高粱抗炭疽病资源筛选及病情与产量损失的关系
徐婧,姜钰,胡兰,刘可杰,徐秀德
高粱抗炭疽病资源筛选及病情与产量损失的关系
徐婧,姜钰,胡兰,刘可杰,徐秀德
(辽宁省农业科学院植物保护研究所,沈阳 110161)
【】针对高粱炭疽病()在生产上突发流行问题,以利用寄主抗病性控制病害为目的,在人工接种条件下筛选高粱抗炭疽病资源,明确病害严重度与产量损失关系,为高粱抗炭疽病育种和田间病害产量损失评估提供科学依据和指导。2016—2017年连续2年以高粱炭疽病菌()菌株SY-1为供试菌株,采用人工接种方法对74份国内外高粱种质资源抗高粱炭疽病特性进行测定和评价;利用已知对高粱炭疽病抗性不同的20份高粱资源作为供试材料,在人工接种条件下测定病害不同程度所造成的产量损失,并采用相关分析的方法分析高粱主要产量性状与炭疽病为害程度之间的关系。从供试的74份资源中,筛选出表现高度抗病(highly resistant,HR)资源21份,占鉴定资源总数的28.38%;抗病(resistant,R)资源12份,占鉴定资源总数的16.22%;中度抗病(moderately resistant,MR)资源26份,占鉴定资源总数的35.14%;感病(susceptible,S)资源8份,占鉴定资源总数的10.81%;高度感病(highly susceptible,HS)资源7份,占鉴定资源总数的9.46%。病害严重程度与产量损失关系研究结果表明,高粱炭疽病对高粱单穗粒重和千粒重影响显著,不同病情级别间的穗粒重及千粒重损失率差异显著;病害严重程度(1—9级)间的穗粒重损失率幅度为0.37%—78.00%,千粒重损失率为0.03%—44.43%。相关分析表明,高粱穗粒重及千粒重损失率与病害级别均呈指数相关,随着病害级别的升高,高粱穗粒重及千粒重损失率逐渐升高,且穗粒重损失率比千粒重损失率升高更快。病害严重度与症状表现关系密切,高度抗病(1级)资源的病株叶片上有不规则圆形小斑点,无分生孢子盘产生;抗病(3级)资源的病株叶片上有不规则圆形及椭圆形小型病斑,有零星分生孢子盘;中抗(5级)资源植株叶片上有大量不规则圆形、椭圆形斑点,有黑色分生孢子盘和孢子形成,穗枝梗亦有椭圆形斑点;感病(7级)资源植株叶片上有不规则圆形、椭圆形、长条形病斑,有大量黑色分生孢子盘和孢子形成,穗部枝梗病斑明显,部分叶片枯死,个别植株近于死亡。高度感病(9级)资源植株叶片上病斑连片,有大量黑色分生孢子盘和孢子,穗部枝梗病斑较多,大部分叶片枯死,植株近于死亡。筛选出21份高抗资源,明确了高粱炭疽病病情级别与高粱产量损失呈指数相关,随着病害级别的增高,高粱穗粒重及千粒重损失率相应升高。此外,高粱炭疽病不同病情级别相对应病害症状明显不同。
高粱;炭疽病;抗性鉴定;产量损失
0 引言
【研究意义】高粱((L.) Moench)是世界上重要的粮食作物之一,被用作饲料、酿制酒和醋的原料以及其他的工业原料。由亚线孢炭疽菌(P. Henn,曾用名为禾生炭疽菌(Ces.)G W Wilson)侵染引起的高粱炭疽病是世界上重要的高粱病害之一,在环境温暖湿润的热带和亚热带地区发生危害更为严重[1],可造成高粱产量损失达30%—100%[2]。【前人研究进展】历史上,中国高粱炭疽病一直被认为是次要病害,尽管在南方高粱种植区有该病发生,但未见有造成严重损失的报道[3]。近年来,该病害在中国高粱产区具有不同程度发生,尤其是四川、云南、贵州、甘肃等省区发生严重,局部地区感病品种几乎绝收,现已成为中国高粱生产上的主要病害[4]。