Ti3C2Tx/Wood Carbon as High-areal-capacity Electrodes for Supercapacitors
Li Teng-Fei, HUANG Lu-Jun, YAN Xu-Dong, LIU Qing-Lei, GU Jia-Jun
Ti3C2T/Wood Carbon as High-areal-capacity Electrodes for Supercapacitors
Li Teng-Fei, HUANG Lu-Jun, YAN Xu-Dong, LIU Qing-Lei, GU Jia-Jun
(State Key Laboratory of Metal Matrix Composites, School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China)
MXenes—two-dimensional (2D) compounds generated from layered bulk materials, have attracted significant attention in energy storage fields. However, low mass loading of MXenes results in low areal capacity and impedes the practical use of MXenes electrodes. Inspired by natural basswood, an ideal architecture with natural, three-dimensionally (3D) aligned open microchannels was developed for high Ti3C2Tmass loading. Compared with reported Ti3C2Telectrode structure, the 3D porous carbon matrix has several advantages including low tortuosity, high conductivity and good structure stability. The Ti3C2Tassembled with the wood carbon can deliver a high areal capacity of 1983 mF/cm2at 2 mV/s with a high Ti3C2Tmass loading of 17.9 mg/cm2when used as electrode for supercapacitors. This work provides a new strategy to develop 3D porous electrodes for MXenes, which can achieve high areal capacity.
Ti3C2T; wood carbon; supercapacitor; high areal capacity
MXenes have gained tremendous attention in supercapacitor[1], lithium battery[2], electromagnetic interference shields[3]and other energy-related fields. They were firstly reported by Michael Naguibselectively etching from MAX phases[4](where M is a transitional metal, A is a III or IV A-group element and X is carbon or nitrogen). The resulting 2D materials,, MXenes, have many chemical and structural forms with high intrinsic electronic/ionic conductivities. When applied to supercapacitors, the fast surface redox makes them possess large energy. However, although much effort have been made to obtain superior gravimetric and volumetric capacity of MXenes[1, 5], the mass loading of MXenes still needs to be further improved in order to get a high areal capacity and meet practial applications in large scale.
In 2017, Chen,[6]reported a fascinating work to utilize natural basswood structure for an all-wood-structured asymmetric supercapacitors design. The electrodes were derived from natural wood and inherited its unique anisotropic structure which has numerous open channels along the growth direction. The wood based devices displayed high desirable thickness (up to ~1 mm) with high mass loading of 30 mg/cm2. With these unique features, such as low tortuosity, high electronic conductivity, enabling high mass loading, low cost and environmentally friendly, the wood-structured electrode represents a promising way to realize high power density and high energy density for green energy storage device[7-9].
Herein, we reported a facile way to make a high mass loading NaOH-Ti3C2T/wood carbon electrode for supercapacitors. The resulted electrode exhibited a high areal capacity of 1983 mF/cm2at 2 mV/s with a high Ti3C2Tmass loading of 17.9 mg/cm2, which surpass three times than that of NaOH-Ti3C2Treported before[10].
1 Experimental
1.1 Synthesis of Ti3C2Tx
Ti3C2T(T=O, OH) was synthesized according to the paper reported before[10]. About 80 mg Ti3AlC2powder (400 mesh (38 μm), purchased from 11 Technology Co. Ltd.) was added into the 35 mL NaOH solution (27.5 mol/L, the water was heated to boiling to reduce the concentration of oxygen) contained in a 50 mL autoclave in argon (Ar) atmosphere. Then the autoclave was heated at 270 ℃ in an electric thermostatic drying oven for 12 h. Finally, the resultant suspension was filtered with deionized water washing for several times. The obtained powder was dried at 60 ℃ for 12 h.
1.2 Synthesis of wood carbon
Natural basswood blocks were cut perpendicularly in the growth direction to afford wood slices. Then the slices were pre-carbonized in air at 250 ℃ for 6 h, followed by carbonization in an argon flow at 1000 ℃ for 6 h. The carbonized slices were carefully polished with 2000 grit sandpaper to obtain a thickness of 700 μm and residual carbon was removed with ultrasonic washing by deionized water and ethanol for three times. To further reduce the weight and activate the wood carbon, the resultant wood carbon slices were activated in a carbon dioxide (CO2) flow at 750 ℃ for 1 h in a gas flow of 0.2 L/min.
