土壤有机碳形成机制的探索历程
周国逸, 熊鑫,2
土壤有机碳形成机制的探索历程
周国逸1, 熊鑫1,2
(1. 中国科学院华南植物园, 广州 510650; 2. 中国科学院大学, 北京 100049)
土壤有机碳(SOC)是生态系统的重要资产,在全球碳平衡中发挥着关键的作用。2015年巴黎气候会议以来,促进SOC在陆地生态系统中的积累受到特别重视,被认为是有效减缓大气CO2浓度上升的最重要地表措施。从服务于这个目标出发,对过去几十年全球在探索SOC形成机制上的历程进行了回顾和总结,从弄清SOC全球分布规律,阐明样地以下尺度的SOC循环过程及其相应的物理、化学与生物机理,到样地及以上尺度的土壤固碳机制,最后给出了成熟森林SOC积累机制的实例。SOC形成机制的探索历程就是寻求为促进土壤固碳提供理论指导的过程。
土壤有机碳;形成;固存;机制
土壤有机碳(SOC)是土壤的重要组成成分,是生态系统的重要资产[1],它不仅是度量土壤肥力的关键因子,更是表征生态系统服务功能大小的综合指标[2]。在特定的区域内,SOC含量高往往意味着该生态系统的服务功能强,根据生态系统SOC储量的大小并结合所处的时空分布特征,可以准确地量化出该生态系统的其他服务功能,诸如固碳释氧功能[3–4]、水土保持与涵养功能[5]、生物多样性保育功能[6]等。
探索SOC形成机制的主要动机不是因为它在形成生态系统服务功能上的中心地位,而在于SOC对全球碳平衡的贡献。全球尺度上,SOC储量是大气和陆地植被各自碳储量的3倍以上[1],中国陆地生态系统的SOC更是生物量碳的3.9倍[7]。SOC储量的微小变化将导致全球碳平衡估算的巨大误差,假设SOC含量变化0.01% a–1[按百分浓度计算,即由%变化到(±0.01)%],在假定土壤容重为1 g cm–3且其年内变化可以忽略的情况下,SOC库的变化将为±1 Mg hm–2a–1,这是一个很大的值。以这个值乘以全球森林面积(3.87×109hm2)得到的全球森林土壤碳储量的年变化量为±3.87 Pg,这个值甚至比1990年的全球碳“失汇”量还要大。土壤固碳潜力巨大,同样地,如果SOC的百分浓度增量为0.01% a–1,则净增加1%需要100 a时间。Tang等[7]通过分析中国森林生态系统7 800个样地的资料证明,森林生态系统由建立到成熟,土壤碳库的增速显著高于生物量碳库的增速;且在生物量保持相对稳定后,土壤碳库依然有相对高的增长速率。Zhou等[8]报道成熟森林土壤可持续积累有机碳,且被全球范围内的大量研究结果所证实[9–12],这便为土壤固碳潜力提供了一个更高的天花板。一个未经证实的理论认为随着生态系统的自然成熟,光合作用固定的有机碳将逐渐被呼吸作用释放的无机碳所平衡[13],成熟生态系统没有碳汇功能。该理论虽然受到成熟森林土壤持续固碳和成熟森林生物量增加等现象的挑战[8,11,14–15],但长时间尺度上并不存在异议, 有争议的是如何定义成熟生态系统。事实上,也只有“成熟森林土壤持续固碳”这个现象被证明是在生态系统演替过程中自然发生的[6],来源于内源驱动、因而是相对长久的;而成熟森林生物量持续增加的现象则是全球环境变化(气温升高、CO2浓度上升、氮沉降加剧等)的结果[16],来源于外源驱动、因而是短暂的。相较于生物量碳,SOC平均周转时间更长[17]、有些甚至能保存上百万年[1],因此,在缓解全球大气CO2浓度上升的举措中,SOC是更为优质的有机碳储存方式。在全球人工林面积持续多年增加的基础上[18],继续依靠植被面积增加从而增加全球陆地生态系统碳储量的模式将很快遇到其壁垒,在陆地生态系统中寻求更优质的固碳方式,是保障社会经济持续发展的同时减少碳净排放的根本途径。
因此,自2015年巴黎气候会议以来,促进SOC在陆地生态系统中的积累受到特别的重视,被认为是有效减缓大气CO2浓度上升的最重要地表措施[19–20], 明显扬弃了过去“造林、再造林”的理念。实现大规模SOC存贮量的提升并为此规划行动方案以及准确模拟全球碳平衡的先决条件是全面阐明SOC积累的驱动机制,为此,全球开展了探索SOC形成机制的广泛研究。我们通过Web of Science查找了过去20年来,以“SOC”为主题的论文数(图1), 从中可以看出,涉及该内容的论文数一直在持续增加,特别是2009年以前和近几年,这反映了探索SOC形成机制历程的曲折性、艰难性和前沿性。本文试图从如下几个方面再现这个历程。
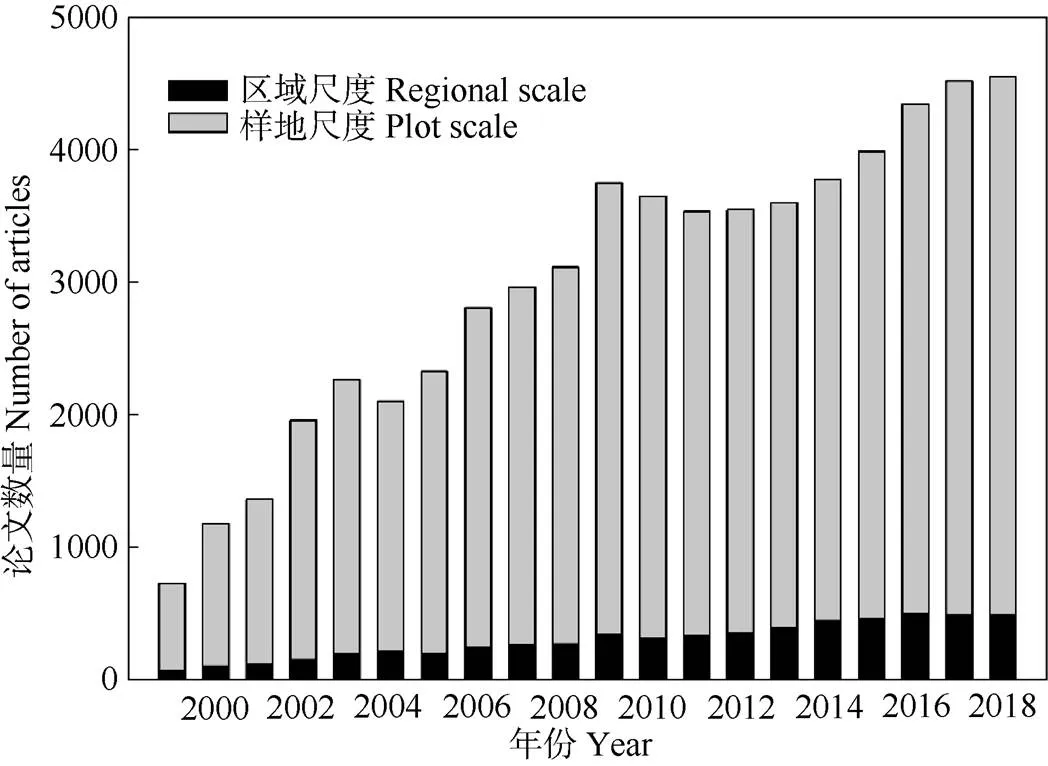
图1 通过Web of Science搜索的全球过去20年以“SOC”为主题的论文数
1 SOC积累现象、空间分布和试验检验
探索SOC形成机制的历程起步于对全球陆地土壤碳库现状大小的评估。认识到SOC在全球碳平衡中的核心地位后,全球范围内广泛开展了与SOC在陆地生态系统分布规律相关的大量工作[21–24]。至今,SOC库在主要生态系统类型及土壤剖面的分布规律已基本清晰,不同的研究结果间没有数量级上的差异[7,25–26]。可以预计,如果没有人为的土地利用变化和气象灾害的发生,陆地植被生态系统碳库大小将是相对稳定且有规律增长的,具有长时间尺度上的参照性;但一些特殊生境类型(如:高寒区、荒漠区、内陆湿地、湖泊、农田、大陆架等)下的土壤碳库状况尚未完全弄清楚[27–28]。
