Co2+/Cd2+复合材料的合成及其催化性质
刘冬宁 王萃娟*, 肖玉美 刘 成 罗 丹 朱子馨 陈 霜 王尧宇
(1西南交通大学生命科学与工程学院化学化工系,成都 610031)
(2西北大学化学与材料学院,合成与天然功能分子化学教育部重点实验室,西安 710069)
0 Introduction
Metal-organic frameworks (MOFs)have great potential in many applications[1-3],such as gas separation and storage[4-9],sensing[10-13],adsorption[14-16],proton conduction[17-20]and drug delivery[21],due to their advantageous properties including structural diversity and large surface area[22-24].MOFs have been used as carriers for metal nanoparticles for catalysis[25-28].The use of MOFs as a carrier for noble metals generally increases the reaction rate[29-31].However,the control of the reaction rate is often achieved by changing the catalyst loading,which may result in a lower utilization efficiency of the catalyst,resulting in waste of the precious metal catalyst.One of the strategies to overcome these shortcomings is to design a catalyst carrier that is similar in structure but functionally different,and the rate of reaction is regulated by the carrier.
The use of isomorphous metal doped MOFs can accomplish the above objectives[32].MOFs doped or incorporated with another one or more metal centers have attracted much attention in recent years[33-35],becausethiskind of MOFsmay enhancetheir particular activity[36-38].However,few people have reported the effect of metal ions(Co2+/Cd2+)on the catalytic behavior of MOFs.Different central metal ions in MOFs play an important role in the catalytic reaction.In order to clarify the relationship,isomorphous metal doped MOFs with different central metal ions and different proportions of metal ions should be studied.In this way,the effects of the ligand can be easily eliminated.At the same time,it is convenient to find the relationship between the central metal ions and the corresponding effects of the reaction.
Here,we reported a series of MOFs doped as a catalyst carrier isomorphic metal,which were loaded into a Co2+/Cd2+doped MOF by a simple immersion method.This process had been applied to five kinds of MOFs with different Co2+/Cd2+ratios,and included five catalyst-loaded complexes(1~5).Powder diffraction showed no change in the crystal structure before and after catalyst loading.We showed that isomorphous metal doped MOF was a potential catalyst support that could be used to regulate the reaction rate of the system.We chose the reduction experiment of pnitrophenol as the target reaction.The Ag@compound 1 catalyst with 14.2%(w/w)Ag had higher catalytic efficiency for the reaction,and the reaction rate decreased with the increased proportion of Co which even had a certain inhibitory effect.By increasing the loading of Ag from 14.2%to 47%(w/w)),the catalytic reaction rate can be greatly improved. Cyclic experiments and SEM showed that Ag@compound 1 had good cyclability,and still had 96% catalytic efficiency after 4 cycles of reaction with the structure of crystal unchanged.
1 Experimental
1.1 Materials and ethods
Solvents, transition metal salts and ligand(Hpypymba=4-((3-(pyrazin-2-yl)-1H-pyrazol-1-yl)-methyl)benzoic acid)were of analytical grade.All chemicals were purchased from commercially available sources and used without further purification.The UV-Vis spectrum of the compounds were determined by UVVis spectroscopy (UV-1800).The powder X-ray diffraction (PXRD)measurements were obtained on D8 ADVANCE diffractometer using Cu Kαradiation(λ=0.154 18 nm),voltage of 40 kV,current of 40 mA,scanning range from 5°to 90°,step size of 0.02°and test speed of 0.1 s·step-1.The scanning electron microscope (SEM)were obtained on Hitachi S-4800 using acceleration voltage of 3 kV,and working distance of 8 mm.
1.2 Synthesis of[Co x Cd1-x(pypymba)2]n (compounds 1~5)(0≤x≤1)
Our previous work had successfully synthesized compounds 1~5,and it had been subjected to PXRD,scanning electron microscope (SEM),inductively coupled plasma (ICP)and thermogravimetric analysis(Fig.S1~S5),which proved that isomorphous metal doping compounds 1~5 had a similar structure and crystal form[39-40].
1.3 Preparation of Ag@compounds material
First,we added 25 mg of silver nitrate inorganic salt to a 5 mL aqueous solution to prepare a 5 mg·mL-1silver nitrate aqueous solution,and then 10 mg of the synthesized MOFs were immersed in 3 mL aqueous solution of silver nitrate.
Second,the mixture was slowly stirred for 60 minutes,and then kept it stand for one day.After the MOFs were sufficiently contacted with the silver ions,they were centrifuged several times with pure aqueous solution to wash.
