模拟大气氮沉降对中国森林生态系统影响的研究进展
鲁显楷,莫江明,张炜,毛庆功,刘荣臻,2,王聪,2,王森浩,2,郑棉海,Mori Taiki,毛晋花,2,张勇群,2,王玉芳,2,黄娟
模拟大气氮沉降对中国森林生态系统影响的研究进展
鲁显楷1,莫江明1,张炜1,毛庆功1,刘荣臻1,2,王聪1,2,王森浩1,2,郑棉海1,Mori Taiki1,毛晋花1,2,张勇群1,2,王玉芳1,2,黄娟1
(1. 中国科学院华南植物园, 退化生态系统植被恢复与管理重点实验室, 广东省应用植物学重点实验室, 广州 510650; 2. 中国科学院大学, 北京 100049)
人类活动加剧了活性氮的生产和排放,并导致氮沉降日益增加并全球化。目前,人类活动对全球氮循环的干扰已经超出了地球系统安全运行的界限。中国已成为全球氮沉降的高发区域,高氮沉降已经威胁到生态系统的健康和安全,并成为生态文明建设过程中亟待理清和解决的热点问题。对国际上和中国森林生态系统模拟氮沉降研究的概况进行了综述,并从生物学和非生物学两大过程重点阐述模拟氮沉降增加对中国主要森林生态系统影响的研究进展。中国自2000年以后才开始重视大气氮沉降产生的生态环境问题,中国科学院华南植物园在国内森林生态系统模拟氮沉降试验研究上做出了开创性的贡献。模拟氮沉降研究表明,持续高氮输入将会显著改变森林生态系统的结构和功能,并威胁生态系统的健康发展,特别是处于氮沉降热点区域的中国中南部。森林生态系统的氮沉降效应依赖于系统的氮状态、土地利用历史、气候特征、林型和林龄等。最后,对未来的研究提出了一些建议,包括加强长期跟踪研究和不同气候带站点之间的联网研究,特别是在森林生态系统对长期氮沉降响应与适应的过程机制、地下碳氮吸存潜力研究、以及与其他全球变化因子的耦合研究等方面,以期为森林生态系统的可持续发展提供理论基础和管理依据。
氮沉降; 全球变化; 森林生态系统; 氮饱和; 氮限制; 氮素生物地球化学循环; 生物多样性; 碳吸存
1 大气氮沉降及其趋势