随后,重点转移到了在样地(生态系统)尺度上SOC积累与某些表观因素间的关系研究,这类工作占据了已有研究的大部分,并且还在进行中。主要特点是试图发现SOC含量随生态系统类型和空间位置不同而发生变化的证据[29–30],包括处于不同恢复阶段的人工恢复生态系统系列的SOC含量差异证据[31–32],以及处于不同演替阶段的自然生态系统系列的SOC含量差异证据等[33],都属于这类研究。同时,在有长期监测数据的支持下, 一些报道阐明了同一生态系统类型SOC储存量随时间的变化趋势[7–8]。这些工作不仅开启了全球范围内探索SOC积累机制的研究,而且为深入探讨该机制提供了重要的实验参照和数据准备。随着探索的不断深入, 很多研究试图阐述SOC与植被生态系统的状态参量(如生物多样性[34]、植被生物量[35]、初级生产力与凋落物量[36–39]、凋落物质[40–41]、根系生物量[42]、根系分泌物[43]等)或生态因子(如光[44]、温[45]、水[46]、土壤质地[47–48]和土壤养分[49]等)的相互关系,并认为这些相互关系可以推广到其他区域或更大尺度上。
对环境因子和生态系统本身的控制实验也在全球范围内广泛地开展起来,多数研究者通过FACE (free-air CO2enrichment)试验[50]、人工升温[51]、改变降水输入[52]、添加氮磷[53–54]等处理对植被生态系统施加影响,观测不同生态系统的响应与适应,包括SOC动态对这些因子变化的响应[55–56];个别研究者通过人工改变植物物种多样性(如草原中的物种剔除试验[57]、森林中的不同物种数混交试验等[58]),以检验生物多样性对生态系统功能包括土壤固碳功能的影响。与此同时,另一些研究者则通过将整个生态系统进行移位,即:将处于某一环境条件下的生态系统尽可能完整地移位至另一环境条件下,以研究同一生态系统在不同环境条件下的响应差异,当然也包括SOC动态响应的差异。如: 将亚热带与温带交界处的土柱整体移位至南亚热带地区[59];在鼎湖山,将海拔600 m处的山地常绿阔叶林生态系统整体移位至海拔300和30 m处, 也把海拔300 m处的针阔叶混交林生态系统整体移位至30 m处, 以检验不同森林生态系统对环境条件改变的响应差异[51,60]。一般认为,生态系统整体移位试验比传统的环境因子控制试验更加贴近真实状况;但如果不在机理上对这些试验结果做更深层次的分析,那么所有的控制试验都只是起到了辨识和检验自然观测结果的作用,从机理探讨的角度来说并没有本质的进步;而且,由于控制试验的条件或多或少地与自然条件存在差异,所得到的结果必然与真实状况存在偏差,甚至存在错误的可能。因此,控制试验的主要优点在于缩短了研究周期,其最终目的是为深层次的机理分析提供便利、而不在于观测到的现象和试验结果本身。
2 样地以下尺度的SOC循环过程及其物理、化学与生物机理
2.1 生物量碳的输入
随着探索SOC形成机制的深入,越来越多的研究试图阐明由植物残体向土壤有机质转化的输入机制。一些研究指出氮含量丰富、碳氮比低的凋落物不仅分解快,而且分解过程中会产生更高比例的可溶性有机碳(DOC)和微小植物残片,有利于传输到整个土层并最终稳定存贮[40,61–64]。Sumiyoshi等[41]的试验证实,木质素含量低的凋落物分解速度快的同时,促进了SOC的积累。另一些研究则证实微生物更能有效地利用氮含量丰富的凋落物以构建微生物自身生物量,从而提高碳的利用效率[65]。
包括根系凋落物和根系分泌物在内的根系输入对SOC的贡献一直是研究者们关心的问题[42–43,66]。由于物理化学的保护机制、微尺度下的物理保护机制、与金属离子的化学作用以及根系组织的化学稳定性,来源于根系的SOC比来源于地上部分的平均驻留时间(residence time)长1.4倍[42]。根系分泌物不仅是SOC的供给者而且给土壤带来了生命活性物质, 这些生命活性物质在植物与土壤生物之间起着重要的纽带作用[67],通过激发微生物对土壤氮循环的反馈机制[68],调节土壤生物化学过程而间接地影响SOC存贮;而其对SOC存贮的直接作用机理仍有待更深入的研究。
生物量碳输入量与SOC积累的关系一直是研究者关心的焦点,这是一个直观的认识,生物量碳输入量决定着SOC积累的观点几乎是全球碳平衡模型的一个基石[69]。然而,有研究发现全球生物量分布与SOC分布并没有关联[35],间接说明了生物量碳输入量对SOC积累没有影响,但对于农田土壤,这个论点似乎并不成立[39];Zhou等[6]通过对中国森林生态系统的观测和全球森林生态系统的meta数据分析,直接证明生物量碳输入量(包括地上地下所有生物残体的输入)相对于生物残体的质(以C/N比来表征)来说,对SOC的贡献是微不足道的。
2.2 SOC的存贮机制
在SOC的自身形态上,依据不同组分的化学顽抗性水平和周转速率,研究者们往往将SOC划分为易分解有机碳和难分解有机碳[70],或是活性组分和惰性组分两大类[71]。不少研究都试图从SOC形态组成的角度探索SOC的存贮机制[1,72],木质素、烷基碳或芳香碳等难分解有机组分或分子结构的多寡常被用来表征土壤有机质碳的稳定性[73–74]。研究者显然认为,易分解有机碳比例高,则SOC平均存在时间短,不利于积累;反之则长,利于积累。这看起来似乎是SOC积累的一种机理,但本质上不过是一个问题的两个方面,因为研究者并没有回答为什么这个比例在不同的土壤中会存在差异、这个差异是如何形成的等问题;实际上,就连“易分解有机碳比例高,则SOC存在时间短”的命题也是有条件才成立的,只是从一个侧面来说明SOC的状况。
土壤质地(soil texture)被认为对SOC的存贮起着重要的作用[48],以至于生物地球化学模型几乎无一例外地根据土壤粘粒的含量来修正土壤有机质的周转速率。然而,Zhou等[6]和Wynn等[47]认为大尺度下的SOC积累并未受控于土壤质地,也许土壤质地只是影响SOC积累的一个局地因子。土壤结构(soil structure)因为可能影响SOC的存贮而被广泛地研究[3,75–76],一般认为随着土壤团粒结构的增大,土壤有机质含量增加[77–78]。同时,土壤团粒结构的形成降低了底物的可接触性,有利于SOC的保存[79]。然而,土壤团粒结构的大小与SOC含量的高低可能也只是一个问题的两个方面,分不清哪个是因、哪个是果,甚至土壤团粒结构的大小更有可能是SOC含量高低的结果,大量的研究结果已经证实土壤有机质是大团聚体形成的主要胶结剂[80–81]。