1.4 Catalytic degradation performance of 4-NP
In the experiment,4-NP was used as the catalyst to degrade the substrate.During the reaction,the change of the characteristic absorption peak of the reactants in the solution was observed to test the catalytic performance of Ag@compounds.The prepared Ag@compounds (5 mg)were added to 8 mL 1×10-4mol·L-14-NP ethanol solution and 5 mg NaBH4,and the change in absorbance was monitored by UV-Vis.We studied the catalytic degradation of 4-NP by using catalyst-free and unsupported Ag ions as control experiment.
2 Results and discussion
2.1 Structural analysis of Ag@compounds
The prepared Ag@compound 1 was tested by PXRD (Fig.1).The obtained PXRD pattern had a higher similarity to the pattern of compound 1,and the decrease of intensity at 22.5°may be related to the crystal lattice of Ag+.The prepared Ag@compound 1 had the same crystal structure as the initial one and had good chemical stability.The supernatant was collected and the sodium chloride solution was added dropwise thereto until the precipitate no longer increased.And we collected the resulting precipitate and calculated the loading of Ag.The newly prepared Ag@compounds were dried by low temperature to remove the aqueous solution.
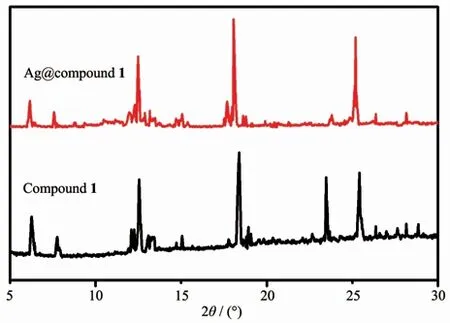
Fig.1 PXRD patterns of Ag@compound 1 and compound 1
2.2 Catalytic degradation of 4-NP by metaldoped Ag@compounds 1~5
First,we studied the catalytic degradation of 4-NP by MOFs in the absence of any catalyst and without the inclusion of Ag+,respectively.The experimental results were shown in Fig.2.The absorbance of the characteristic absorption peak of 4-NP before and after the reaction did not change significantly,indicating that the MOFs themselves had no catalytic degradation to 4-NP.
In addition to the inconsistencies in the central metal elements,complexes 1~5 had the same spatial structure,and they can be used as carriers for Ag+to study the effect of the metal center of the complex on the catalysis.
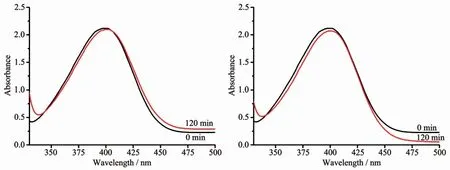
Fig.2 Degradation of 4-NPwithout catalyst(left)and with compound 1(right)
According to the experimental protocol,a certain amount and concentration of 4-NP solution was added to monitor the change of absorbance.The UV-visible scan spectra of the supernatant were shown in Fig.3.The catalytic reduction efficiency decreased gradually with the time.When Ag@compound 1(~14.2%(w/w))was used as the catalyst,the intensity of the absorption peak at 400 nm was gradually reduced,and 60%of the substrate was reduced after 60 min(Fig.3a).The catalytic effect of Ag@compound 1 was higher than other groups,but the reaction rate was significantly reduced,which may be related to the decrease of substrate concentration.After 120 min,the degradation of 4-NP was completed,which proved that Ag@compounds 1 had good catalytic degradation to 4-NP.At the same time,the degra-dation rate gradually decreased as the increase of Cd content in the structure.When Ag@compound 5 was used as the catalyst,84%remained after 120 min (Fig.3e).This further indicated that the Ag@compound 1 composite prepared by compound 1 and Ag+had good catalytic degradation and showed that the composite had a high catalytic rate under low concentration of catalyst.
By comparison,it was found that the compound 1 was most suitable as a silver ion carrier when the silver ion loading was equivalent.In order to verify this inference,the silver ion loading of Ag@compounds was calculated by the precipitation method.The calculation results showed that the loading of compounds 1~5 on silver ions were basically the same(14.2%(w/w)).
Since the concentration of NaBH4was much higher than the amount of the reaction substrate,the excess can be regarded as a constant amount,and the catalytic reaction can be regarded as a quasi-firstorder reaction,and the expression for the reaction is(1):

Where k is the reaction rate constant,min-1;t is the catalytic reduction reaction time,min;P0and Ptare the mass concentration of 4-NP at the initial reaction time and t,respectively;A0and Atare the absorbance of 4-NPat the initial reaction time and t,respectively.
