锌卟啉自组装在染料敏化太阳能电池中的应用
贾海浪 彭智杰 李珊珊 龚炳泉 关明云
(江苏理工学院化学与环境工程学院,常州 213001)
0 Introduction
In recent years,the rapid development of economy has also brought about serious environmental pollution and energy shortage,and people show more and more interest in the development and utilization of new energy.New energy has incomparable advantages compared with traditional energy,such as abundant sources,easy development,environmental friendliness,recycling and so on.Among them,solar energy has great application potential,it has become one of the important areas of new energy[1-2].Many researchers are devoted to the research of DSSCs(dye-sensitized solar cells),the DSSCs have many advantages compared with traditional energy,such as low cost,simple fabrication process,wide source of raw materials,multi-color transparency,high photoelectric conversion efficiency and environmental friendliness[3-5].After more than 20 years of development,DSSCs have made great progress,up to now,the device of ADEKA-1 exhibited the highest PCE of 14.3%[6].
Dyes play an important role in DSSCs,due to TiO2is a wide band material,it can only absorb ultraviolet rays and the utilization of sunlight is very low,on the contrary,dyes can broaden the absorption of DSSCs to visible and even near infrared regions[7-8].So far,many research groups have designed and synthesized many new dyes.Generally speaking,ideal dyes need to meet the following conditions:(1)dyes can absorb sunlight in visible and even infrared regions,(2)dyes contain carboxyl(-COOH),sulfonic acid(-SO3H),phosphoric acid(-PO3H2),pyridine and other anchoring groups,(3)high quantum efficiency,(4)the excited state energy level of dyes should match theconduction level of TiO2,(5)dyesshould have high oxidation potential and high regeneration efficiency,(6)dyes have good stability and can be recycled 105times[9-12].So far,there are two kinds of efficient dyes for DSSCs,metal complexes and metal-free organic dyes[13-16].Many excellent dyes have been prepared,the PCE of some of these dyes has exceeded 10%[17-20].In 2005,ruthenium complex dye N719 was prepared,the PCE of N719 achieved 11.18%[21].In 2010,Wang prepared ruthenium complex C106,the value of PCE was up to 11.7%[22].In addition,many metalloporphyrin dyes have excellent photoelectric properties,Grätzel and co-workers reported an excellent porphyrin dye YD2-o-C8,the PCE of the device reached 12.3%[23-27].In 2014,Grätzel and co-workers synthesized a more efficient dye SM315,under the condition of using cobalt-based redox electrolyte,the PCE of the device was up to 13%[28].Organic dyes have also developed rapidly,these dyes have good application prospects,which has obvious advantages compared with metal complexes,such as easy modification,low price,good plasticity and easy degradation[29-32].Some excellent dyes have been successfully prepared,such as C275,DTS-CA,HL7[33-35].
Nevertheless,this is still far from the theoretical efficiency(33%),how to achieve higher PCE on the basis of the previous is a major challenge for DSSCs.Many dyes require complex synthetic steps,which have been troubling researchers,in addition,the monolayer structure of dye molecules always restricts the development of DSSCs,thus,we need to develop some new structures and methods.Construction of multilayer dyes by supramolecular self-assembly strategy may be a promising way to improve the performance of DSSCs.The method is easy to operate,by adjusting the antenna molecules and anchoring groups,the light-harvesting ability of dyes can be improved and charge recombination can be reduced,and complex synthesis is not required[36-38].What′s more,the amount of dye adsorbed will not decrease,this may change the limitation of monolayer dyes of DSSCs.Saha and co-workers used ZnPc and PyPMI as antenna molecules and anchoring groups,respectively,after supramolecular self-assembly,the PCE of PyPMIZnPc was 3 times than that of device of PyPMI[39].