人们还试图寻求SOC在垂直分布上的差异对其稳定性的贡献[26],一般认为处于缺氧环境下的深层SOC周转时间慢,更易于保存[82]。
2.3 环境因子的调控机制
SOC积累涉及一系列连续的物理、化学和生物反应,这些反应可以被粗略地包含在输入和存贮两个紧密联系的过程中。仅凭快速的凋落物分解并不一定会带来SOC的积累,只是为SOC的积累创造了条件;同样,仅仅依靠良好的存贮环境,也不会增加SOC。要正确地阐明SOC的积累机理,必须将这两个过程综合考虑。环境因子、特别是全球变化因子如何影响这一连串的物理、化学、生物反应从而控制SOC积累是众多研究者密切关注的热点。
气温上升和适度的降水将加速植物残体的分解[83],从而控制生物量碳输入过程,这个结论在全球范围内是一致的,不管植物残体的类型和性质如何变化[84]。尽管如此,却很少有研究将凋落物分解速率表述为气温与降水函数,可见,这方面的研究至今并不深入,已有的全球和区域性凋落物交叉分解试验并不多[84–85]。在降水稀少的干旱区草地,推动凋落物分解的气候因子是紫外辐射[82]。SOC矿化速率(土壤呼吸速率)明显与分解者的活性规律相一致,随气温的上升而加快,虽然不同区域的Q10值差异很大[86],但总体上呈指数增加的模式。与降水相比,土壤水分状况是影响土壤呼吸更为直接的因子,大多数相关研究都将土壤水分而不是降水量作为考量因素,研究发现土壤呼吸作用与土壤水分呈单峰曲线,即在土壤水分含量低的时候,土壤呼吸速率随土壤水分含量增加而单调地增大,达到峰值后, 又单调地减小。土壤水分含量高便于有机碳向土壤深层运输,增加土壤剖面有机碳含量的均匀性,同时维持土壤的厌氧环境[82],有利于SOC的保存。
植物残体分解速率和土壤呼吸速率对氮沉降的响应则比较复杂,Mo等[53]报道氮沉降水平的增加延缓了“氮饱和”的成熟森林生态系统凋落物分解和土壤呼吸、而对未达到“氮饱和”的先锋群落和过渡群落则相反;这种现象似乎是由于氮沉降对不同成熟度森林土壤的酸化程度存在差异,土壤酸化增加了土壤阳离子(cation)交换量从而促进了SOC的积累[87];同时土壤酸化也降低了分解者的活性[88–89]。
一般认为,微生物活性在调控凋落物分解速率上起着重要的作用[83];但全球尺度上的凋落物分解交互试验结果表明,与气候作用比较,分解者的作用是局部的、不会改变凋落物分解速率由热带向寒带逐渐减小的全球格局[84]。有研究表明植物多样性上升将导致分解者生物量和活性增加[34,90],从而间接地调控SOC的积累,因此,植物多样性也是影响SOC积累的环境因子之一。
3 样地及以上尺度的土壤固碳机制
在样地及以上尺度检验和校正样地以下尺度的个例研究(case study)所得到的SOC平衡过程及其机理并归纳出普遍规律,是探索SOC形成机制历程中的一个关键节点。如前言中所述,探索SOC形成机制的根本目的在于建立适合评估区域或全球碳平衡的模型、促进全球陆地生态系统SOC积累以减缓大气CO2浓度上升。多年来特别是2015年巴黎气候会议以来,在这个目的驱动下,全球广泛开展了样地尺度以上的土壤固碳机制的探索工作,试图建立SOC与大尺度环境因子之间的关系,诸如SOC储量与气候和植被[25–26,91–93]、土壤类型[94]和管理方式[35]的关系等等。然而,尽管人们对样地尺度以下的SOC平衡过程如前所述已有了充分的了解,但目前还没有普遍认可的样地尺度以上的土壤固碳机制[95],这种现状呼吁人们在对不同时空尺度和土壤类型的SOC进行大量测定的基础上,继续开展进一步的研究[96]。
基于中国科学院战略先导专项课题“中国森林生态系统固碳现状、速率、机制和潜力(森林课题)”在国家尺度上所布置的森林样地及其调查数据、结合全球已经发表的研究结果,Zhou等[6]报道样地及以上尺度的SOC动态与气候、初级生产者、土壤之间存在如下规律:(1) 与湿润指数P/PET (P-年降水量,PET-年蒸散潜力)呈显著正相关关系;(2) 与凋落物(泛指输入到土壤的所有初级产品,包括地上地下凋落物及粗死木、根系分泌物)碳氮比(C/N)呈显著负相关关系;(3) 植物多样性只直接影响表层SOC动态,对深层SOC动态没有直接影响,但是在自然状况下,植物多样性的上升将导致群落凋落物平均C/N比下降,从而间接影响SOC;(4) 与凋落物量没有显著关系;(5) 与土壤质地没有显著关系。这些结果表明,任何能够引起凋落物C/N比下降的自然过程和人为措施(如自然演替或人工引进C/N比低的物种到群落中)、以及能导致湿润指数P/PET上升的气候变化事件都将促进SOC的积累。这可以解释很多先前难以理解的现象。如:FACE试验表明, CO2浓度上升促进了生物量的积累且增加了凋落物产量, 却并不能增加SOC含量[55,97–98],这显然是因为CO2浓度上升并没有降低(甚至增加)凋落物C/N比[6]。与此类似,氮肥添加试验虽然增加了初级生产者的生产力和生物量,但能否增加SOC含量则取决于是否降低了凋落物的C/N比[99–100]。营林工作者通过引进豆科植物以增加原有林分的土壤肥力[101],也是因为豆科植物的加入降低了原有林分的凋落物C/N比[6]。
4 成熟森林生态系统土壤固碳机制
自发现成熟森林土壤可持续固碳现象以来[8],通过13年的研究,其固碳机理已基本清晰。作为本文主题下的一个实例,这里将成熟森林生态系统土壤固碳机制总结如下。
4.1 内源驱动机制
“内源驱动机制”是指由生态系统自身演替所导致的气候、初级生产者、土壤理化性质改变所驱动的SOC积累。受这种机制作用,成熟与未成熟生态系统SOC的反应是完全不同的。