该病菌可通过土壤、种子和气流传播[5-6],能侵染高粱叶片、茎秆和穗部枝梗等所有地上部分[7]。该病菌具有明显致病性分化现象[8-11],易发生致病性变异,一旦新的生理小种产生,将导致病害突发流行,采用药剂等防治措施难以奏效。生产实践证明,种植抗性品种是防治高粱炭疽病的既经济又有效的措施,而抗病资源筛选是成功选育抗病品种的关键。国外学者高度重视利用寄主抗性防治高粱炭疽病,开展了抗病基因资源挖掘和抗性品种选育,Buiate等[12]分别用12个炭疽病菌株对87份高粱资源和63个杂交种进行接种,发现有14.94%的资源和19.04%的杂交种表现抗病性,且病菌不同菌株间的致病性有很大差别;Prom等[13]于2005—2006年,对40份来自中国的高粱试材进行抗性鉴定,筛选出PI430471、PI563905、PI563924和PI563960等抗高粱炭疽病材料,同年对来自乌干达的高粱资源进行抗性鉴定,筛选出一些抗病资源[14];此后,Prom等[15]于2007和2008年又对来自埃塞俄比亚、马里、苏丹和乌干达的72份高粱资源进行抗炭疽病鉴定,筛选出26份抗性资源,研究认为广泛收集不同地区的高粱资源进行抗性鉴定将有助于筛选和获得新的抗病资源。诸多高粱抗炭疽病资源鉴定研究采用自然发病进行[16-19],一些以抗病性遗传和抗性基因标记为目的的研究多采用人工接种[20],但因所鉴定的试验材料、试验环境不同,及接种病菌小种和接菌量不同[21],资源抗感性评价结果不尽一致,育种者难以直接参考和利用。【本研究切入点】中国高粱炭疽病是高粱生产上新流行的病害,有关高粱炭疽病研究资料甚少,仅有利用自然发病条件对当地高粱资源的抗性进行观察的报道[22-23],由于缺乏适宜的高粱抗炭疽病资源筛选和评价方法,人工接种条件下进行高粱抗炭疽病鉴定筛选工作开展困难,育种上抗病资源缺乏,致使生产上应用的高粱品种抗性水平低,病害加重流行。【拟解决的关键问题】本研究以利用寄主抗病性控制病害为出发点,利用高粱抗炭疽病鉴定技术[24]进行高粱抗炭疽病资源鉴定和抗源筛选,使该技术得到进一步完善,筛选出抗病资源供育种者利用。同时研究明确病害严重度与产量损失关系,为高粱抗炭疽病育种和田间病害产量损失评估提供科学依据和技术支撑。
1 材料与方法
1.1 供试材料
1.1.1 种质资源 供鉴定的高粱种质资源74份,来源于国内外收集引进及辽宁省农业科学院植物保护研究所旱粮病害防控课题组在高粱抗病资源研究中新创自选品系。用已知高抗炭疽病材料L414B和高感材料NR10分别作为抗病和感病对照。上述高粱资源均经严格套袋自交、性状观察,获得纯种子备用。
1.1.2 菌种 供试高粱炭疽病菌()菌株SY-1,采自辽宁省沈阳市田间自然发病的高粱炭疽病病叶,经形态学、分子生物学鉴定和致病力测定获得的强致病力菌株,于辽宁省农业科学院植物保护研究所保存。
1.2 试验方法
1.2.1 接种物制备 将供试菌株转接到酪蛋白-乳糖水解液培养基(lactose casein hydrolysate medium)上,24℃光暗交替培养10—15 d,直至产生大量分生孢子。用无菌水将孢子从培养基上洗下,两层纱布过滤后,将孢悬液浓度调节为1×105个/ml,供接种用。
1.2.2 抗病性鉴定 试验于2016—2017年连续2年在辽宁省辽阳市试验地进行。鉴定材料采用随机排列,小区行长5 m,2行区,行距0.6 m,小区保苗50株以上。试验设年度间重复,田间管理按照常规高粱生产田进行。病害接种于高粱植株喇叭口末期,即喇叭口中可见旗叶尖端时期,用喷雾器将上述制备的孢子悬浮液喷洒于植株喇叭口中及周围叶片上,每株用量10 ml,接种后24 h进行人工辅助喷雾、保湿。接种40 d后目测调查并记载鉴定材料的发病情况,重点调查接种部位叶片,病情级别及其抗性评价标准见表1。