Hererin,we prepared two antenna molecules zinc porphyrin P2 and P3.The zinc porphyrin was choosed as antenna molecule due to its excellent photoelectric properties,which has good absorption in a range of 400~450 nm(Soret band)and 500~700 nm(Q band),this will be very helpful to improve the lightharvesting ability of devices.4-Pyrid-4-ylbenzoic acid(A)was used as the anchoring group,which can coordinate with zinc porphyrin,after supramolecular self-assembly,the DSSCs based on A-P show obvious photovoltaic performance. The device of A-P2 exhibited the PCE of 1.68%,and the Vocis 526 mV,the Jscis 5.39 mA·cm-2,which is better than that of A-P3.We also studied the optical properties,electrochemical properties,photovoltaic performance to analyze the differences between them.
1 Experimental
1.1 Synthesis
The structures of P2,P3 were shown in Fig.1,and the synthesis method of the two antenna molecules was shown in Fig.2.All solvents were treated by standard methods before use and all chemicals were purchased from commercial suppliers and used without further purification unless indicated otherwise.The1H NMR were recorded on a Bruker DRX NMR spectrometer with tetramethylsilane(TMS)as the internal standard.

Fig.1 Self-assembly of A(4-pyrid-4-ylbenzoic acid)with P2 or P3

Fig.2 Synthesis procedure of P2 and P3
1.1.1 Synthesis of compound 2
The compound 1 (2.00 g,2.05 mmol)was were dissolved in DCM (500 mL),then the temperature is cooled to 0℃,and the NBS(0.75 g,4.20 mmol,in 30 mL DCM)was dropped slowly.After 6 h,the mixture was quenched with acetone,and evaporated in vacuo.Then the residue was dissolved in DCM(200 mL)and MeOH(100 mL),the Zn(OAc)2·2H2O(2.25 g,10.25 mmol)was added,the mixture was stirred at room temperature for 2 h.The mixture was washed with brine,dried over MgSO4,and evaporated in vacuo.The residue was purified by silica gel column chromatography (VPE∶VEA=10∶1)to give compound 2(1.83 g,75%).1H NMR (CDCl3,500 MHz):δH9.66~9.67(m,4H),8.92~8.95(m,4H),7.72(t,J=8.5 Hz,2H),7.02 (d,J=8.5 Hz,4H),3.86(t,J=6.0 Hz,8H),0.96~0.99(m,8H),0.80~0.84(m,8H).0.38~0.63(m,44H).
1.1.2 Synthesis of P2
A mixture of compound 2 (1.00 g,0.84 mmol),triisopropylacetylene(0.46 g,2.51 mmol),CuI(32 mg,0.17 mmol)in THF(60 mL)and Et3N(10 mL).Then the Pd(PPh3)2Cl2(0.20 g)was added under N2,the mixture was heated under 80℃for overnight.The reaction mixture was cooled to room temperature and evaporated in vacuo.The residue was purified by silica gel column chromatography(VDCM∶VPE=1∶4)to give P2(0.71 g,61%).1H NMR(CDCl3,500 MHz):δH9.66(d,J=4.5Hz,4H),8.87(d,J=4.5 Hz,4H),7.69(t,J=8.5 Hz,2H),7.00 (d,J=6.0Hz 4H),3.85 (t,J=6.0 Hz,8H),1.45~1.51(m,42H),0.92~0.98(m,8H),0.80~0.84(m,8H).0.39~0.63(m,44H).
1.1.3 Synthesis of P3
Under nitrogen,compound 2(100 mg,84μmol),compound 3(110 mg,250μmol),K2CO3(46 mg,334 μmol)and Pd(PPh3)4(30 mg)were dissolved in 1,4-dioxane (30 mL)and H2O (5 mL).The mixture was heated under 90℃for overnight.The reaction mixture was cooled to room temperature and extracted by DCM (3×30 mL).The combined organic layers were washed with brine,dried over MgSO4,and evaporated in vacuo.The residue was purified by silica gel column chromatography(VDCM∶VPE=1∶4)to give P3(101 mg,73%).1H NMR (CDCl3,400 MHz):δH8.76~8.83(m,8H),7.93~7.97(m,4H),7.64~7.68(m,2H),7.22~7.28(m,3H),7.16(d,J=8.4 Hz,2H),6.96~7.04(m,9H),4.08(t,J=7.2 Hz,4H),3.80(t,J=6.4 Hz,8H),2.01~2.08(m,4H),1.55~1.63(m,4H),1.26~1.47(m,16H),0.83~0.97(m,24H),0.68~0.73(m,8H),0.45~0.59(m,34H).