对季风常绿阔叶林(南亚热带地带性顶级群落)及其演替系列的研究发现,在先锋群落向顶级群落自然演替过程中及达到顶级群落后,虽然凋落物量很快趋向稳定,但土壤水分[102]和植物多样性[103–104]持续上升,凋落物C/N比和木质素含量持续降低,推动着凋落物分解速率的加快以及流向土壤的有机碳比例增大[40],为土壤提供了越来越丰富的有机碳源。与此同时,随着演替的进行,土壤有效磷含量的下降和有效氮含量的上升阻碍了SOC的分解从而有利于SOC的保存[53,105–106];而且,由于土壤水分随森林成熟度增加而逐步提高,一方面使得成熟森林土壤表层有机碳较易于向土壤深层运输并在厌氧环境下得到保存[82],另一方面森林的顺行演替有利于驱动微生物朝着促进SOC积累的方向发挥功能[102]。
上述成熟森林SOC持续积累的内源驱动机制可以被Zhou等[6]近期的研究结果完美诠释并相互印证。
4.2 外源驱动机制
“外源驱动机制”是指生态系统受外力(人类经营管理、自然灾害和全球及区域环境变化)作用所导致的SOC积累。这种机制对所有生态系统SOC积累都起作用,但成熟与非成熟生态系统的反应程度可能有所差异。
对季风常绿阔叶林及其演替系列的研究发现,该区域长期受酸沉降和氮沉降上升的胁迫,导致土壤酸化和成熟森林土壤氮含量饱和,酸化土壤和氮饱和土壤都将抑制土壤呼吸[53,107],增加土壤阳离子交换量(CEC)[87],有利于SOC的保存,这种效应在成熟森林土壤更为显著[53]。
过去几十年来,季风常绿阔叶林区域气温持续上升、降水强度两极化,这在导致土壤水分下降的同时[104,108],也增大了成熟与未成熟生态系统土壤水分的年内变幅差异,成熟生态系统土壤水分的年内变幅小于未成熟生态系统的年内变幅,更有利于SOC的积累[102]。
[1] SCHMIDT M W I, TORN M S, ABIVEN S, et al. Persistence of soil organic matter as an ecosystem property [J]Nature, 2011, 478(7367): 49–56. doi: 10.1038/nature10386.
[2] LIU X D, QIAO Y N, ZHOU G Y. Controlling action of soil organic matter on soil moisture retention and its availability [J]Chin J Plant Ecol, 2011, 35(12): 1209–1218. doi: 10.3724/SP.J.1258.2011.01209.刘效东, 乔玉娜, 周国逸. 土壤有机质对土壤水分保持及其有效性的控制作用 [J]. 植物生态学报, 2011, 35(12): 1209–1218. doi: 10. 3724/SP.J.1258.2011.01209.
[3] OADES J M. Soil organic matter and structural stability: Mechanisms and implications for management [J]Plant Soil, 1984, 76(1/2/3): 319– 337. doi: 10.1007/BF02205590.
[4] NADPOROZHSKAYA M A, MOHREN G M J, CHERTOV O G, et al. Dynamics of soil organic matter in primary and secondary forest succession on sandy soils in The Netherlands: An application of the ROMUL model [J]Ecol Modell, 2006, 190(3/4): 399–418. doi: 10. 1016/j.ecolmodel.2005.03.025.
[5] GUPTA S C, LARSON W E. Estimating soil water retention charac- teristics from particle size distribution, organic matter percent, and bulk density [J]Water Resour Res, 1979, 15(6): 1633–1635. doi: 10.1029/ WR015i006p01633.
[6] ZHOU G Y, XU S, CIAIS P, et al. Climate and litter C/N ratio constrain soil organic carbon accumulation [J]Natl Sci Rev, 2019: nwz045. doi: 10.1093/nsr/nwz045.
[7] TANG X L, ZHAO X, BAI Y F, et al. Carbon pools in China’s terrestrial ecosystems: new estimates based on an intensive field survey [J]Proc Natl Acad Sci USA, 2018, 115(16): 4021–4026. doi: 10.1073/ pnas.1700291115.
[8] ZHOU G Y, LIU S G, LI Z, et al. Old-growth forests can accumulate carbon in soils [J]Science, 2006, 314(5804): 1417. doi: 10.1126/ science.1130168.