1.3 Synthesis of Ti3C2Tx@WC
Ti3C2Twas loaded into the channelsvacuum- assisted infiltration. Briefly, 160 mg amount of prepared Ti3C2Tpowder was dispersed in 10 mL ethanolsonication for 30 min. The as-prepared wood carbon was placed on a filter funnel and then the obtained homogeneous ink was dripped into the wood carbon matrix along the microchannels after vacuum filtration using a turbo pump. The wood carbon filled with Ti3C2Twas treated by freeze-drying for 48 h. The mass ratio of Ti3C2Twith wood carbon was ~ 1 : 2.4.
1.4 Characterization
XRD test was conducted using a Rigaku Ultima IV Powder Diffractometer (Cu Kα radiation, sweeping speed: 5(°)/min). TEM analysis was performed on a JEM-2100F transmission electron microscope (200 kV, JEOL, Japan). SEM studies were carried out on a Mira3 scanning electron microscope (5 kV for morphology observation, Tescan, Czech).
1.5 Electrochemical measurements
All electrochemical measurements were performed using a traditional three electrode system with 1 mol/L H2SO4solution as electrolyte. Ti3C2T/wood carbon electrode, carbon rod, and Hg/Hg2SO4in saturated K2SO4aqueous solution were used as working electrode, counter electrode, and reference electrode, respectively. Cyclic voltammograms (CVs) and galvanostatic charge-discharge(GCD) analysis were performed on an electrochemical workstation (Biologic VMP3). The scan rates for CV analysis were 1, 2, 5, 10 and 20 mV/s. The scan range (-0.5 ~ 0 V) was determined by series of CV scans at 10 mV/s.
2 Results and discussion
2.1 Characterizations of Ti3C2Tx
Ti3C2Twas synthesized from bulk material Ti3AlC2hydrothermal method (27.5 mol NaOH, Ar atmosphere, 270 ℃). The raw material Ti3AlC2was shown in Fig. 1(a). The bulk Ti3AlC2material was made from Ti2AlC and TiC by a hot pressing sintering method[11].There were some impurities such as Al2O3(See XRD patterns of Ti3AlC2, Fig. 1(c)) during the synthesis process. After alkali treatment, sheet structure emerged in obtained Ti3C2T(Fig. 1(b)), which indicated the successful etching of Al atoms. It was also supported by X-ray diffraction (XRD) that the rising peak at 2≈7.5°corresponding to (002) plane of Ti3C2T. The morphology of NaOH-Ti3C2Twas different from accordion-like HF-Ti3C2Thigh concentration (50wt%) HF treatment but similar to low concentration (5wt%) HF treatment[11]. It is noted that the position of (002) peak in NaOH route is similar to HF etching route followed by sodium intercalation[12], which indicates that NaOH treatment has the same effect. Furthermore, we also performed Transmission electron microscopy (TEM) analysis to observe the morphology of Ti3C2T. Lamellar stripes can be observed in the edge of Ti3C2Tsheets and the interlayer space was ~1.2 nm. The space was larger than that of hydrofluoric acid (HF) method, which can be explained by sodium intercalation during the hydrothermal process.
2.2 Morphology of Wood carbon and Ti3C2Tx/ wood carbon
SEM was used to observe the structure of wood carbon. Fig. 2(a) showed the top-view of wood carbon, where multi big channels (30-60 μm) were surrounded by numerous small channels (5-10 μm). From the cross- sectional view (Fig. 2(b)), we can clearly observe that almost all channels were aligned straight from top to bottom. Furthermore, there were some mesopores (high magnification image in Fig. 2(b)) existed in channels, which were beneficial to diffusion of electrolyte. Next, Ti3C2Tink was madesonication in ethanol for 30 min and then dripped into the wood carbon with a vacuum filtration method. SEM images showed that multilayer Ti3C2Twas successfully injected (Fig. 2(c, d)). However, Ti3C2Twas not uniformly distributed in the channels of wood carbon. Some channels were filled with Ti3C2T, while some were empty, which may not take full use of wood structure and every single Ti3C2T.