1.2 Fabrication of DSSCs
The working electrode (active area is 0.196 cm2)was prepared by screen printing the TiO2paste on Fluorine-doped tin oxide(FTO)glass plates(15Ω·m-2).For preparation of a DSSC,FTO glass plates were cleaned in a detergent solution using an ultrasonic bath for 30 min for two times and then rinsed with water and ethanol.Then,the plates were immersed into 40 mmol·L-1TiCl4(aqueous)at 70℃for 30 min and washed with water and ethanol.The TiO2paste consisted of 12μm thick film (particle size,20 nm,pore size 32 nm).The TiO2films were performed with a programmed procedure:(1)80℃for 15 min;(2)135℃for 10 min;(3)325℃for 30 min;(4)375℃for 5 min;(5)450℃for 15 min,and (6)500℃for 15 min.Then the films were treated again with TiCl4at 70℃for 30 min and sintered at 500℃for 30 min.Then the electrode was immersed into 2 mmol·L-1A solution (methanol)for 2 h at room temperature,then rinsed with ethanol,and then was immersed into 1 mmol·L-1P solution (VTHF∶VEtOH=4∶1)for 18 h to form supramolecules on the TiO2surface and dried in air.The working electrode and the Pt counter electrode were then sealed with a Surlyn film (25μm)by heating the sandwich-type cell at 110℃.The electrolyte was introduced through pre-drilled holes in the counter electrode and was driven into the cell via vacuum backfilling,and the hole was sealed with a Surlyn film and a thin glass(0.1 mm thickness)cover by heating.The electrolyte was composed of 0.6 mol·L-11-butyl-3-methylimidazolium iodide (BMII),50 mmol·L-1I2,50 mmol·L-1LiI,0.5 mol·L-1tertbutylpyridine and 0.1 mol·L-1guanidiniumthiocyanate(GuNCS)in acetonitrile.
1.3 Characterizations of DSSCs
The photocurrent-voltage (I-V)curves of the DSSCs were measured on a Keithley 2400 source meter under standard global AM 1.5G solar irradiation supplied by a xenon light source(Oriel).The incident photo-to-electron conversion efficiency(IPCE)spectra of the DSSCs were measured by a DC method.The light source was a 300 W xenon lamp (Oriel 6258)coupled with a flux controller to improve the stability of the irradiance.The single wavelength was selected by a monochromator (Cornerstone 260 Oriel74125).Light intensity was measured by a NREL traceable Si detector(Oriel 71030NS)and the short circuit currents of the DSSCs were measured by an optical power meter(Oriel 70310).
1.4 UV-Vis spectroscopy,electrochemical properties and amounts of dye loading
The UV-Vis absorption spectra were recorded on a Shimadzu UV-3600 spectrometer.The cyclic voltammograms(CV)of the dyes and electrochemical impedance spectroscopy (EIS)were studied using a Chenhua CHI760Emodel Electrochemical Workstation(Shanghai).The amounts of dye loading were estimated according to the following methods:the sensitized electrodes were immersed into a 0.1 mol·L-1NaOH solution in a mixed solvent (VH2O∶VTHF=1∶4),which resulted in desorption of each dye.The amounts of dye loading can be estimated according to the following formula:C=AV/(εS),where C stands for the amounts of dye loading,A is optical absorbance of the dye,V is volume of desorption solution,εis molar extinction coefficients,S iseffectivearea of TiO2films.