氮素是一种重要的生命元素,是蛋白质、维生素和核酸(DNA)的重要组成部分。氮素虽然无处不在,但主要以惰性状态(N2)存在,不能被生物直接利用,所以也是陆地生态系统净初级生产力最为关键的限制性营养元素[1–2]。然而人类活动改变了氮素的存在形态,即具有生物活性的氮化合物(活性氮)的产生和排放显著增加。活性氮主要包括还原态NHx (包括NH3、RNH2和NH4+)和氧化态NOx两种化学形式。活性氮排放增加诱发了大气氮沉降格局的改变。工业化革命(1860年)前,活性氮主要来自雷电作用和生物固氮,大气氮沉降量也非常低, 如陆地系统氮沉降量普遍在0.5~1 kg N hm–2a–1,最高不超过10 kg N hm–2a–1。然而,自20世纪中叶以来,随着化石燃料的燃烧、化学氮肥的生产和使用和畜牧业的迅猛发展等人类活动向大气中排放的活性氮化合物激增,大气氮沉降也呈现出迅猛增加的趋势,并达到工业化革命前的两倍以上,并且愈来愈呈现出全球化趋势[3]。目前全球氮沉降的分布中心正在发生变化:从欧美等发达国家转向发展中国家,从温带区域扩展到热带亚热带区域。
目前中国已成为世界上活性氮生产和排放量最大的国家[4]。近30年来(1980-2010),中国人为源活性氮产生量增加了近3倍(从1.68×107t增加到4.82×107t),其中主要增长来自于化肥和工业用氮(从1.14×107t增加到3.71×107t)。如此高的活性氮排放势必导致陆地和水域大气氮沉降量的急剧增加[5–7]。Lü等[5]对中国的网络监测数据研究表明,中国中南部是氮沉降的高发区域,最高达到65 kg N hm–2a–1。如今,华南大部分地区的大气湿沉降已超过30 kg N hm–2a–1[8],远高于严重威胁欧美森林生态系统健康和安全的氮沉降临界负荷值[9]。Yu等[10]通过构建1980–2015年间的中国区域大气干沉降和湿沉降全组分动态变化数据集,提出了中国大气氮沉降总量和沉降模式转型变化的三个重要特征:(1)近年来虽然中国区域的NO3–氮沉降还在持续增加,但NH4+湿沉降显著降低,使全国氮沉降总量由快速增长转型为趋稳状态;(2)大气干沉降增加导致了干湿沉降比的变化,由以湿沉降为主逐步转型为湿沉降与干沉降并重; (3)大气沉降中的NH4+/NO3–比减小,NO3–氮沉降贡献在持续增加,NH4+氮沉降的贡献则降低,逐渐由以NH4+沉降为主的氮沉降模式转换为NH4+和NO3–氮沉降贡献并重的新模式。