2.3 Supercapacitor performance of Ti3C2Tx/ wood carbon
Since wood displayed anisotropic structure with vertical micro-channels serving as the high transport pathway of ion transport in the electrode, we thus tested the supercapacitor performance of Ti3C2T/wood carbon. The supercapacitor performance was evaluated using a three-electrode cell with 1 mol/L H2SO4aqueous solution serving as the electrolyte. To the best of our knowledge, Ti3C2Tis stable from –1.0–0 V. Hg/HgSO4while the wood carbon is stable from –0.5–0.5 V. Hg/HgSO4according to our experiments (Fig. 3(a)). Therefore, –0.5–0 V was chosen as this potential range which was suitable for both of them. Fig. 3(b) showed the CV curves of Ti3C2T/wood carbon. Different scanning rates ranging from 1 to 20 mV/s were applied. For wood carbon, the CV curves were nearly like rectangle shape which indicated fast charge transfer in the electrode and the wood carbon exhibited high areal capacity of 1030 mF/cm2at 2 mV/s. For Ti3C2T/wood carbon (mass loading: 17.9 mg/cm2), the area of CV curves were relatively larger than that of bare wood at the same potential range and the performance was 1983 mF/cm2at 2 mV/s, representing good values among Ti3C2Telectrodes reported before[10]. Even at 20 mV/s, the capacity of Ti3C2T/wood carbon electrode still maintained 1539 mF/cm2. GCD curves of Ti3C2T/wood carbon were showed in Fig. 3(c). The linear GCD curves with triangular shape suggested ideal capacitive behavior, while the repeated GCD curves at 10 mA/cm2for 104cycles indicated good stability (maintained ~92%) for Ti3C2T/wood carbon electrode (Fig. 3(d)).
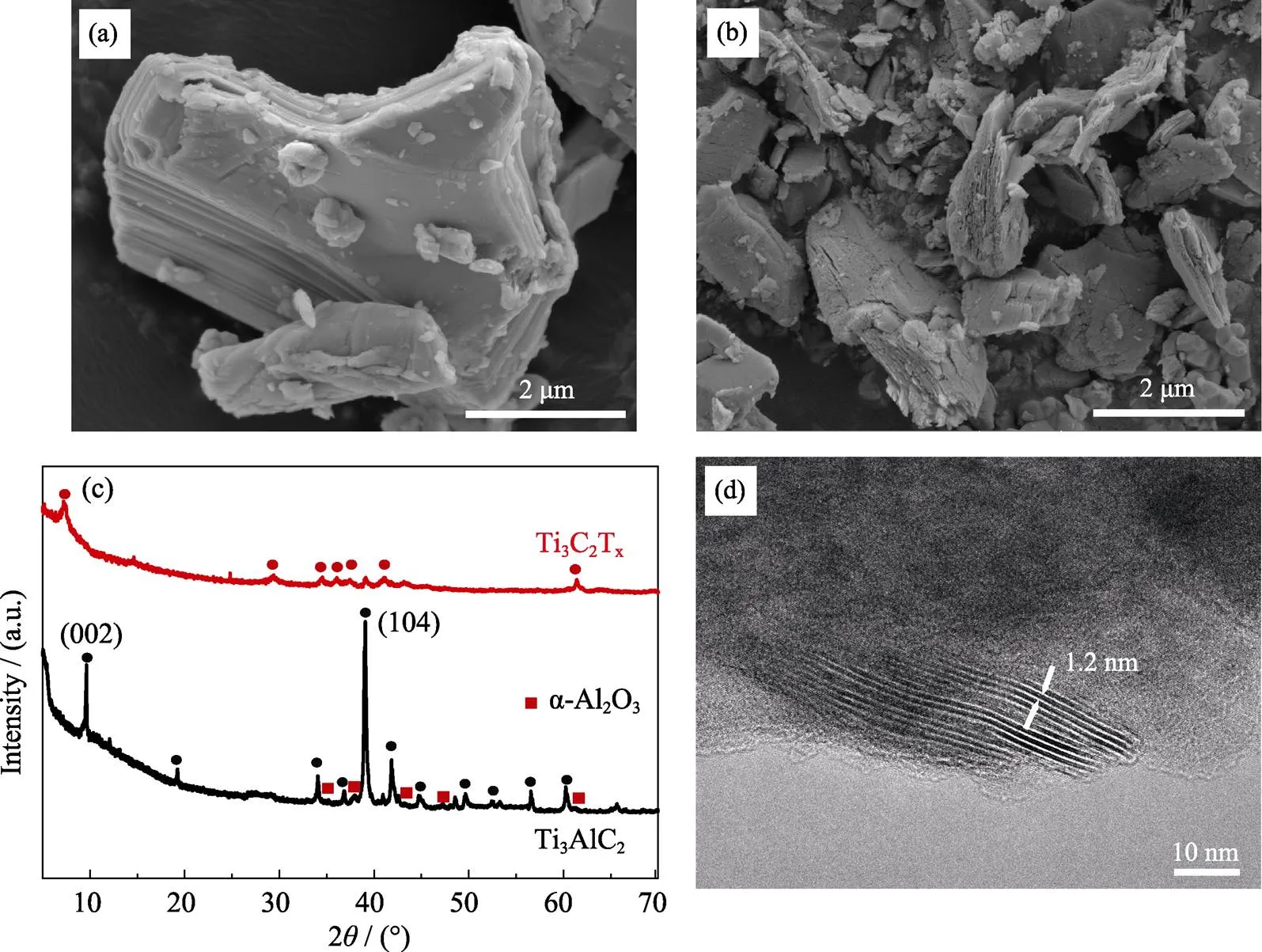
Fig. 1 Characterizations of Ti3C2Tx
(a) SEM image of raw material Ti3AlC2, (b) SEM image of Ti3AlC2NaOH etching, (c) XRD patterns of Ti3AlC2and Ti3C2T, (d) TEM image of NaOH-Ti3C2T