表1 高粱炭疽病鉴定评价标准[24]
HR:高抗;R:抗;MR:中抗;S:感;HS:高感。下同
HR: Highly resistant; R: Resistant; MR: Moderately resistant; S: Susceptible; HS: Highly susceptible. The same as below
1.2.3 发病程度与产量损失的关系 依据高粱种质资源抗炭疽病鉴定结果,选取抗性不同的资源共20份(每抗性级别4份)作为供试材料,在人工接种条件下测定病害造成产量损失。田间试材采用随机排列,小区行长8 m,4行区,行距0.6 m,定苗时株距0.16 m,小区保苗200株,试验重复3次。每小区一端3 m行长接种,另一端不接种作为对照。于高粱植株喇叭口末期,即在植株喇叭口中可见旗叶尖端时期,将上述方法(详见1.2.1)制备的孢子悬浮液进行全株喷洒接种,喷液量以雾滴不流淌为宜。接种时,为了防止接种物飞溅感染未接种区,将接种区植株用塑料布遮盖24 h保湿。接种后,田间管理按照常规进行,40 d后目测调查并记载发病情况,病情级别及其抗性评价标准见表1。
在高粱籽粒成熟期收获,每小区接种一端和不接种一端各按顺序收获大小较为均匀的高粱穗10穗,接种植株和未接种穗分别以病穗和健穗表示。将收获的穗带回室内充分晾晒后,分别测量并记录穗粒重及千粒重等指标。千粒重测定去除不成型的瘪粒和破损籽粒,仅测定有效籽粒。以单穗粒重损失作为产量损失,分析高粱炭疽病病情级别(严重度)与产量损失的关系。
1.2.4 数据分析 采用DPS 7.5软件,利用邓肯新复极差法分别在<0.05和<0.01水平对穗粒重损失率和千粒重损失率进行差异显著性分析;采用excel软件,对发病级别与粒重损失率的关系进行线性回归分析。
2 结果
2.1 田间抗性鉴定
通过田间接种鉴定试验结果(表2)可见,从供试的74份资源中,筛选出表现1级、高抗(HR)的有21份,占鉴定资源总数的28.38%;表现3级、抗病(R)的12份,占鉴定资源总数的16.22%;表现5级、中抗(MR)的资源26份,占鉴定资源总数的35.14%;表现7级、感病(S)的资源8份,占鉴定资源总数的10.81%;表现9级、高感(HS)的资源7份,占鉴定资源总数的9.46%。由鉴定结果可知,74份高粱资源2年的抗性表现基本一致,最终结果以抗性级别高的年份为准,表现1级抗性数量相对较多,是因为人为选择具有抗性的材料作为供试材料的缘故。试验所用的抗感对照资源L414B和NR10分别表现出其各自的抗病和感病特性,分别为1级和9级,说明试验接菌量、接种方法、评价技术以及环境条件适宜高粱炭疽病发生、资源抗性评价结果有效。
2.2 病害级别与产量损失的关系
2.2.1 产量损失测定 高粱收获后,对供试资源产量进行测量及分析结果表明,高粱炭疽病对单穗粒重和千粒重影响显著(表3),供试20份资源对高粱炭疽抗性表现明显不同,不同抗性级别间的穗粒重、千粒重损失率差异显著;病级(1—9级)间的穗粒重损失率幅度为0.37%—78.00%,而千粒重损失率为0.03%—44.43%。病级1级的4份资源接种区穗粒重降低幅度0.37%—1.13%,千粒重降低幅度0.03%—0.57%,且与对照差异不显著,其产量损失可以忽略不计。病级为3级的4份资源粒重降低幅度2.27%—4.4%,千粒重降低幅度1.39%—3.29%;5级的4份资源穗粒重降低幅度8.03%—13.02%,千粒重降低幅度2.69%—6.57%;7级的4份资源穗粒重降低幅度35.47%—44.57%,千粒重降低幅度12.09%—16.04%;9级的4份资源穗粒重降低幅度56.18%—78.00%,千粒重降低幅度25.86%—43.44%。