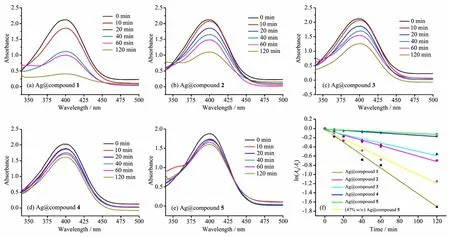
Fig.3 Degradation of 4-NPwith catalyst Ag@compound 1~5(a~e),and effect of Ag+loading on catalytic degradation performance(f)
Ag@compounds 1~5 had an Ag+loading of 14.2%(w/w).The effect of different compounds excess on the catalytic reduction effect was shown in Fig.3f.ln(At/A0)had a linear relationship with t in the case of equivalent silver ion loading in different MOFs materials,and the fitting degree was higher(0.98 or more);when the composite material was Ag@compound 1,the reaction rate constant was higher(0.014 min-1)compared with that of the Ag@compound 5(0.000 9 min-1).The results showed that the catalytic effect of the complex of the central metal element being cobalt as the silver ion carrier on the degradation reaction was stronger than that of cadmium,which may be beneficial to the electron transfer in the reaction with the d-layer electron of the cobalt element.
2.3 Effect of Ag+loading on catalytic reduction
The c omposite material with higher Ag+loading obtained by treating MOFs with 3 mL of silver nitrate aqueous solution(10 mg·mL-1)was calculated to have a load of 47%(w/w)Ag+,and the composite was calculated as 47%(w/w)Ag@compounds 5.When the other catalytic conditions were the same,the catalytic reduction effect was shown in Fig.3f.When the composite material was Ag@compounds 5,the reaction rate constant was 0.000 9 min-1,and after increasing the loading of Ag+to 47%(w/w),the rate of catalytic reduction reached to 0.009 6 min-1,and the reaction rate increased significantly,indicating that increasing the loading of Ag+was one of the ways to improve its catalytic efficiency.However,as the Ag+loading increased,the composite material and the solution quickly turned black,affecting its recovery for recycling experiments.This indicated that it was advantageous to increase the loading of Ag+within an appropriate range.
2.4 Recycling effect of catalyst
Since the precious metal was loaded in the composite material,the use of the material was lowered by considering its recycling.Ag+without MOFs also had high catalytic efficiency,but a single silver ion cannot be recycled.After the reaction,the composite material was washed with ethanol and added to the solution as in the previous scheme to detect the change in the absorbance of p-nitrophenol,and the repeated use effect of the degradation reaction was shown in the Fig.4.When used for the first time,the removal rate of 4-NP for Ag@compound 1 was 99.8%.After recycling 3 times,the catalytic efficiency was still 96%(Fig.4).Although the activity of catalyst was stable,the weight loss during the collection of the complex was unavoidable as the number of cycles increased.The results showed that Ag@compounds 1 had high stability and can be used for multiple cycles.After recycling,the morphology of the composite did not change significantly(Fig.5),indicating the stability of the structure(Fig.6).