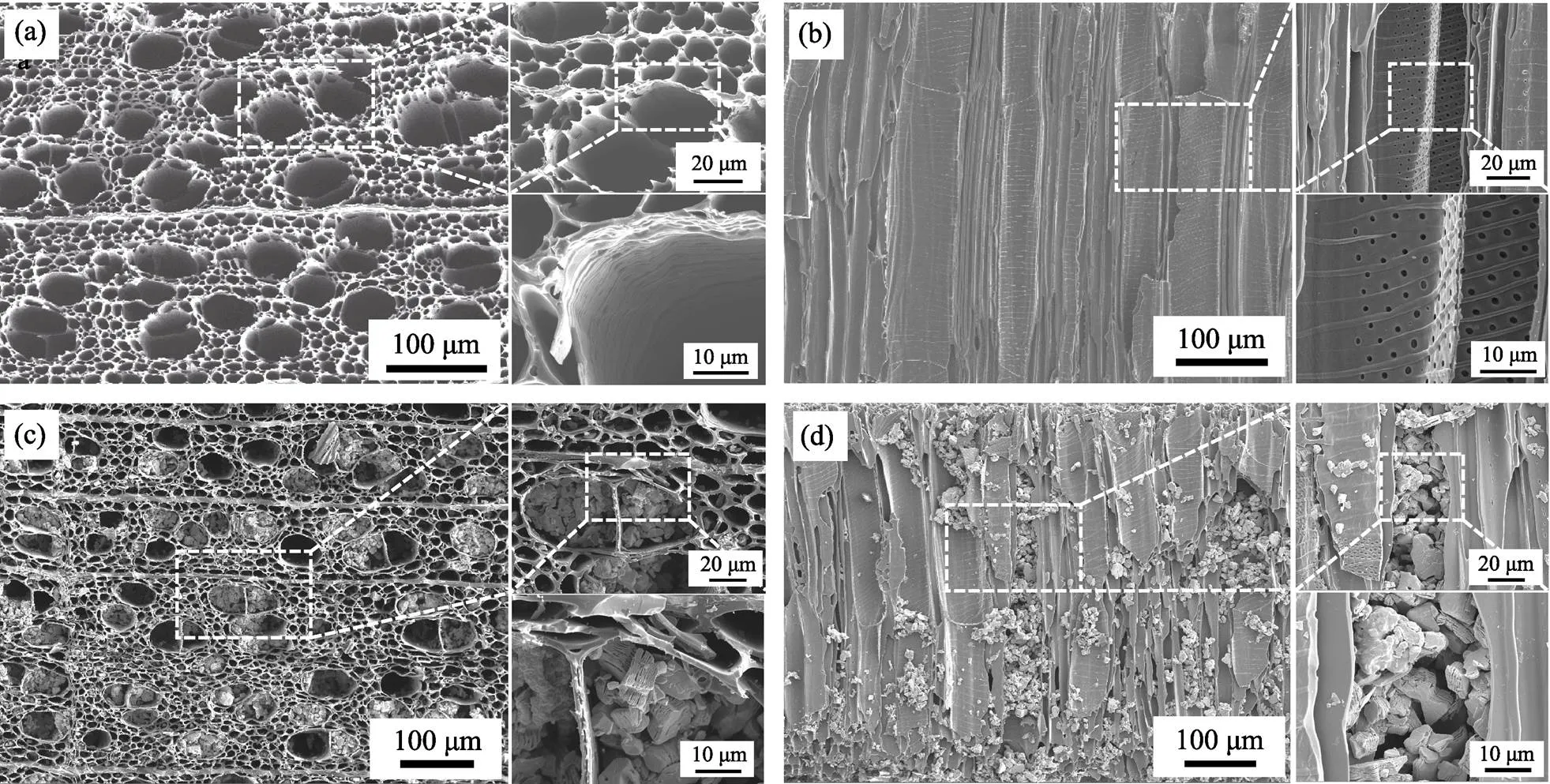
Fig. 2 SEM images of bare wood carbon ((a, b)) and Ti3C2Tx/wood carbon ((c, d))
(a, c) Top-view; (b, d) Cross-sectional view
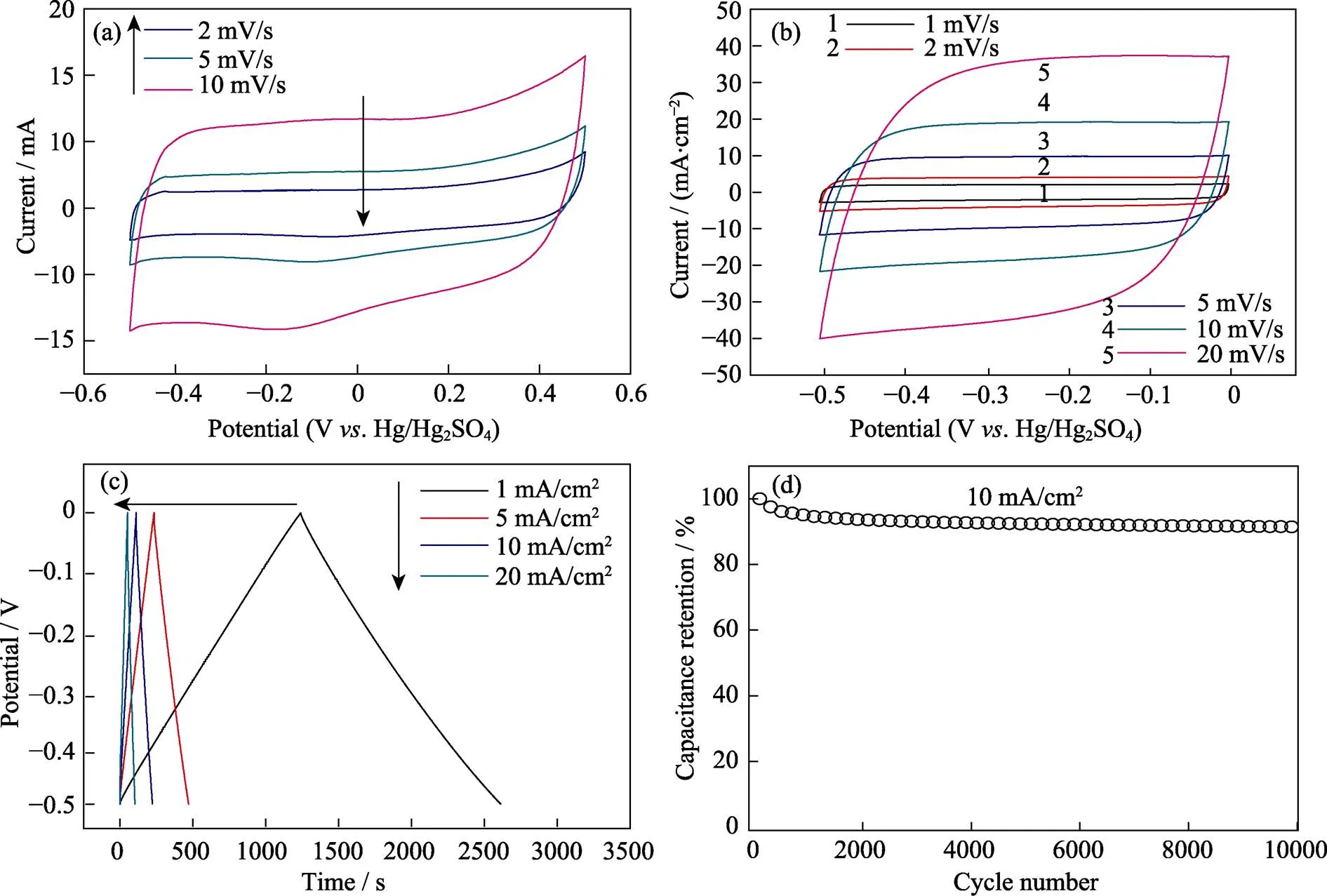
Fig. 3 CV curves of bare wood carbon (a) and Ti3C2Tx/wood carbon (b), GCD cycling profiles (c)of the Ti3C2Tx/wood carbon electrode collected at 1, 5, 10, and 20 mA/cm2, and capacitance retention test at 10 mA/cm2 (d)
3 Conclusions
In conclusion, we have demonstrated wood-based Ti3C2Telectrode a unique structure for supercapacitor to achieve high areal capacity. Open channels of wood benefit fast and efficient transportation of ions. When Ti3C2Twas combined with wood carbon, a high areal capacity (1983 mF/cm2at 2 mV/s) of with high mass loading (17.9 mg/cm2) was achieved. This work also inspires us to explore other natural structure, which may possess fascinating properties in combination with other materials.