2.2.2 高粱主要产量性状与病情级别的关系 相关分析表明,高粱穗粒重损失率与高粱炭疽病发病级别(严重度)之间关系密切,随着病害级别的升高,高粱穗粒重损失率相应升高,且病害级别越高,穗粒重损失率越高(图1),呈指数相关(=0.6018e0.558x,2=0.9782)。高粱千粒重损失率与高粱炭疽病级别之间关系与穗粒重损失率与病害级别之间关系基本相同(图2),同样呈指数相关(=0.2314e0.5804x,2=0.96),随着病害级别的升高,千粒重损失率亦相应升高。但随着病害级别的升高,穗粒重损失率比千粒重损失率升高更快,可能发病严重程度不仅影响籽粒的灌浆充实,还影响穗粒数的多少。田间观察发现,感病(7级)和高感(9级)植株除了叶片上有大量病斑,大部分叶片近于枯死,而且小穗枝梗上病斑明显,有的小穗枝梗已经枯萎,明显影响养分输导和光合作用,这可能与高病情级别植株籽粒灌浆不充实,穗粒数降低较大有关。
2.2.3 病害症状与病情级别 2016—2017年连续2年对田间高粱炭疽病观察发现,接种区发病较为理想,未接种区不发病。接种区不同级别资源仍表现出原有抗性(图3),对照区未发病。高度抗病(1级)和抗病(3级)资源的接种行(3 m)末端未见向外扩展,高抗(1级)资源病株叶片上有不规则圆形及椭圆形小斑点,无分生孢子盘产生,抗病(3级)资源的病株叶片上有不规则圆形及椭圆形小型病斑,有零星分生孢子盘,个别资源穗枝梗偶见小红褐色斑点。中抗(5级)资源种植行末端的病害已向外蔓延约1 m左右,病害症状为植株叶片上有大量不规则圆形、椭圆形斑点,有黑色分生孢子盘和孢子形成,穗枝梗亦有椭圆形斑点。感病(7级)和高感(9级)的接种行末端病害向外扩展明显(2—3 m),且发病严重,感病(7级)资源植株叶片上有不规则圆形、椭圆形、长条形病斑,有大量黑色分生孢子盘和孢子形成,部分叶片枯死,个别植株近于死亡,穗部枝梗病斑明显,已明显影响光合和养分输导。高度感病(9级)资源植株叶片上病斑连片,有大量黑色分生孢子盘和孢子,穗部枝梗病斑较多,大部分叶片枯死,植株近于死亡。

表2 高粱资源对炭疽病抗性鉴定结果
*:自行创新选育材料 *: Selection of materials for self-innovation

表3 高粱炭疽病对高粱主要产量性状的影响
大小写字母分别表示在5%和1%水平差异显著 Capital letters and lower-case letters are differences significant at 5% and 1% level, respectively
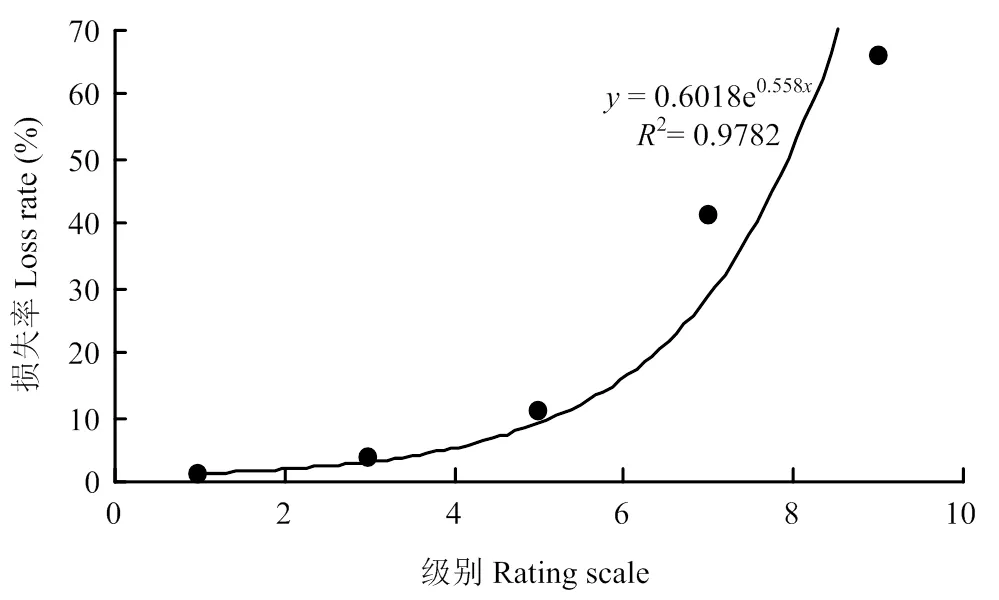
图1 高粱穗粒重损失率与病害级别的关系
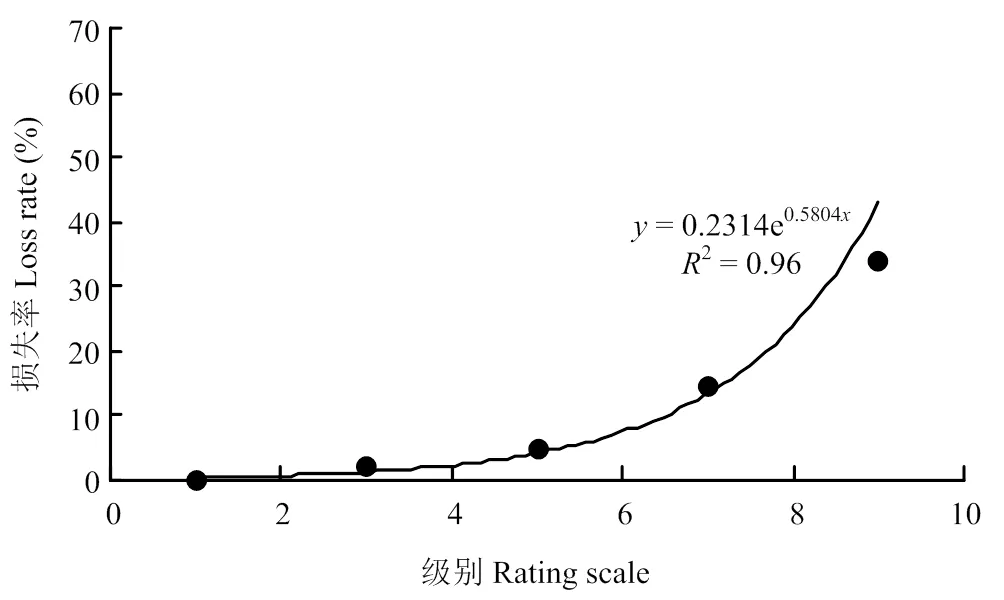
图2 高粱千粒重损失率与病害级别的关系

A:1级;B:3级;C:5级;D:7级;E:9级 A: 1 scale; B: 3 scale; C: 5 scale; D: 7 scale; E: 9 scale
3 讨论