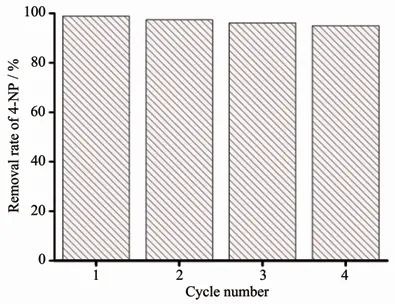
Fig.4 Catalytic cycle test of Ag@compound 1
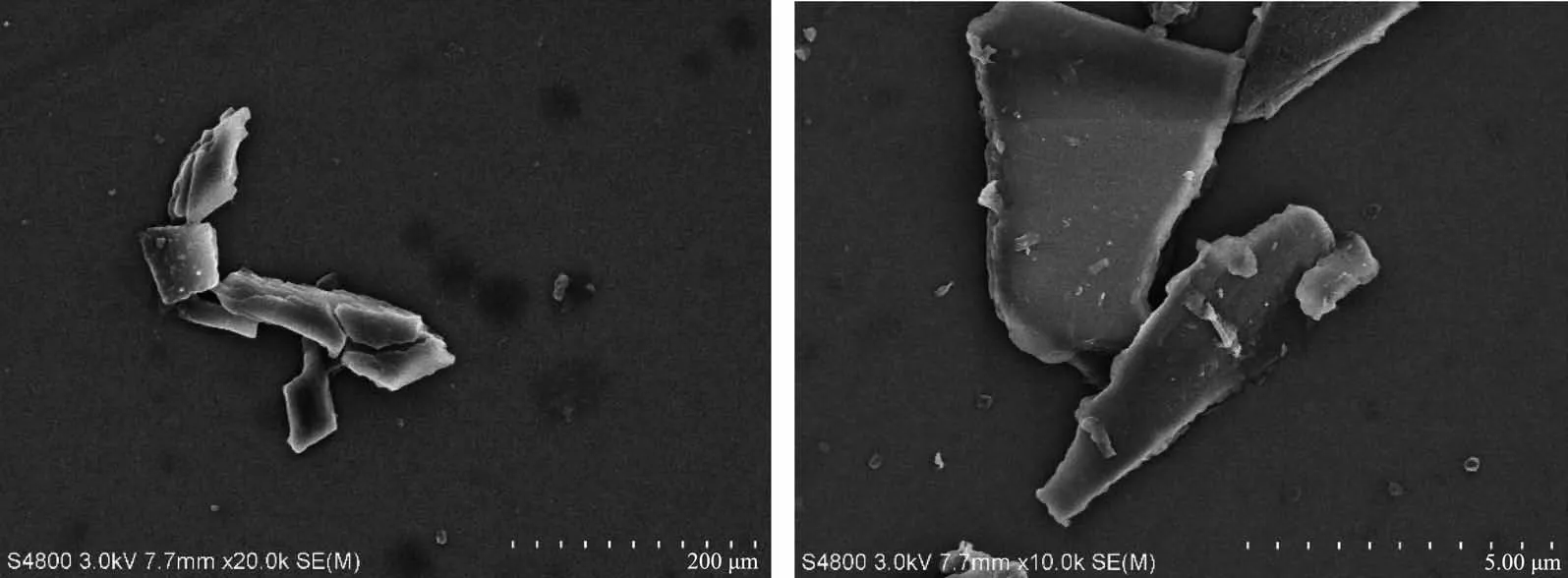
Fig.5 SEM images of Ag@compound 1 before(left)and after(right)the reaction
Although the composite material synthesized under thisstrategy took 2 hoursto degrade 4-NP,which was longer than the complete reaction time of other studies[41],but the reaction time can be reduce by appropriately adding Ag+.Thereaction timewasreduced and the effect of metal center ions on the catalysis was investigated by isomorphic coordination.It had been shown that the prepared Ag@compound 1 with cyclic stability can be used as an excellent catalyst,and we can also control the rate of reaction by using different ratios of Co/Cd compounds.
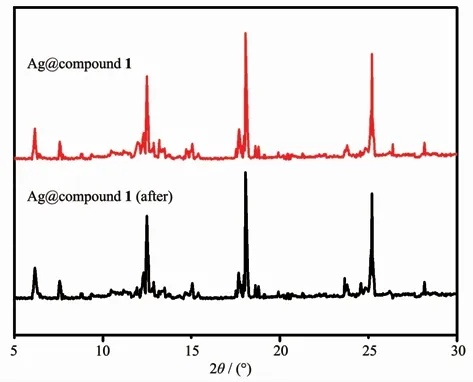
Fig.6 PXRD patterns of Ag@compound 1 before and after the reaction
3 Conclusions
The composite catalyst was prepared by adsorption method.When the Ag+loading was 14.2%(w/w),the Ag@compound 1 had high catalytic activity in catalytic reduction of 4-NPby NaBH4,and the activity increased within a certain range as the increased Ag+loading.The increase of Ag+loading increased the reaction rate.Compared with the complex with the same structure,the catalytic effect of the central metal element Co was higher than that of Cd and the catalytic effect was proportional to the content of Co element.The composite has good stability and the catalytic efficiency was basically unchanged after repeated use for 4 times.It is proved that the reaction rate can be adjusted by the proportion of doping metal of MOFs,which provides a new idea for the preparation of new catalyst carriers.
Acknowledgments:This work was supported by the Fund of National Natural Science Foundation of China (Grant No.21401151),Science and Technology Support Program of Sichuan Province (Grant No.2015GZ0233),Science and Technology Huimin Program of Chengdu(Grant No.2015-HM01-00336-SF),Supported by the Innovative&Practice Project of Graduate School of Southwest Jiaotong University(Grant No.2018CYPY07).Supporting information isavailable at http://www.wjhxxb.cn