[1] LUKATSKAYA M R, KOTA S, LIN Z,Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides., 2017, 2(8): 17105.
[2] NAGUIB M, COME J, DYATKIN B,MXene: a promising transition metal carbide anode for lithium-ion batteries., 2012, 16(1): 61-64.
[3] SHAHZAD F, ALHABEB M, HATTER C B,Electromagnetic interference shielding with 2D transition metal carbides (MXenes)., 2016, 353(6304): 1137-1140.
[4] NAGUIB M, KURTOGLU M, PRESSER V,Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2., 2011, 23(37): 4248-4253.
[5] GHIDIU M, LUKATSKAYA M R, ZHAO M Q,Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance., 2014, 516(7529): 78.
[6] CHEN C, ZHANG Y, LI Y,All-wood, low tortuosity, aqueous, biodegradable supercapacitors with ultra-high capacitance., 2017, 10(2): 538-545.
[7] LU L L, LU Y Y, XIAO Z J,Wood-inspired high- performance ultrathick bulk battery electrodes., 2018, 30(20): 1706745.
[8] JIANG J, ZHANG L, WANG X,Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes., 2013, 113: 481-489.
[9] SHEN F, LUO W, DAI J,Ultra-thick, low-tortuosity, and mesoporous wood carbon anode for high-performance sodium-ion batteries., 2016, 6(14): 1600377.
[10] LI T, YAO L, LIU Q,Fluorine-free synthesis of high-purity Ti3C2T(T=OH, O)alkali treatment., 2018, 57(21): 6115-6119.
[11] ALHABEB M, MALESKI K, ANASORI B,Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2TMXene)., 2017, 29(18): 7633-7644.
[12] LUKATSKAYA M R, MASHTALIR O, REN C E,Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide., 2013, 341(6153): 1502-1505.
碳化钛/椴木多孔碳复合材料用于超级电容器性能的研究
李腾飞, 黄璐君, 闫旭东, 刘庆雷, 顾佳俊
(上海交通大学 材料科学与工程学院, 金属基复合材料国家重点实验室, 上海 200240)
碳化钛作为一种新兴的层状二维材料具有一些独特的物理化学性质, 近年来引起了科研工作者广泛的注意。它是由化学选择性刻蚀的方法获得, 在电化学如锂电池, 超级电容器等领域展现出极好的应用前景。目前研究中碳化钛的电极往往活性物质负载量较低, 导致面容量不佳, 从而限制了其在大规模生产中的应用。本工作受自然界中椴木结构的启发, 利用其多孔道、孔道弯曲度低、导电性好、低价环保等特点, 将碳化钛与椴木活性炭复合, 获得了一种具有高面电容且稳定的超级电容器, 该电容器在2 mV/s的扫速下具有1983 mF/cm2的面容量, 同时活性材料负载量可以达到17.9 mg/cm2。本研究为后续利用自然界构型材料与功能材料的复合提供了一定的借鉴。
碳化钛; 椴木活性炭; 超级电容器; 面电容
TQ174
A
2019-05-31;
2019-09-10
National Natural Science Foundation of China (51672175, 51772187, 51572169, 51271116)
LI Teng-Fei (1993-), male, PhD candidate. E-mail: ltf1993@sjtu.edu.cn
李腾飞(1993-), 男, 博士研究生. E-mail: ltf1993@sjtu.edu.cn
GU Jia-Jun, professor. E-mail: gujiajun@sjtu.edu.cn
顾佳俊, 教授. E-mail: gujiajun@sjtu.edu.cn
1000-324X(2020)01-0126-05
10.15541/jim20190267