高粱炭疽病是世界性的重要高粱病害,国内外研究表明,高粱炭疽病具有突发性和爆发性,一旦发生蔓延迅速,对高粱危害极大,种植抗性品种是防治该病害最有效的措施。世界上许多国家针对该病害开展高粱基因资源挖掘、抗病性遗传和抗性基因标记研究[25-26],取得诸多重要研究结果。Cuevas等[27]对来自津巴布韦的68份高粱资源进行表型研究及抗性鉴定,发现供试资源的表型特征相差很大,且筛选出25份对高粱炭疽病表现抗性的资源,之后又对80份来自布基纳法索(Burkina Faso)和南非(South Africa)的高粱资源进行抗炭疽病鉴定,筛选出12份抗炭疽病材料,其中10份来自BFA,可见来自BFA的高粱抗源丰富[28],这些结果的取得得益于抗源筛选和评价技术研究等基础,相比之下,中国在此方面研究资料较少。本研究以利用寄主抗病性控制病害为目的,在人工接种条件下进行高粱抗炭疽病资源鉴定,并提供育种者利用,克服了目前育种上利用病害自然发生进行资源抗性评价易受环境影响,结果不准确的弊端,解决了育种上抗性资源缺乏和针对性不强的问题,这对于高粱抗病育种及病害深入研究至关重要。
有关病害所致产量损失研究报道较少,Ali等[29]选用4个高粱炭疽病抗感性不同品种,在人工接菌条件研究了病害与产量损失关系,认为高度感病品种产量损失可达30%。Thomas等[30]报道,采用同一试验区种植2个抗感性不同品种,在自然发病条件下,以药剂控制区作为对照测定了病害所致的产量损失,研究认为病害所致产量损失可达67%;Cota等[2]采用在病害严重流行区和轻病区种植相同品种进行病害调查和产量损失测定,结果表明,病害所致产量损失高达86%。不同学者所得出的结果不同,结论差别较大,这可能与供试病菌的致病力、环境条件及试验方法不同有关,尽管如此,这些结果很有价值。本文总结前人研究方法的优点,在人工接种条件下,明确了高粱炭疽病病情级别(1—9级)各级别相对应病害所致产量损失,为病害预警及高粱田间产量损失评估提供了科学依据。然而,高粱炭疽病发生严重程度和造成的损失受多种因素影响[31],由于本研究供试材料数量有限,获得结果有一定局限性,病害级别与穗粒重变化关系尚需多年资料积累逐步完善。
4 结论
筛选出高度抗病资源21份。明确了高粱穗粒重和千粒重损失率与病害级别均成指数相关,病害级别越高,穗粒重和千粒重损失率越大。此外,高粱炭疽病不同病情级别相对应的病害症状明显不同。
致谢:本研究承蒙国家谷子、高粱产业技术体系专家提供部分试验资源,在此谨表谢意。
[1] Chala A, Alemu T, Prom L K, Tronsmo A M. Effect of host genotypes and weather variables on the severity and temporal dynamics of sorghum anthracnose in Ethiopia., 2010. 9(1): 39-41.
[2] Cota L V, Souza A G C, Costa R V, Silva D D, Lanza F E, Aguiar F M, Figueiredo J E F. Quantification of yield losses caused by leaf anthracnose on sorghum in Brazil., 2017: 1-7.
[3] 白金铠. 杂粮作物病害. 北京: 中国农业出版社, 1997: 219-222.
