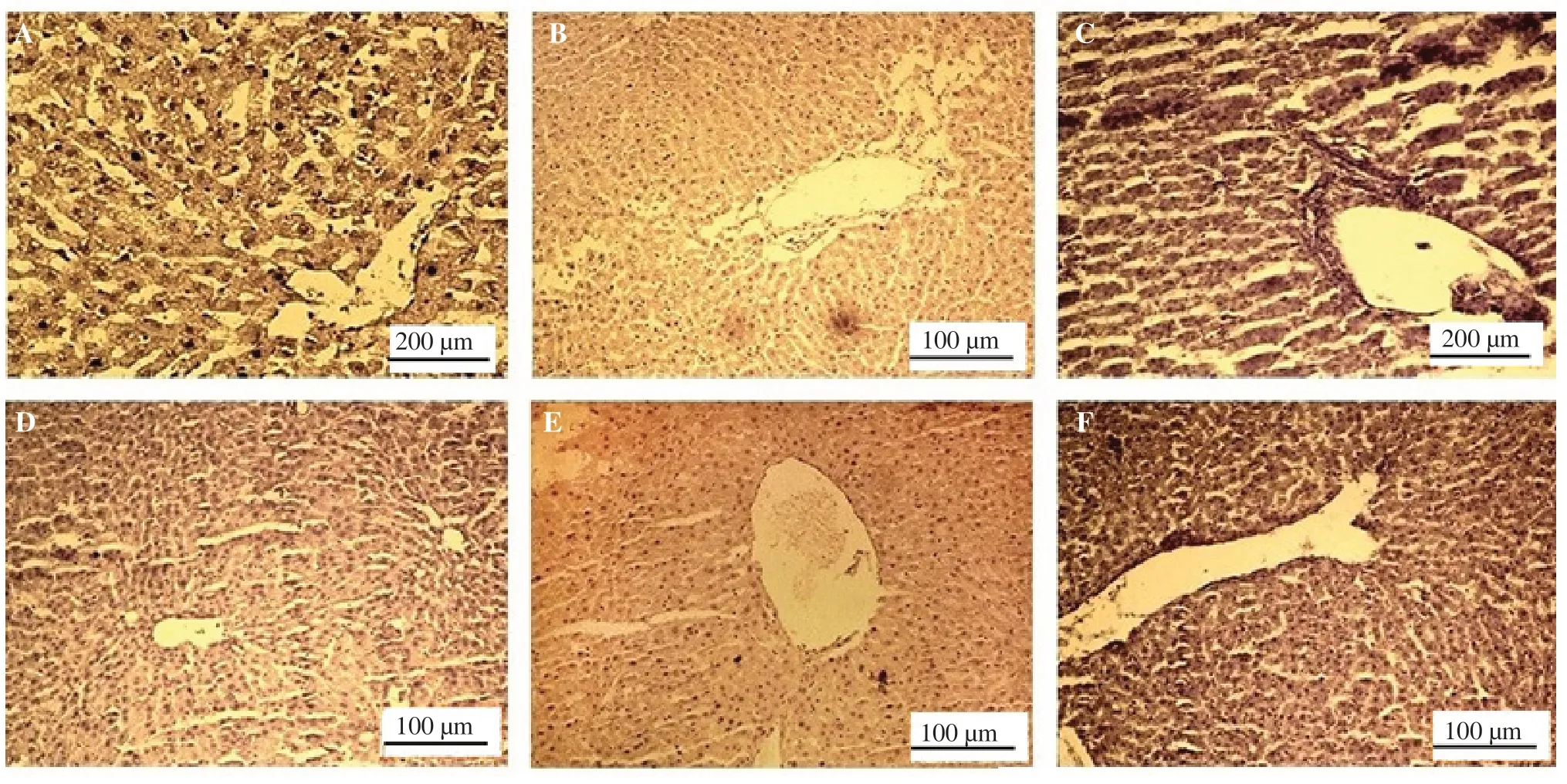
Figure 3. Effect of syringic acid on histological result of the liver of streptozotocin (STZ)-induced diabetic rats in various experimental groups. A: control normal; B: syringic acid (SYR), the result is the same as the control normal; C: STZ group shows damage of the hepatocytes, leukocyte infiltration,inflammation and infiltration of inflammatory cells in the portal vein; D: STZ+ SYR 25 mg/kg group ameliorates leukocyte infiltration and inflammation,and recovers the hepatocytes and normal central vein; E: STZ+ SYR 50 mg/kg group ameliorates the leukocyte infiltration and inflammation, and shows normal hepatocytes and central vein; F: STZ+ SYR 100 mg/kg group shows absence of infiltration and inflammatory cells in the portal vein, normal hepatocytes, and central vein (hematoxylin and eosin were used for staining; scale bar: 100 and 200 μm).