2 Results and discussion
2.1 Optical properties
As shown in Fig.3a,we measured the absorption spectra of P2 and P3 in DCM.It is obvious that all the antenna molecules show standard porphyrin absorption peaks,they have characteristic absorption in the range of 400~450 nm (Soret band)and 500~700 nm(Q band),the band of 400~700 nm can be attributed to electronic transitions fromπ-π*and intramolecular charge transfer(ICT).The antenna molecule porphyrin P2 displays high absorption coefficient(2.26×105L·mol-1·cm-1)at 438 nm in Soret band,and medium absorption coefficient at 580 nm (8.20×103L·mol-1·cm-1)and 631 nm(2.20×104L·mol-1·cm-1),respectively.From this,we can see that antenna molecule P2 has good spectral response in visible region,this in favor of enhancing the light-harvesting capability of dyes,thereby increasing Jsc(short-circuit current density)of DSSCs.When the Rgroup isreplaced by phenothiazine,the absorption spectrum of P3 shows a slight hypsochromic-shift compared with P2,this may be due to the non-planar steric effect between porphyrin ringand phenothiazine.Theantennamoleculeporphyrin P3 displays high molar extinction coefficient(2.39×105L·mol-1·cm-1)at 436 nm,with medium absorption coefficient at 567 nm(1.16×104L·mol-1·cm-1)and 614 nm(1.12×104L·mol-1·cm-1),respectively.
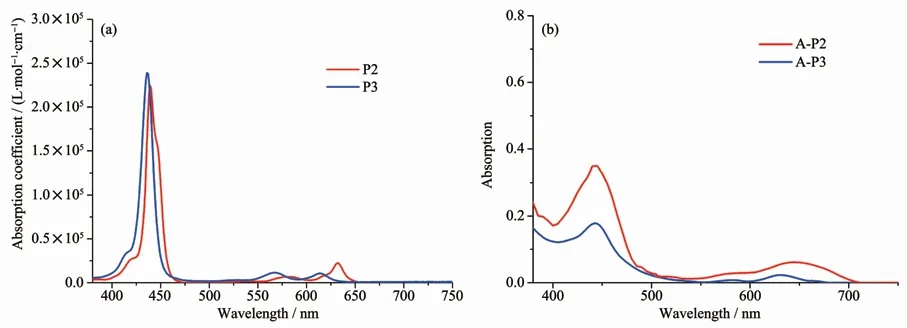
Fig.3 (a)UV-Vis absorption spectra of P1,P2 and P3 in DCM,(b)UV-Vis absorption spectra of A-P1,A-P2 and A-P3 anchored on TiO2 surface
In order to study the changes of absorption spectra,we further measured the spectral response performance of the three supermolecules anchored on 12μm TiO2films (Fig.3b).As shown in Fig.4,The TiO2film was first immersed into 2 mmol·L-1A solution for 2 h at room temperature and rinsed with ethanol,then was immersed into 1 mmol·L-1Psolution to form supramolecules on the TiO2surface and dried in air.From Fig.3b,we can see that the absorption spectra have changed a lot compared with those of P2,P3 in DCM,the absorption spectra have been significantly improved,their absorption ranges all are over 650 nm,even up to 700 nm.This indicates that the light-harvesting ability of dyes has been improved greatly after supramolecular self-assembly,this will improve the Jscof the DSSCs.From Fig.3a,we can see that there is little difference in spectral response between P2 and P3,after self-assembly,the absorption spectrum of A-P2 was obviously better than that of AP3,we infer that this should be attributed mainly to the difference in the antenna molecules loading amounts.To better analyze the differences between their absorption spectra,the amounts of dye loading were further measured.After self-assembed with porphyrin chromophores,the value of loading amount of P2 is about 1.12×10-8mol·cm-2,and the value of P3 isonly about 0.51×10-8mol·cm-2,thelargest loading amount of P2 can enhance the light-harvesting ability of the device well.