BAI J K.. Beijing: China Agricultural Press, 1997: 219-222. (in Chinese)
[4] 徐秀德, 刘志恒. 高粱病虫害原色图鉴. 北京: 中国农业科学技术出版社, 2012: 112-118.
Xu X D, Liu Z H.. Beijing: China Agricultural Science and Technology Press, 2012: 112-118. (in Chinese)
[5] GwaryD M, MailafiyaD M,JibrinT J. Survival ofand other seed-borne fungi in sorghum seeds after twenty months of storage., 2006, 8(5): 676-679.
[6] Cardwell K F, Hepperly P R, Frederiksen R A. Pathotypes ofand seed transmission of sorghum anthracnose., 1989, 73: 255-257.
[7] Frederiksen R A. Anthracnose stalk rot//Frederiksen A(Ed.).. St. Paul, MN: APS Press, 1986: 27.
[8] Casela C R,Fredriksen R A. Pathogenic variability in monoconidial isolates of the sorghum anthracnose fungusfrom single lesions and from monoconidial cultures., 1994, 19: 149-153.
[9] Marley P S, Thakur R P, Ajayi O. Variation among foliar isolates ofof sorghum in nigeria., 2001, 69(2): 133-142.
[10] Prom LK, Perumal R, Erattaimuthu S R, Little C R, No E G,Erpelding J E, Rooney W L, Odvody G N, MagillC W. Genetic diversity and pathotype determination ofisolates causing anthracnose in sorghum., 2012, 133(3): 671-685.
[11] Costa R V, Zambolim L, Cota L V, Silva D D, ParreiraD F, Lanza F E, Souza A G C. Pathotypes ofin response to sorghum populations with different levels of genetic diversity in sete lagoas-mg., 2015, 163(7/8): 543-553.
[12] Buiate E A S, Souza E A D, Vaillancourt L, Resende I, Klink U P. Evaluation of resistance in sorghum genotypes to the causal agent of anthracnose., 2010, 10(10): 166-172.