2 生态系统的氮沉降效应
目前,人类活动干扰下的大气氮沉降已成为全球氮素生物地球化学循环的一个重要组成部分,也是全球变化的重要驱动因子。大气氮沉降增加改变了生态系统氮循环模式和进程,加剧了氮循环的速率,并通过耦合效应驱动其他元素循环(如碳、磷等)发生改变[11–13]。
作为营养源和酸源,大气氮沉降急剧增加将会威胁到生态系统的健康和安全,对农田、森林、草地等陆地生态系统以及湖泊和海洋等水域生态系统都会造成影响。大气氮沉降的负面效应主要有:导致生态系统土壤酸化、养分流失,造成水体污染并富营养化,改变生态系统元素计量化学,降低生物多样性并改变物种组成,降低生态系统生产力和削弱生态系统稳定性,加剧温室气体(N2O)排放等[3,9,14–19]。大气氮沉降的持续增加,最终会导致生态系统氮饱和(nitrogen saturation),即氮的输入超过了生物对氮素的需求,从而加剧上述生态学效应,并影响系统的健康发展[20]。此外,氮沉降还会与其他全球变化驱动因子(如CO2浓度升高、全球变暖、O3浓度增加等)相耦合,并引发一系列的连锁效应(cascade effect),相关的生态后果还有待于未来深入研究。目前,人类活动对全球氮循环的干扰已经超出了地球系统安全运行的界限[21]。因此,氮素在大气-土壤-植物-水循环的生物地球化学过程成为科学家、公众和决策者关注的主要环境问题之一。
森林是陆地生态系统的主体。中国陆地系统的气候复杂性和空间的变异性产生了丰富森林生态系统类型,如各类针叶林、针叶阔叶混交林、落叶阔叶林、常绿阔叶林和热带雨林等。这些森林在维护生物多样性和生态平衡、并为社会发展提供各种供给服务中扮演着重要角色。在氮沉降全球化的背景下,本综述主要关注中国森林生态系统对氮沉降增加的响应及其机制。
3 国际上森林生态系统模拟氮沉降研究的历史概况