[9] KNOHL A, SCHULZE E D, KOLLE O, et al. Large carbon uptake by an unmanaged 250-year-old deciduous forest in central Germany [J]Agric For Meteorol, 2003, 118(3/4): 151–167. doi: 10.1016/S0168- 1923(03)00115-1.
[10] ZHANG J H, HAN S J, YU G R. Seasonal variation in carbon dioxide exchange over a 200-year-old Chinese broad-leaved Korean pine mixed forest [J]Agric For Meteorol, 2006, 137(3/4): 150–165. doi: 10. 1016/j.agrformet.2006.02.004.
[11] LUYSSAERT S, SCHULZE E D, BÖRNER A, et al. Old-growth forests as global carbon sinks [J]Nature, 2008, 455(7210): 213–215. doi: 10.1038/nature07276.
[12] TAN Z H, ZHANG Y P, SCHAEFER D, et al. An old-growth subtropical Asian evergreen forest as a large carbon sink [J]Atmos Environ, 2011, 45(8): 1548–1554. doi: 10.1016/j.atmosenv.2010.12.041.
[13] ODUM E P. The strategy of ecosystem development [J]Science, 1969, 164(3877): 262–270. doi: 10.1126/science.164.3877.262.
[14] PHILLIPS O L, MALHI Y, HIGUCHI N, et al. Changes in the carbon balance of tropical forests: Evidence from long-term plots [J]Science, 1998, 282(5388): 439–442. doi: 10.1126/science.282.5388.439.
[15] LEWIS S L, LOPEZ-GONZALEZ G, SONKÉ B, et al. Increasing carbon storage in intact African tropical forests [J]Nature, 2009, 457(7232): 1003–1006. doi: 10.1038/nature07771.
[16] MULLER-LANDAU H C. Carbon cycle: Sink in the African jungle [J]Nature, 2009, 457(7232): 969–970. doi: 10.1038/457969a.
[17] WANG J S, SUN J, XIA J Y, et al. Soil and vegetation carbon turnover times from tropical to boreal forests [J]Funct Ecol, 2018, 32(1): 71– 82. doi: 10.1111/1365-2435.12914.
[18] KEENAN R J, REAMS G A, ACHARD F, et al. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015 [J]For Ecol Manage, 2015, 352: 9–20. doi: 10.1016/j.foreco. 2015.06.014.
[19] SMITH P. Soil carbon sequestration and biochar as negative emission technologies [J]Glob Change Biol, 2016, 22(3): 1315–1324. doi: 10. 1111/gcb.13178.
[20] RUMPEL C, AMIRASLANI F, KOUTIKA L S, et al. Put more carbon in soils to meet Paris climate pledges [J]Nature, 2018, 564(7734): 32– 34. doi: 10.1038/d41586-018-07587-4.
[21] POST W M, EMANUEL W R, ZINKE P J, et al. Soil carbon pools and world life zones [J]Nature, 1982, 298(5870): 156–159. doi: 10.1038/ 298156a0.
[22] ESWARAN H, van den BERG E, REICH P. Organic carbon in soils of the world [J]Soil Sci Soc Amer J, 1993, 57(1): 192–194. doi: 10. 2136/sssaj1993.03615995005700010034x.
[23] LAL R. Forest soils and carbon sequestration [J]For Ecol Manage, 2005, 220(1/2/3): 242–258. doi: 10.1016/j.foreco.2005.08.015.
[24] TARNOCAI C, CANADELL J G, SCHUUR E A G, et al. Soil organic carbon pools in the northern circumpolar permafrost region [J]Glob Biogeochem Cycle, 2009, 23(2): GB2023. doi: 10.1029/2008GB003327.
[25] BATJES N H. Total carbon and nitrogen in the soils of the world [J]Eur J Soil Sci, 1996, 47(2): 151–163. doi: 10.1111/j.1365-2389.1996. tb01386.x
[26] JOBBAGY E G, JACKSON R B. The vertical distribution of soil organic carbon and its relation to climate and vegetation [J]Ecol Appl, 2000, 10(2): 423–436. doi: 10.1890/1051-0761(2000)010[0423:TVD OSO]2.0.CO;2.
[27] YU Z C. Northern peatland carbon stocks and dynamics: A review [J]Biogeosciences, 2012, 9(10): 4071–4085. doi: 10.5194/bg-9-4071- 2012.
[28] HUGELIUS G, STRAUSS J, ZUBRZYCKI S, et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps [J]Biogeosciences, 2014, 11(23): 6573–6593. doi: 10.5194/bg-11-6573-2014.
[29] DIXON R K, SOLOMON A M, BROWN S, et al. Carbon pools and flux of global forest ecosystems [J]Science, 1994, 263(5144): 185– 190. doi: 10.1126/science.263.5144.185.
[30] POST W M, KWON K C. Soil carbon sequestration and land-use change: Processes and potential [J]Glob Change Biol, 2000, 6(3): 317–327. doi: 10.1046/j.1365-2486.2000.00308.x.
[31] PAUL K I, POLGLASE P J, NYAKUENGAMA J G, et al. Change in soil carbon following afforestation [J]For Ecol Manage, 2002, 168 (1/2/3): 241–257. doi: 10.1016/S0378-1127(01)00740-X.
[32] DENG L, WANG K, TANG Z, et al. Soil organic carbon dynamics following natural vegetation restoration: Evidence from stable carbon isotopes (13C) [J]Agric, Ecosyst Environ, 2016, 221: 235–244. doi: 10.1016/j.agee.2016.01.048.
[33] DIOCHON A, KELLMAN L. Natural abundance measurements of13C indicate increased deep soil carbon mineralization after forest distur- bance [J]Geophys Res Lett, 2008, 35(14): L14402. doi: 10.1029/2008 GL034795.
[34] LANGE M, EISENHAUER N, SIERRA C A, et al. Plant diversity increases soil microbial activity and soil carbon storage [J]Nat Commun, 2015, 6: 6707. doi: 10.1038/ncomms7707.
[35] SCHARLEMANN J P W, TANNER E V J, HIEDERER R, et al. Global soil carbon: Understanding and managing the largest terrestrial carbon pool [J]Carbon Manag, 2014, 5(1): 81–91. doi: 10.4155/cmt.13.77.
[36] SAYER E J, HEARD M S, GRANT H K, et al. Soil carbon release enhanced by increased tropical forest litterfall [J]Nat Clim Change, 2011, 1(6): 304–307. doi: 10.1038/nclimate1190.