Fig.4 Schematic diagram of self-assembly
2.2 Electrochemical properties
In the process of dye design,the excited state energy level of dyes should match the conduction level of TiO2,and dyes should have high oxidation potential and high regeneration efficiency[40-41].In order to study the related properties,we measured the cyclic voltammetry curves of A-P2 and A-P3 anchored on TiO2(Fig.5).As shown in Fig.5,the EOX(ground state oxidation potentials)of A-P2 and A-P3 were 0.93 and 0.79 V (versus NHE),respectively.As we known,the redox potential of the I-/I3-is 0.4 V,while the EOXof the two supramolecules all are much positive than 0.4 V,the result indicates that the oxidized dyes can be effectively recycled.We estimated the energy gap (E0-0)by their absorption spectra,and the values of A-P2 and A-P3 are 1.77 and 1.82 eV,respectively.Thus,the excited oxidation potentials (EOX*)of A-P2 and A-P3 are-0.84 and-1.03 V,respectively.They are all negative than the conduction band of TiO2(-0.5 V versus NHE),this indicates that the electron from the excited dyes of A-P2 and A-P3 all have high electron injection efficiency[42-43].
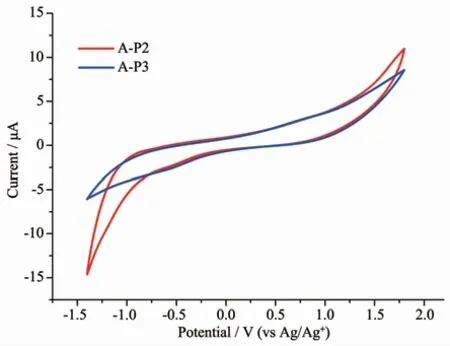
Fig.5 Cyclic voltammogram of A-P2 and A-P3
2.3 Photovoltaic performance of DSSCs
The photovoltaic performance of the devices based on supramolecular self-assembly were measured,the J-V(photocurrent-density-photovoltage)curves of the devices were measured(Fig.6),and Table 1 shows the related parameters.As designed,the antenna molecules P2,P3 can coordinate with the anchoring groups (A)to form supermolecules A-P2 and A-P3,after self-assembly,the DSSCs based on these supermolecules exhibit remarkable photovoltaic performance.The device of A-P2 shows the highest PCE of 1.68%,with a value of Vocis 526 mV,a value of Jscis 5.39 mA·cm-2,and a value of FF is 59.20%.Compared with A-P2,the performance of A-P3 has slightly decreased,the PCE droped down to 0.79%.The main reason is that the Vocdecreased from 526 to 478 mV and the Jscdecreased from 5.39 mA·cm-2to 3.02 mA·cm-2.In addition,the loading amounts of P2,P3 have been measured,and the value of P2(1.12×10-8mol·cm-2)is much higher than that of P3 (0.51×10-8mol·cm-2).We can see that the loading amount has a great influence on the performance of DSSCs,the highest loading amount of P2 can make up for the deficiency of spectral response,this will not only enhance the light-harvesting capability of devices,on the other hand,close alignment of antenna molecules may can effectively prevent the I3-penetrating into the TiO2surface,thus,the charge recombination can be reduced.
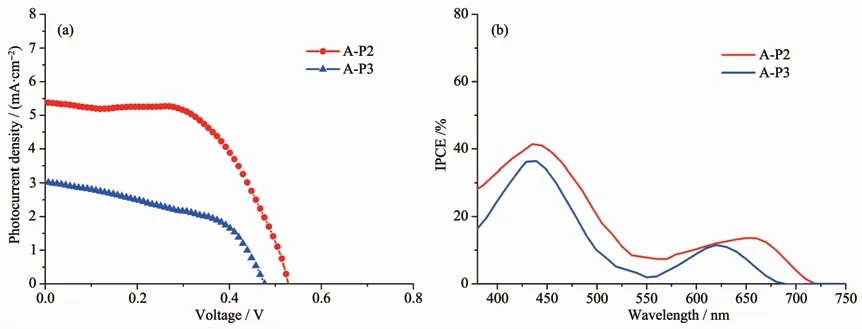
Fig.6 (a)I-V curves of A-P2 and A-P3,(b)IPCE curves of A-P2 and A-P3
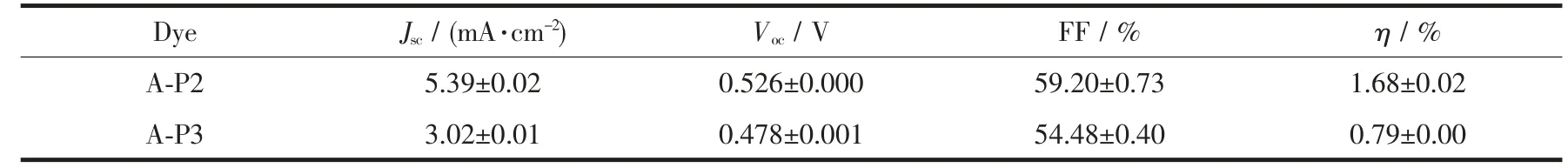
Table 1 Photovoltaic parameters of the DSSCs obtained from the J-V curves