在国际上,氮沉降对森林生态系统结构和功能影响的研究始于20世纪80年代,到90年代发展为定位研究,并逐渐形成网络,研究内容也不断细化和深化。这些研究主要集中在欧洲和北美,特别是经历高氮沉降输入的区域。欧共体委员会于20世纪80年代末资助了两大研究项目,即氮饱和试验项目(Nitrogen saturation experiments, NITREX)和欧洲森林生态系统试验控制项目(Experimental manipulation of forest ecosystems in Europe, EXMA)[22]。其中NITREX项目在7个国家的8个试验点进行,主要研究增加或减少大气氮沉降对欧洲森林生态系统,特别是针叶林生态系统的影响。而EXMAN项目也涉及4个国家的6个试验站点,通过实验改变周边大气氮沉降的化学组成和数量进而探讨森林生态系统的响应。在美国,长期试验研究站点有马萨诸塞州的哈佛森林长期氮素增加试验(Chronic nitrogen amendment in Harvard forest)和缅因州Bear brook集水区和Mt. Ascutney森林的模拟氮沉降试验[20,23]。这些研究对理解温带和北方森林生态系统对大气氮沉降的响应及其机理有着重要贡献。
截至2008年,全球范围内有关森林生态系统氮沉降研究的站点共计31个,绝大部分在温带区域,热带区域仅有2个:中美洲巴拿马和中国广东鼎湖山(图1)[24]。
4 中国森林生态系统模拟氮沉降研究概况
中国氮沉降问题起源于20世纪80年代初期的酸沉降监测研究[25],但自2000年后才逐步受到的重视。为了更好地理解和预测大气氮沉降对中国森林生态系统的影响,中国科学院华南植物园(以下简称华南园)莫江明研究团队于2002年在广东鼎湖山国家级自然保护区确立了我国首批森林生态系统长期氮沉降研究样地(图1);该样地包含了热带亚热带区域代表性的3种森林类型:季风常绿阔叶林、针叶阔叶混交林和马尾松针叶林[13,18,26–27]。此后, 从南到北涌现出更多的模拟氮沉降研究样地。