[37] LEFF J W, WIEDER W R, TAYLOR P G, et al. Experimental litterfall manipulation drives large and rapid changes in soil carbon cycling in a wet tropical forest [J]Glob Change Biol, 2012, 18(9): 2969–2979. doi: 10.1111/j.1365-2486.2012.02749.x.
[38] XU S, LIU L L, SAYER E J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments [J]Biogeosciences, 2013, 10(11): 7423–7433. doi: 10.5194/bg-10-7423-2013.
[39] LUO Z K, FENG W T, LUO Y Q, et al. Soil organic carbon dynamics jointly controlled by climate, carbon inputs, soil properties and soil carbon fractions [J]Glob Change Biol, 2017, 23(10): 4430–4439. doi: 10.1111/gcb.13767.
[40] HUANG Y H, LI Y L, XIAO Y, et al. Controls of litter quality on the carbon sink in soils through partitioning the products of decomposing litter in a forest succession series in south China [J]For Ecol Manage, 2011, 261(7): 1170–1177. doi: 10.1016/j.foreco.2010.12.030.
[41] SUMIYOSHI Y, CROW S E, LITTON C M, et al. Belowground impacts of perennial grass cultivation for sustainable biofuel feedstock production in the tropics [J]GCB Bioenergy, 2017, 9(4): 694–709. doi: 10.1111/gcbb.12379.
[42] RASSE D P, RUMPEL C, DIGNAC M F. Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation [J]Plant Soil, 2005, 269(1/2): 341–356. doi: 10.1007/s11104-004-0907-y.
[43] KEILUWEIT M, BOUGOURE J J, NICO P S, et al. Mineral protection of soil carbon counteracted by root exudates [J]Nat Clim Change, 2015, 5(6): 588–595. doi: 10.1038/nclimate2580.
[44] FOEREID B, BELLARBY J, MEIER-AUGENSTEIN W, et al. Does light exposure make plant litter more degradable? [J]Plant Soil, 2010, 333(1/2): 275–285. doi: 10.1007/s11104-010-0342-1.
[45] CONANT R T, RYAN M G, ÅGREN G I, et al. Temperature and soil organic matter decomposition rates-synthesis of current knowledge and a way forward [J]Glob Change Biol, 2011, 17(11): 3392–3404. doi: 10. 1111/j.1365-2486.2011.02496.x.
[46] HUANG W J, HALL S J. Elevated moisture stimulates carbon loss from mineral soils by releasing protected organic matter [J]Nat Commun, 2017, 8(1): 1774. doi: 10.1038/s41467-017-01998-z.
[47] WYNN J G, BIRD M I, VELLEN L, et al. Continental-scale measure- ment of the soil organic carbon pool with climatic, edaphic, and biotic controls [J]Glob Biogeochem Cycle, 2006, 20(1): GB1007. doi: 10. 1029/2005GB002576.
[48] RASMUSSEN C, HECKMAN K, WIEDER W R, et al. Beyond clay: Towards an improved set of variables for predicting soil organic matter content [J]Biogeochemistry, 2018, 137(3): 297–306. doi: 10.1007/s 10533-018-0424-3.
[49] KIRKBY C A, RICHARDSON A E, WADE L J, et al. Nutrient availability limits carbon sequestration in arable soils [J]Soil Biol Biochem, 2014, 68: 402–409. doi: 10.1016/j.soilbio.2013.09.032.
[50] AINSWORTH E A, LONG S P. What have we learned from 15 years of free-air CO2enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2[J]New Phytol, 2005, 165(2): 351–371. doi: 10.1111/j. 1469-8137.2004.01224.x.
[51] FANG X, ZHOU G Y, LI Y L, et al. Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest [J]Biol Fertil Soils, 2016, 52(3): 353–365. doi: 10.1007/s00374-015-1081-5.
[52] HE D, SHEN W J, EBERWEIN J, et al. Diversity and co-occurrence network of soil fungi are more responsive than those of bacteria to shifts in precipitation seasonality in a subtropical forest [J]Soil Biol Biochem, 2017, 115: 499–510. doi: 10.1016/j.soilbio.2017.09.023.
[53] MO J M, ZHANG W, ZHU W X, et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China [J]Glob Change Biol, 2008, 14(2): 403–412. doi: 10.1111/j.1365-2486.2007.01503.x.
[54] MAYOR J R, WRIGHT S J, SCHUUR E A G, et al. Stable nitrogen isotope patterns of trees and soils altered by long-term nitrogen and phosphorus addition to a lowland tropical rainforest [J]Biogeo- chemistry, 2014, 119(1/2/3): 293–306. doi: 10.1007/s10533-014-9966-1.
[55] van GROENIGEN K J, QI X, OSENBERG C W, et al. Faster decom- position under increased atmospheric CO2limits soil carbon storage [J]Science, 2014, 344(6183): 508–509. doi: 10.1126/science.1249534.
[56] LI Y Y, ZHOU G Y, HUANG W J, et al. Potential effects of warming on soil respiration and carbon sequestration in a subtropical forest [J]Plant Soil, 2016, 409(1/2): 247–257. doi: 10.1007/s11104-016-2966-2.
[57] PAN Q M, TIAN D S, NAEEM S, et al. Effects of functional diversity loss on ecosystem functions are influenced by compensation [J]Ecology, 2016, 97(9): 2293–2302. doi: 10.1002/ecy.1460.
[58] HUANG Y Y, CHEN Y X, CASTRO-IZAGUIRRE N, et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment [J]Science, 2018, 362(6410): 80–83. doi: 10.1126/science. aat6405.
[59] YAN J H, ZHANG W, WANG K Y, et al. Responses of CO2, N2O and CH4fluxes between atmosphere and forest soil to changes in multiple environmental conditions [J]Glob Change Biol, 2014, 20(1): 300–312. doi: 10.1111/gcb.12327.
[60] LIU J X, LIU S G, LI Y Y, et al. Warming effects on the decomposition of two litter species in model subtropical forests [J]Plant Soil, 2017, 420(1/2): 277–287. doi: 10.1007/s11104-017-3392-9.
[61] BERG B. Litter decomposition and organic matter turnover in northern forest soils [J]For Ecol Manage, 2000, 133(1/2): 13–22. doi: 10.1016/ S0378-1127(99)00294-7.
[62] SANDERMAN J, AMUNDSON R. A comparative study of dissolved organic carbon transport and stabilization in California forest and grassland soils [J]Biogeochemistry, 2008, 89(3): 309–327. doi: 10. 1007/s10533-008-9221-8.