We find that the Jscof these DSSCs decreases in turn along the following trend:A-P2(5.39 mA·cm-2)>A-P3 (3.02 mA·cm-2).The incident photon-to-current conversion efficiency (IPCE)spectra of the DSSCs were measured to analysis the differences.From Fig.6b,the IPCE curves of A-P2 exhibited the highest value of 41.9%at 437 nm,and the photocurrent generation up to about 720 nm.The IPCE curves of A-P3 exhibited the photocurrent generation up to about 680 nm,the value at 434 nm was only 36.5%.In the long wave region,we also found that photocurrent of A-P2 was better than that of A-P3,this should be attributed to the larger loading amount of A-P2.

Fig.7 (a)Nyquist plots of A-P2 and A-P3,(b)Bode phase plots of DSSCs based on A-P2 and A-P3
2.4 EISmeasurements
Electrochemical Impedance Spectroscopy(EIS)is an important method for studying the charge transfer properties at the interface of DSSCs,in this work,we measured the EIS to analyze charge recombination dynamics under dark[44-47].As we known,the charge recombination rate has an important influence on Voc.The EIS was measured under the applied voltage of-0.6 V to analyze the difference between Voc.From Fig.7,two semicircles were found in the plots,the small semicircle means the transport resistance at the Pt/electrolyte,and the large semicircle means the charge transfer resistance at the TiO2/dye/electrolyte interface.We can see that the radius of the large semicircle of A-P2 is larger than that of A-P3,this means that the device of A-P2 effectively reduces the electron recombination rate after self-assembly,it is conducive to suppressing dark current and improving Voc,this also explains why Vocof A-P2 has the largest value of 526 mV.In addition,the electron lifetimes of the DSSCs were further measured by bode phase plots.The DSSCwith longer electron lifetime indicates that there will be low dark current for the device,this helps to enhance the Voc.The peak frequency(f)at lower frequency region can be readed from Fig.7b,and the electron lifetime(τ)can be calculated byτ=1/(2πf)[48-49].The f of A-P2 and A-P3 are 63.2 and 76.5 Hz,respectively.As a result,the electron lifetime values of A-P2 and A-P3 are 2.51 and 2.08 ms,respectively.The trend is consistent with the Vocof DSSCs.
3 Conclusions
In summary,we used a simple method to improve the performance of DSSCs,two antenna molecules zinc porphyrin P2,P3 were prepared and then self-assembly was carried out by coordination with anchoring group (A,4-pyrid-4-ylbenzoic acid).This method shows obvious advantages,it can avoid complex synthesis steps and improve the lightharvesting ability of dyes and reduce charge recombination by adjusting the antenna molecules and anchoring groups.After supramolecular self-assembly,the device of A-P2 showed a PCE of 1.68%,and a Vocof 526 mV,a Jscof 5.39 mA·cm-2,and a FFof 59.20%,in contrast,the device of A-P3 showed a PCE of 0.79%,the main reason should be that the different structures of antenna molecules lead to different loading amount.The results show that self-assembly strategy has been successfully used in this work,this will be a potential and effective way to improve the performance of DSSCs.
Acknowledgements:This work was supported by grants from the National Natural Science Foundation of China(21701060),Changzhou Sci Tech Program(CJ20190079),China and Natural Science Foundation of the Higher Education Institutions of Jiangsu Province(17KJB150015,18KJA150003),China.