2010年北京大学牵头建立了中国森林生态系统养分添加试验网络(Nutrient enrichment experiments in China’s forests project, NEECF),拥有从南(海南尖峰岭的热带山地雨林)到北(内蒙古根河的寒温带针叶林)分布在7个研究站点的10个森林类型[28]。到目前为止,全国范围森林生态系统氮沉降研究样地共有25个,基本上涵盖了中国的主要森林类型(图2)。
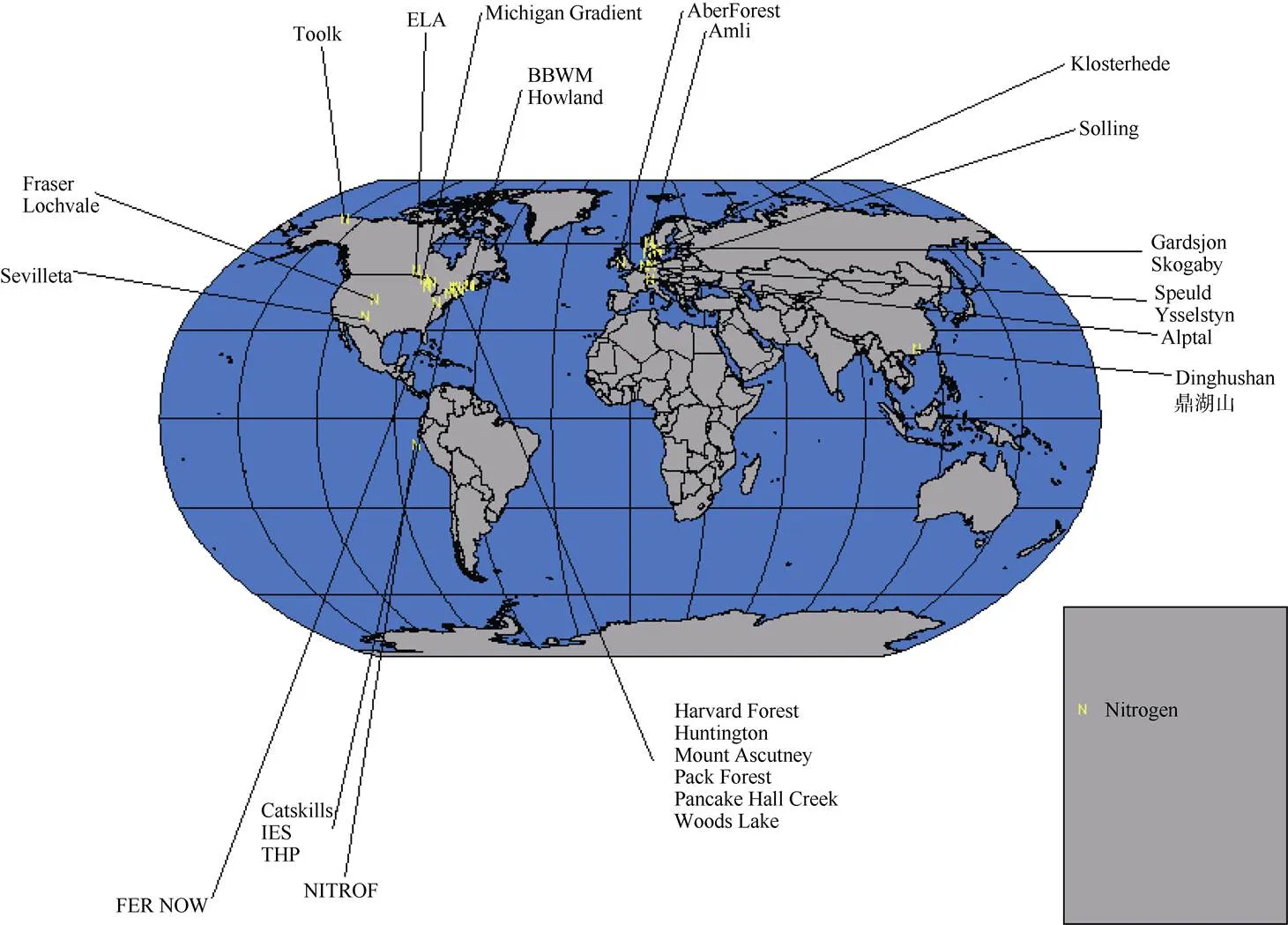
图1 氮沉降试验研究站点国际分布图[24]
模拟氮沉降研究的技术方法主要体现在4个方面:氮沉降的种类、数量、施加频率和方式。氮沉降的种类主要有4种类型:NH4NO3、NaNO3、(NH4)2SO4和NH4Cl,其中NH4NO3溶液是应用最为广泛的施加方式,硝态氮(NaNO3)和铵态氮(NH4Cl)常用来评估氧化态和还原态氮沉降带来的影响。施氮数量通常在当地背景氮沉降基础上进行不同梯度的倍增处理,鼎湖山样地的氮沉降处理水平分别为0、50、100和150 kg N hm–2a–1[17]。施氮频率通常为每月1次或每两月1次,也有少数为一季度1次或者半年1次。施氮方式主要有林下和林冠施加两种,目前绝大多数为林下施氮。2013年,华南园率先在国内(广东石门台和河南鸡公山)开展了林冠模拟氮沉降试验,并与传统的林下施氮进行对比研究,以期更为真实地反映大气氮沉降对森林生态系统的影响、以及氮沉降与林冠的交互作用[35]。
中国自2000年以后才开始重视大气氮沉降产生的生态环境问题。截至2018年12月,森林生态系统依托模拟氮沉降研究样地发表的研究论文共计720篇(图3)。华南园在模拟氮沉降试验研究上做出了开创性的贡献,2004-2005年以华南园为第一单位发表的研究论文占国内发表论文总数的100%。随着国内科学家对氮沉降问题关注度的增加,发表的研究论文也越来越多,特别是自2010年以后,同期来自华南园的论文发表数量相对稳定,但质量显著提升。如今,中国社会和经济高速发展诱发的高氮沉降已经威胁到了生态系统的健康和安全,并成为生态文明建设过程中亟待理清和解决的热点问题。
5 模拟氮沉降增加对中国森林生态系统的影响
5.1 氮沉降对土壤酸化的影响