[63] COTRUFO M F, WALLENSTEIN M D, BOOT C M, et al. The microbial efficiency-Matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? [J]Glob Change Biol, 2013, 19 (4): 988–995. doi: 10.1111/gcb.12113.
[64] COTRUFO M F, SOONG J L, HORTON A J, et al. Formation of soil organic matterbiochemical and physical pathways of litter mass loss [J]Nat Geosci, 2015, 8(10): 776–779. doi: 10.1038/ngeo2520.
[65] MANZONI S, ČAPEK P, MOOSHAMMER M, et al. Optimal metabolic regulation along resource stoichiometry gradients [J]Ecol Lett, 2017, 20(9): 1182–1191. doi: 10.1111/ele.12815.
[66] CLEMMENSEN K E, BAHR A, OVASKAINEN O, et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest [J]Science, 2013, 339(6127): 1615–1618. doi: 10.1126/science.1231923.
[67] BAIS H P, WEIR T L, PERRY L G, et al. The role of root exudates in rhizosphere interactions with plants and other organisms [J]Annu Rev Plant Biol, 2006, 57: 233–266. doi: 10.1146/annurev.arplant.57.032905. 105159.
[68] PHILLIPS R P, FINZI A C, BERNHARDT E S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2fumigation [J]Ecol Lett, 2011, 14(2): 187–194. doi: 10.1111/j.1461-0248.2010.01570.x.
[69] LAJTHA K, TOWNSEND K L, KRAMER M G, et al. Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems [J]Biogeo- chemistry, 2014, 119(1/2/3): 341–360. doi: 10.1007/s10533-014-9970-5.
[70] ROVIRA P, VALLEJO V R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach [J]Geoderma, 2002, 107(1/2): 109–141. doi: 10.1016/S0016-7061(01)00143-4.
[71] KNORR W, PRENTICE I C, HOUSE J I, et al. Long-term sensitivity of soil carbon turnover to warming [J]Nature, 2005, 433(7023): 298– 301. doi: 10.1038/nature03226.
[72] SOLLINS P, HOMANN P, CALDWELL B A. Stabilization and desta- bilization of soil organic matter: Mechanisms and controls [J]Geo- derma, 1996, 74(1/2): 65–105. doi: 10.1016/S0016-7061(96)00036-5.
[73] CHEN X M, LIU J X, DENG Q, et al. Effects of elevated CO2and nitrogen addition on soil organic carbon fractions in a subtropical forest [J]Plant Soil, 2012, 357(1/2): 25–34. doi: 10.1007/s11104-012-1145-3.
[74] CHENG X L, YANG Y H, LI M, et al. The impact of agricultural land use changes on soil organic carbon dynamics in the Danjiangkou Reservoir area of China [J]Plant Soil, 2013, 366(1/2): 415–424. doi: 10.1007/s11104-012-1446-6.
[75] BRONICK C J, LAL R. Soil structure and management: A review [J]Geoderma, 2005, 124(1/2): 3–22. doi: 10.1016/j.geoderma.2004.03.005.
[76] RABOT E, WIESMEIER M, SCHLÜTER S, et al. Soil structure as an indicator of soil functions: A review [J]Geoderma, 2018, 314: 122– 137. doi: 10.1016/j.geoderma.2017.11.009.
[77] SIX J, PAUSTIAN K, ELLIOTT E T, et al. Soil structure and organic matter: I. Distribution of aggregate-size classes and aggregate-asso- ciated carbon [J]Soil Sci Soc Amer J, 2000, 64(2): 681–689. doi: 10. 2136/sssaj2000.642681x.
[78] TANG X Y, LIU S G, LIU J X, et al. Effects of vegetation restoration and slope positions on soil aggregation and soil carbon accumulation on heavily eroded tropical land of Southern China [J]J Soil Sediment, 2010, 10(3): 505–513. doi: 10.1007/s11368-009-0122-9.
[79] LÜTZOW M V, KÖGEL-KNABNER I, EKSCHMITT K, et al. Stabili- zation of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions: A review [J]Eur J Soil Sci, 2006, 57(4): 426–445. doi: 10.1111/j.1365-2389.2006.00809.x.
[80] ABIVEN S, MENASSERI S, CHENU C. The effects of organic inputs over time on soil aggregate stability: A literature analysis [J]Soil Biol Biochem, 2009, 41(1): 1–12. doi: 10.1016/j.soilbio.2008.09.015.
[81] KARAMI A, HOMAEE M, AFZALINIA S, et al. Organic resource management: Impacts on soil aggregate stability and other soil physico- chemical properties [J]Agric Ecosyst Environ, 2012, 148: 22–28. doi: 10.1016/j.agee.2011.10.021.
[82] SCHUUR E A G, CHADWICK O A, MATSON P A. Carbon cycling and soil carbon storage in mesic to wet Hawaiian montane forests [J]Ecology, 2001, 82(11): 3182–3196. doi: 10.1890/0012-9658(2001)082 [3182:CCASCS]2.0.CO;2.
[83] PRESCOTT C E. Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? [J]Biogeo- chemistry, 2010, 101(1/2/3): 133–149. doi: 10.1007/s10533-010-9439-0.
[84] MAKKONEN M, BERG M P, HANDA I T, et al. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient [J]Ecol Lett, 2012, 15(9): 1033–1041. doi: 10.1111/j.1461-0248.2012.01826.x.
[85] ZHOU G Y, GUAN L L, WEI X H, et al. Factors influencing leaf litter decomposition: An intersite decomposition experiment across China [J]Plant Soil, 2008, 311(1/2): 61–72. doi: 10.1007/s11104-008-9658-5.
[86] ZHOU T, SHI P J, HUI D F, et al. Global pattern of temperature sensi- tivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback [J]J Geophys Res-Biogeo, 2009, 114(G2): G02016. doi: 10.1029/2008JG000850.
[87] LU X K, MAO Q G, GILLIAM F S, et al. Nitrogen deposition contri- butes to soil acidification in tropical ecosystems [J]Glob Change Biol, 2014, 20(12): 3790–3801. doi: 10.1111/gcb.12665.
[88] HOBBIE S E. Nitrogen effects on decomposition: A five-year experi- ment in eight temperate sites [J]Ecology, 2008, 89(9): 2633–2644. doi: 10.1890/07-1119.1.