In our study, weight loss was significant as it was predicted in the diabetic group. Furthermore, SYR administration led to a reduction in mortality during the experiment. Effects of SYR on body weight and reduced mortality can be attributed to the control of hyperglycemic conditions in the diabetic group.
In this study, TG was increased in the diabetic group and similar results were reported in a previous study on STZ induced diabetes, in which TG and LDL rose by indirectly increasing the blood glucose level[32]. There was a strong relationship between lipids and the pathogenesis of diabetes. Reduced insulin in diabetic cases had a significant effect on the mobilization of fat from their storage, which led to a rise in their plasma concentration[32]. Based on the current findings, the TG level was reduced in the group treated with SYR 100 mg/kg.
Elevated levels of SGOT and SGPT in the blood are liver abnormal function indices, which is due to their release from the hepatic damaged cells into the blood stream[33]. Treatment of diabetic rats with SYR(100 mg/kg) significantly reduced the levels of SGPT and SGOT in comparison with the untreated ones. A similar research also reported the reducing effect of ellagic acid on SGOT and SGPT levels in diabetic rats[34].
The SYR-only group with 100 mg/kg showed an increase in the SGOT level; however, the difference of SGOT level between this group and the control was not statistically significant. For this reason, SYR is not hepatotoxic at least up to 100 mg/kg dose in rats.
There is no consensus between studies on antioxidant enzyme activities in different organs of diabetic rats. A study reported a reduction in the levels of these enzymes[35]while another showed an increase in the activity of these enzymes in diabetic rats[36].
In the current study, diabetes induction reduced the antioxidant defense system factors by reducing CAT activities while SOD activity was significantly increased in the diabetic group. According to the results, treatment of diabetic groups with SYR led to increased CAT and decreased SOD activities; however, there was no significant effect on the GSH content, which might be related to sub-chronic oxidative stress condition in the liver, leading to cell compensation by the synthesis of GSH at a constant level. SYR might have a role in the radical scavenging of oxygen radicals. Therefore, it might inhibit the increase of SOD activity in hepatic tissues of diabetic rats.
In this study, the diabetic group showed reduced CAT activity, which can be associated with the hepatic inability to eliminate hydrogen peroxide. Punithavathi et al. reported that gallic acid protects the pancreatic tissue from oxidative stress damages during diabetes by scavenging the free radicals and by improving the activity of these enzymes[30].
MDA is a product of lipid peroxidation in oxidative stress, which can be due to protein and DNA damages in different tissues[37]. Thus, it can be a proper marker to predict the severity of liver damages. In this study, the MDA level in the hepatic tissue was increased in the diabetic groups while significantly decreased in the diabetic group treated with 50 and 100 mg/kg SYR. These results are in agreement with those showing an increased lipid peroxidation in the liver by the induction of hyperglycemia[38]. It shows that SYR can reduce lipid hydroperoxide levels in the liver.
SYR has a phenolic structure with moderate free radical scavenging antioxidant activity. It is suggested that SYR plays an indirect antioxidant role in genes expression or enzyme activity, such as SOD and CAT to reduce the effects of diabetic complications.
The present findings are in agreement with studies that introduced gallic acid as an attenuator of MDA in the pancreatic tissue and caffeic acid phenethyl ester as the inhibitor of lipid peroxidation in the diabetic liver[30].
Apart from glucose and lipid, mtDNA is the other target of oxidative damage[39]. A previous study showed that the reduction of mtDNA is an important factor in diabetes pathogenesis[39]. Thus, an effective strategy improves mitochondrial energy impairment, which increases the number of mitochondria[40]. Mitochondrial biogenesis can be defined as the process of increasing the mass and copy number of cellular mitochondria[41]. Insulin resistance could be due to the reduction of mitochondrial biogenesis. However, mitochondrial irregularities might increase insulin resistance by producing more ROS[42]. Therefore,improving mitochondrial function could result in improved insulin resistance[11].