目前,随着中国氮沉降量的增加和对硫排放的控制,人为氮源对酸沉降的贡献越来越大[29,36]。据估计,在我国华南林区由氮沉降引起的土壤酸化已占沉降因素的36%以上[37]。氮沉降主要通过H+输入、NH4+硝化和NO3–的淋溶流失、植物吸收等直接或间接过程增加土壤酸度。理论上,1 mol NH4+通过微生物的硝化作用转化成NO3–将释放2 mol H+[38]。由于H+与土壤胶体具有更强的亲和性(相比于其他盐基离子),因此氮沉降诱导的H+增加会替代胶体颗粒上的盐基离子(Ca2+和Mg2+等),并使它们随NO3+的淋溶而流失。当氮沉降持续增加,土壤pH进一步降低,导致Al3+、Fe3+或Mn2+等离子的移动性增加,威胁生物生长。
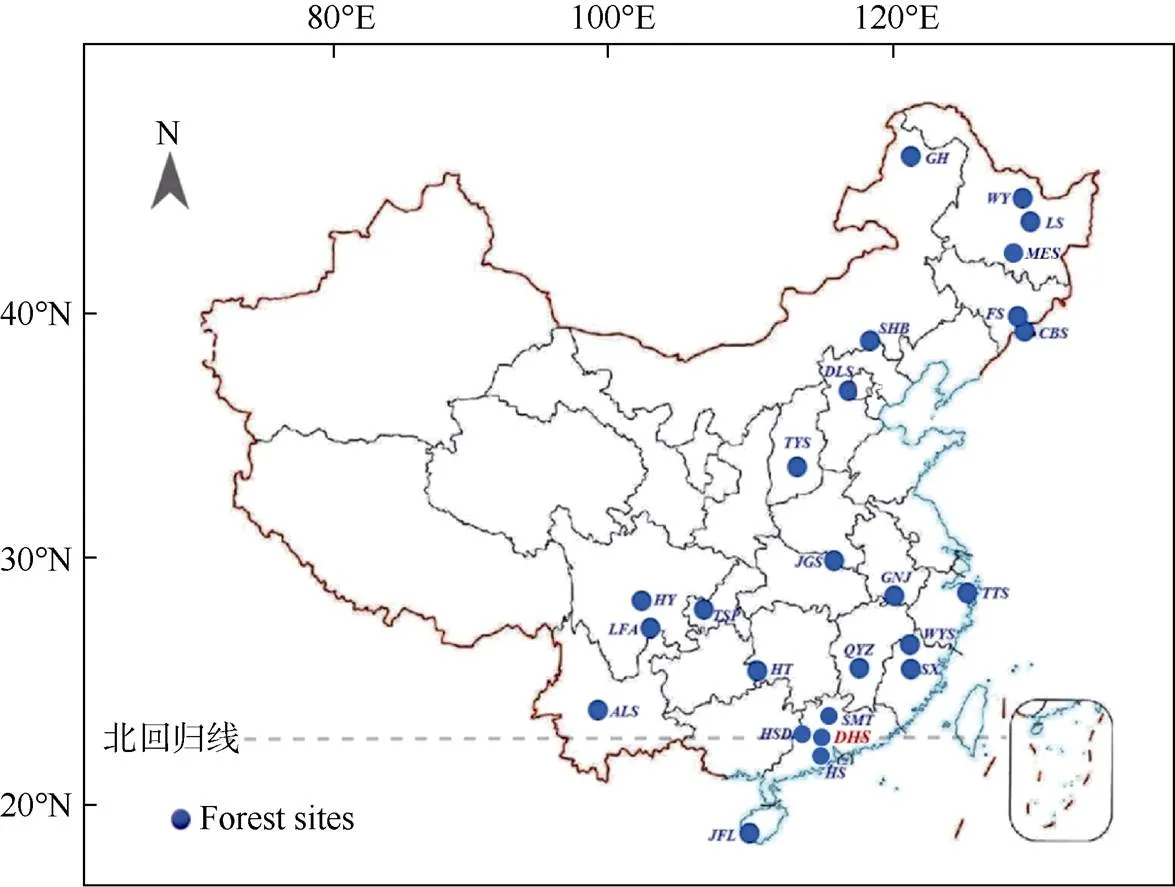
图2 中国森林生态系统模拟氮沉降研究站点分布[13,29–34]。GH: 根河; WY: 五营; LS: 凉水; MES: 帽儿山; FS: 抚松; CBS: 长白山; SHB: 塞罕坝; DLS:东灵山; TYS: 太岳山; JGS: 鸡公山; GNJ: 牯牛降; TTS: 天童山; WYS: 武夷山; SX: 沙县; QYZ: 千烟洲; SMT: 石门台; HSD: 黑石顶; DHS: 鼎湖山; HS: 鹤山; JFL: 尖峰岭; HT: 会同; ALS: 哀牢山; TSP: 铁山坪; HY: 洪雅; LFA: 凉风坳。
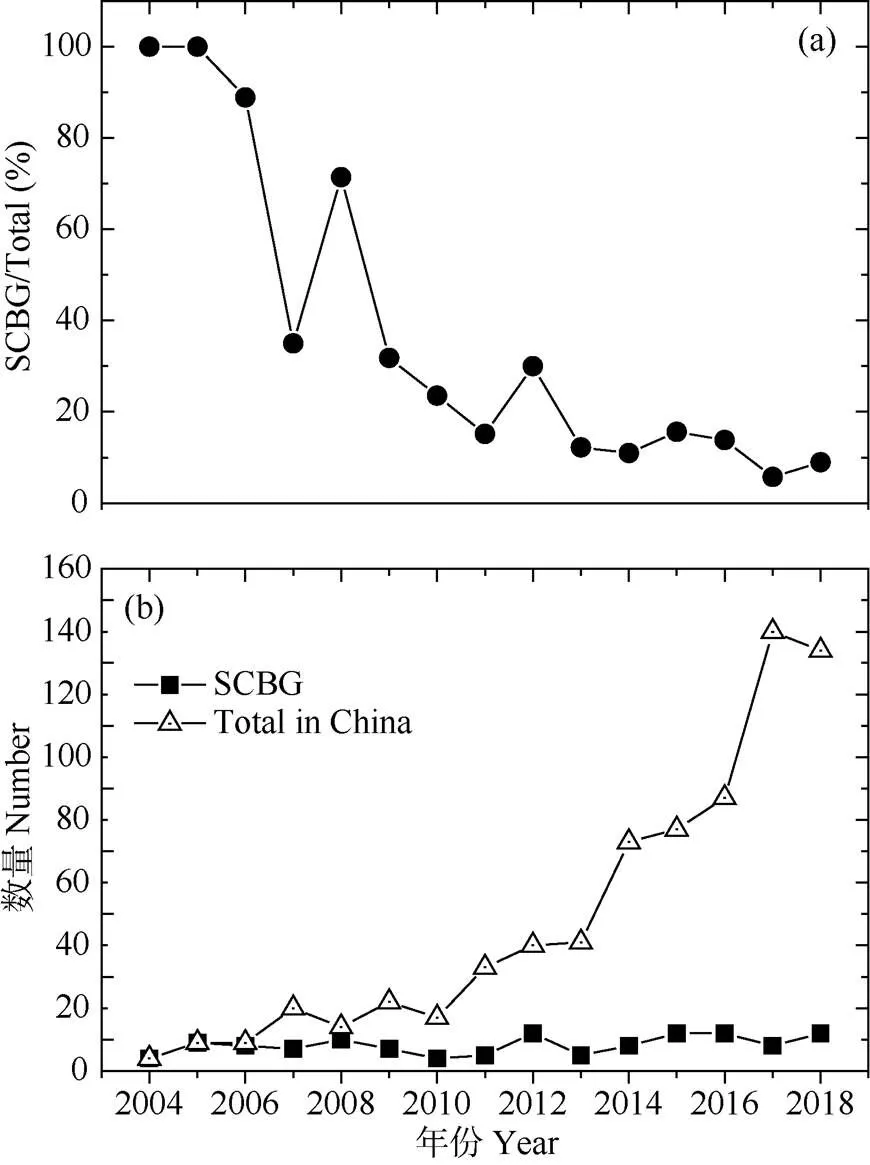
图3 模拟氮沉降试验研究中以华南植物园(SCBG)为第一单位发表的论文在全国的比重(a)及其绝对数量(b)
中国温带和亚热带森林均面临着氮沉降升高引起的土壤酸化问题[39]。目前仅有少数研究报道了氮沉降导致中国温带森林土壤酸化,可能是该区域土壤pH本底值较高,土壤缓冲能力较强[15]。然而,热带亚热带森林生态系统土壤缓冲能力普遍较低,且对酸源沉降更加敏感。在珠三角地区,土壤pH值沿着农村到城市的氮沉降增加梯度而降低[40]。