[13] Prom L K, Erpelding J E, Garcia N M. Chinese sorghum germplasm evaluated for resistance downy mildew and anthracnose., 2007, 2(1): 26-31.
[14] Prom L K, Isakeit T, Perumal R, Erpelding J E, Rooney W, Magill C W. Evaluation of the Ugandan sorghum accessions for grain mold and anthracnose resistance., 2011, 30(5): 566-571.
[15] Prom L K, Erpelding J, Perumal R, Isakeit T, Cuevas H. Response of sorghum accessions from four African countries against, causal agent of sorghum anthracnose., 2012, 3(1): 125-129.
[16] Erpelding J E. Field assessment of anthracnose disease response for the sorghum germplasm collection from the Mopti Region., 2010, 5(3): 363-369.
[17] Derese S A, Shimelis H, Mwadzingeni L, Laing M. Agro-morphological characterisation and selection of sorghum landraces., 2018: 1-11.
[18] Mofokeng M A, Shimelis H, Laing M, Shargie N. Sorghum [(L.) Moench] breeding for resistance to leaf and stalk anthracnose,, and improved yield: progress and prospects., 2017, 11(9): 1078-1085.
[19] Mofokeng M A, Shimelis H, Laing M, Shargie N G. Genetic variability, heritability and genetic gain for quantitative traits in South African sorghum genotypes., 2019, 13(1): 1-10.
[20] Biruma M, Martin T, Fridborg I, Okori P, Dixelius C. Two loci in sorghum with NB-LRR encoding genes confer resistance to., 2012, 124(6): 1005-1015.
[21] Rosewich U L, Pettway R E, McDonald B A, Duncan R R, Frederiksen R A. Genetic structures and temporal dynamics ofpopulation in sorghum disease nursery., 1998, 88: 1087-1093.
[22] 张长伟, 潘学贤, 汪远宏, 程开禄, 黄富, 曾富言. 高粱炭疽病的严重度和品种抗性分级标准. 云南农业大学学报, 1998, 13(1): 37-42.
Zhang C W, Pan X X, Wang Y H, Cheng K L, Huang F, Zeng F Y. A proposal standard for the classification of sorghum anthracnose rating index and resistant index., 1998, 13(1):37-42. (in Chinese)
[23] 邓小锋, 彭秋, 刘天友, 李青风. 贵州地方高粱资源炭疽病害田间抗性评估. 西南农业学报, 2017, 30(5): 1074-1077.
Deng X F, Peng Q, Liu T Y, Li Q F. Resistance to anthracnose of local sorghum varieties in Guizhou., 2017, 30(5): 1074-1077. (in Chinese)
[24] 徐婧, 刘可杰, 胡兰, 姜钰, 王岩, 张明会, 尤广兰, 徐秀德, 王艳红, 黄欣阳. 高粱抗炭疽病鉴定技术规程DB 21/T 2807-2017. 辽宁省质量技术监督局. 2017.
XU J, LIU K J, HU L, JIANG Y, WANG Y, ZHANG M H, YOU G L, XU X D, WANG Y H, HUANG X Y. Rule for evaluation of sorghum resistance to anthracnose. Liaoning Quality and Technical Supervision Bureau, 2017. (in Chinese)
[25] Ramasamy P, Menz M A, Mehta P J, Katil S, Gutierrez- Rojas L A, Klein R R, Klein P E, Prom L K, Schlueter J A, Rooney W L, Magill C W. Molecular mapping of Cg1, a gene for resistance to anthracnose () in sorghum., 2009, 165: 597-606.
[26] BURRELL A M, Sharma A, Patil N Y, Collins S D, Anderson W F, Rooney W, Klein P. Sequencing of an anthracnose-resistant sorghum genotype and mapping of a major QTL reveal strong candidate genes for anthracnose resistance., 2015, 55(2): 790.
[27] Cuevas H E, Prom L K, Erpelding J E, Brotons V. Assessments of genetic diversity and anthracnose disease response among Zimbabwe sorghum germplasm., 2014, 133(2): 234-242.
[28] Cuevas H E, Prom L K, Isakeit T, Radwan G. Assessment of sorghum germplasm from Burkina Faso and South Africa to identify new sources of resistance to grain mold and anthracnose., 2016, 79: 43-50.