[89] RIGGS C E, HOBBIE S E. Mechanisms driving the soil organic matter decomposition response to nitrogen enrichment in grassland soils [J]Soil Biol Biochem, 2016, 99: 54–65. doi: 10.1016/j.soilbio.2016.04.023.
[90] ZAK D R, HOLMES W E, WHITE D C, et al. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? [J]Ecology, 2003, 84(8): 2042–2050. doi: 10.1890/02-0433.
[91] BATJES N H, SOMBROEK W G. Possibilities for carbon sequestration in tropical and subtropical soils [J]Glob Change Biol, 1997, 3(2): 161–173. doi: 10.1046/j.1365-2486.1997.00062.x.
[92] de DEYN G B, CORNELISSEN J H C, BARDGETT R D. Plant functional traits and soil carbon sequestration in contrasting biomes [J]Ecol Lett, 2008, 11(5): 516–531. doi: 10.1111/j.1461-0248.2008.01164.x.
[93] CARVALHAIS N, FORKEL M, KHOMIK M, et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems [J]Nature, 2014, 514(7521): 213–217. doi: 10.1038/nature13731.
[94] DOETTERL S, STEVENS A, SIX J, et al. Soil carbon storage controlled by interactions between geochemistry and climate [J]Nat Geosci, 2015, 8(10): 780–783. doi: 10.1038/ngeo2516.
[95] O’ROURKE S M, ANGERS D A, HOLDEN N M, et al. Soil organic carbon across scales [J]Glob Change Biol, 2015, 21(10): 3561–3574. doi: 10.1111/gcb.12959.
[96] HARDEN J W, HUGELIUS G, AHLSTRÖM A, et al. Networking our science to characterize the state, vulnerabilities, and management opportunities of soil organic matter [J]Glob Change Biol, 2018, 24(2): e705-e718. doi: 10.1111/gcb.13896.
[97] KING J S, KUBISKE M E, PREGITZER K S, et al. Tropospheric O3compromises net primary production in young stands of trembling aspen, paper birch and sugar maple in response to elevated atmospheric CO2[J]New Phytol, 2005, 168(3): 623–636. doi: 10.1111/j.1469-8137. 2005.01557.x.
[98] TALHELM A F, PREGITZER K S, ZAK D R. Species-specific responses to atmospheric carbon dioxide and tropospheric ozone mediate changes in soil carbon [J]Ecol Lett, 2009, 12(11): 1219–1228. doi: 10.1111/j. 1461-0248.2009.01380.x.
[99] BAER S G, BLAIR J M. Grassland establishment under varying resource availability: A test of positive and negative feedback [J]Ecology, 2008, 89(7): 1859–1871. doi: 10.1890/07-0417.1.
[100]FORNARA D A, TILMAN D. Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition [J]Ecology, 2012, 93(9): 2030–2036. doi: 10.1890/12-0292.1.
[101]JOHNSON D W, CURTIS P S. Effects of forest management on soil C and N storage: Meta analysis [J]For Ecol Manage, 2001, 140(2/3): 227–238. doi: 10.1016/S0378-1127(00)00282-6.
[102]WANG G S, HUANG W J, MAYES M A, et al. Soil moisture drives microbial controls on carbon decomposition in two subtropical forests [J]Soil Biol Biochem, 2019, 130: 185–194. doi: 10.1016/j.soilbio. 2018.12.017.
[103]ZHOU G Y, GUAN L L, WEI X H, et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon ever- green broadleaved forests in Guangdong, China [J]Plant Ecol, 2007, 188(1): 77–89. doi: 10.1007/s11258-006-9149-9.
[104]ZHOU G Y, PENG C H, LI Y L, et al. A climate change-induced threat to the ecological resilience of a subtropical monsoon evergreen broad- leaved forest in southern China [J]Glob Change Biol, 2013, 19(4): 1197–1210. doi: 10.1111/gcb.12128.
[105]HUANG W J, ZHOU G Y, LIU J X. Nitrogen and phosphorus status and their influence on aboveground production under increasing nitrogen deposition in three successional forests [J]Acta Oecol, 2012, 44: 20– 27. doi: 10.1016/j.actao.2011.06.005.
[106]LIU L, GUNDERSEN P, ZHANG T, et al. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China [J]Soil Biol Biochem, 2012, 44(1): 31–38. doi: 10.1016/j.soilbio.2011.08.017.
[107]LIANG G H, LIU X Z, CHEN X M, et al. Response of soil respiration to acid rain in forests of different maturity in southern China [J]PLoS One, 2013, 8(4): e62207. doi: 10.1371/journal.pone.0062207.
[108]ZHOU G Y, WEI X H, WU Y P, et al. Quantifying the hydrological responses to climate change in an intact forested small watershed in southern China [J]Glob Change Biol, 2011, 17(12): 3736–3746. doi: 10.1111/j.1365-2486.2011.02499.x.
Exploration History of Soil Organic Carbon Formation Mechanisms
ZHOU Guo-yi1, XIONG Xin1,2
(1. South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China; 2. University of Chinese Academy of Sciences,Beijing 100049, China)
Soil organic carbon (SOC) is an important asset of ecosystems and plays a key role in the global carbon balance. Since the 2015 Paris Climate Conference, promoting SOC accumulation in terrestrial ecosystems has received special attention and is considered to be the most important land-based action of mitigating the rising atmospheric CO2concentration. Starting from serving this goal, this paper reviews and summarizes the history of the global exploration of SOC formation mechanisms over the recent decades. Including the global distribution law of SOC, the SOC cycle processes and the corresponding physical, chemical and biological mechanisms at scales that are smaller than plot, and the mechanisms of soil carbon sequestration at scales that are larger than plot. Finally, an example of the SOC accumulation mechanisms of mature forest is given. So, the exploration process of the SOC formation mechanisms is actually the process of seeking theoretical guidance for promoting soil carbon sequestration.
SOC; Formation; Sequestration; Mechanism
10.11926/jtsb.4094
2019–05–16
2019–07–05
中国科学院前沿科学重点研究项目(DYZDJ-SSW-DQC003);国家自然科学基金项目(41430529, 41573077)资助
This work was supported by the Key Research Projects in Frontier Science of Chinese Academy of Sciences (Grant No. DYZDJ-SSW-DQC003), and the National Natural Science Foundation of China (Grant No. 41430529, 41573077).
周国逸(1963~ ),男,研究员,博士生导师,研究方向为生态系统生态学和生态水文学。E-mail: gyzhou@scib.ac.cn