Mitochondrial biogenesis can be induced by peroxisome proliferatoractivated receptor-c coactivator 1 alpha (PGC-1α), nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2), and mitochondrial transcription factor A (TFAM) contributions[43]. PGC-1α and NRFs are able to play a role in TFAM expression which is a nuclear-encoded factor involved in the activation, replication, and transcription of the mitochondria[44].In brief, PGC-1α activation results in NRF-1 stimulation, following an increase in the synthesis of TFAM that ultimately leads to mitochondria production[43]. A previous study showed that reduced PGC-1α expression in the hepatic tissues might contribute to NRF dependent genes accounting for insulin resistance and diabetes. In line with the aforementioned, DNA microarray analysis indicates that PGC-1α expression could result in metabolic disorders such as diabetes and insulin resistance[11].
Vinayagam et al. determined NRF-1 as a critical factor in oxidative stress and inflammation in the hepatic tissues. Moreover, they suggested that NRF-1 deficient hepatocytes are more exposed to intracellular ROS levels, raised lipids that eventually lead to neoplastic growth[27].According to the current study results, mRNA expression levels of PGC-1α, NRF-2, NRF-1, and TFAM were reduced in the hepatic tissue of the diabetic group. In addition, a significant decrease was observed in the mtDNA/nDNA ratio in diabetic rats, but there was an increase in the mitochondria copy number in the liver following supplementation of SYR. These results indicate that SYR improves mitochondrial biogenesis. Previously, Rayamajhi et al. suggested quercetin, another phenol compound, as the stimulator of mtDNA copy number in the HepG2 cells[44]. The current results exhibit that SYR has the potential to enhance the PGC-1α mRNA expression, a key factor of the transcriptional system in the mitochondrial biogenesis regulation in the diabetic liver[44].
This study showed that SYR treatment ameliorated the functional and histological abnormalities and mitochondria biogenesis in the liver of diabetic rats. These effects should principally contribute to the improvement of oxidative stress via normalization of antioxidant and mitochondrial function by SYR.
Anti-diabetic effects of natural products, especially phenolic compound, were confirmed in another study[45], but the effect of these compounds on the mitochondrial biogenesis in diabetes is limited[40,44].As mentioned earlier, mitochondrial biogenesis is affected by an insulin-resistant state[11,42]. Subsequently, interventions that can improve the mitochondrial function are novel strategies to fight insulin resistance and to protect organs against dysfunction[11].
In this study, there were several limitations and further investigations are warranted to reveal the relationship between mRNA level and protein expression of the mitochondrial biogenesis markers in the liver tissues.
Dietary supplementation of SYR in diabetes protects the hepatic tissue against hyperglycaemia and lipid peroxidation. This compound provides protection by ameliorating the impairment of mitochondrial biogenesis in diabetic rats. The results of this study suggest the role of SYR in the mitochondrial biogenesis and its antioxidant activity in diabetes complications.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
The authors wish to thank Mr. H. Argasi at the Research Consultation Center of Shiraz University of Medical Sciences for his invaluable assistance in editing this manuscript. This article was extracted from the thesis written by Zahra Ghasempour Farsani and Hoda Mogholi Gerashi
Funding
This study was financially supported by Shiraz University of Medical Sciences (Grant number: 95-01-70-12474).
Authors’ contributions
ZS and MR conceived and desiged the work, analysed data, wrote and revised the article, final approved of the version to be published.MJK conceived and desiged the work, and analysed data. BK, SSH,ZGF and HMG collected and analysed data.
 Asian Pacific Journal of Tropical Biomedicine2020年3期
Asian Pacific Journal of Tropical Biomedicine2020年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Information for AuthorsAsian Pacific Journal of Tropical Biomedcine
- Mayaro fever: A brief review on the immune profile
- Effect of protease inhibitor from Agaricus bisporus on glucose uptake and oxidative stress in 3T3-L1 adipocytes
- Deoxyelephantopin induces ROS-mediated autophagy and apoptosis in human colorectal cancer in vitro and in vivo
- Thai Perilla frutescens fruit oil alleviates carbon tetrachloride-induced hepatotoxicities in rats