[29] ALI M E K, Warren H L, Lantin R X. Relationship between Anthracnose leaf blight and losses in grain yield of sorghum., 1987, 71: 803-806.
[30] Thomas M D, Sissoko I S, Scko M. Development of leaf anthracnose and its effect on yield and grain weight of sorghum in West Africa., 1996, 80: 151-153.
[31] Chala A, Brur M B, Tronsmo A M. Incidence and severity of sorghum anthracnose in Ethiopia., 2010, 9(1): 23-30.
Evaluation of sorghum accessions resistance againstand Relationship between severity and yield loss on Sorghum
XU Jing, JIANG Yu, HU Lan, LIU KeJie, Xu XiuDe
(Institute of Plant Protection, Liaoning Academy of Agricultural Sciences, Shenyang 110161)
Base on the new problems of sorghum anthracnose () prevalent recent years in sorghum production, screening sorghum disease-resistant resources under artificial inoculation conditions was carried out with the purpose of use host resistance to control the disease. The results of this study were expected to provide scientific basis and guidance for sorghum anthracnose resistance breeding and yield loss assessment in the field.During the year of 2016 and 2017, 74 sorghum accessions from home and abroad were identified for disease resistance by artificial inoculation with pathogen isolate of(SY-1). Twenty accessions with different rating scale were used as test materials to determine yield losses caused by anthracnose diseases under artificial inoculation. The relationship between rating scale (severity) of disease and the main yield traits of sorghum was clarified by using the method of correlation analysis.The results showed that there were 21 accessions rated as highly resistant (HR) and accounted for 28.38% of the total of 74 sorghum accessions, 12 accessions rated as resistant (R) and accounted for 16.22%, 26 accessions rated as moderate resistant (MR) and accounted for 35.14%, 8 accessions rated as susceptibility (S) and accounted for 10.81%, and 7 accessions rated as high susceptibility (HS) and accounted for 9.46% of the total accessions. The relationship between the severity of disease and yield loss was clarified. The results showed that 1000-grain weight and grain weight per panicle of sorghum are significantly affected by sorghum anthracnose. The reduction of grain weight per panicle between 1- 9 grade diseased plants was 0.37%-78.00%, and the reduction of 1000-grain weight was 0.03%-44.43%. Correlation analysis showed that both of grain weight per panicle and 1000-grain weight had an exponential relationship with the rating scale of disease. The reduction rate of grain weight per panicle and 1000-grain weight of sorghum rise significantly with the increase of rating scale of disease, and the rise of reduction rate of grain weight per panicle is more rapid than that of 1000-grain weight. There was a close relationship between the severity and symptoms of disease. Accessions rated as HR showed symptoms of red spots on inoculated leaves, but no acervuli in the lesions and R accessions showed small circular to oval spots and acervuli development. MR accessions showed symptoms of abundant circular to oval spots on inoculated leaves and black acervuli development and some lesions also appear on panicle-stalk. S accessions showed circular to oval and long strip spots on inoculated leaves with abundant black acervuli development. Lesions on panicle-stalk were visible and part of leaves wilt and some diseased plants were died. On the HS accessions lesions join to cover a large proportion of the leaf and panicle-stalk surface with abundant black acervuli. Most leaves of diseased plants wilt and the whole plants were near death.In this study, 21 accessions with highly resistant (HR) were screened. Both of grain weight per panicle and 1000-grain weight had an exponential relationship with the rating scale of the disease, and with the increase of rating scale, the loss rate of grain weight per panicle and 1000-grain weight of sorghum were increased correspondingly. In addition, the disease symptoms of sorghum anthracnose were obviously different with disease rating scale.
sorghum;; evaluation of resistance; yield loss
10.3864/j.issn.0578-1752.2019.22.012

2019-06-12;
2019-09-20
现代农业产业技术体系建设专项资金(CARS-06)、辽宁省博士启动基金(20180540112)
徐婧,Tel:13478839929;E-mail:mljasmine2004@163.com。通信作者姜钰,Tel:15804050906;E-mail:jiangyumiss@163.com
(责任编辑 李莉)