在南亚热带广东鼎湖山自然保护区,Lu等[41]报道长期氮添加显著降低了原始森林土壤的缓冲能力并加剧土壤酸化,但是次生林和人工林响应不明显;不同土地利用历史导致的氮状态不同是响应差异的重要原因, “富氮的”成熟林更容易发生氮素流失和土壤酸化。过量氮输入将会显著降低土壤中有效阳离子(如Ca2+和Mg2+)含量,进而导致养分失衡[13,42–43]。考虑到这些热带亚热带生态系统大多已经处于铝化合物缓冲阶段,在未来氮沉降增加背景下应更关注Ca2+和Mg2+的缺乏而非铝毒效应[18]。
5.2 氮沉降对植物化学元素的影响
大气氮沉降会直接作用于植物,或者通过改变土壤化学元素的组成来间接影响植物元素平衡[44–45],并最终导致生态系统水平上的元素失衡[17,29]。关于氮沉降对森林植物元素化学影响的研究主要集中于地上部分,对于植物地下部分的研究十分匮乏。
关于地上部分,国内最先见于李德军的研究[46],他总结前人研究认为,氮沉降会造成植物体内养分元素比例的失衡;对南亚热带3种乔木幼苗的研究表明,氮施肥增加了植物N含量,增加了N与P、K、Ca、Mg和Mn等元素的比值。除N/P比外, N/K比也可以作为植物对氮沉降响应的敏感指标[47]。对温带森林的研究表明,氮素输入普遍增加了植物N含量[48],进一步验证了温带区域的氮素限制性[49]。但也有研究报道5年的N添加对温带落叶松()各器官N、P、K、Ca和Mg含量的影响不明显[50]。最近的整合分析(meta-analysis)表明,在中国随着氮沉降的增加,木本和草本植物的叶氮含量增加,但是叶磷绝对含量变化不显著[51–52]。对元素比值的整合分析表明,氮沉降普遍降低了中国各生态系统植物的C/N比,增加了N/P比,但是对C/P的效应不显著,根本原因是氮沉降增加了植物内氮含量[53–55]。然而,Lu等[13]在“富氮”的热带成熟林的研究表明,为期10年的高氮输入并没有显著改变乔木植物叶片N、K、Ca、Mg和Al等元素的含量,这主要是由于该生态系统已经达到氮饱和以及植物产生的自我适应性调整;并由此提出了植物适应性新假说:即“富氮”生态系统植物可以通过提升自身蒸腾能力适应高氮沉降来维持养分平衡。这更新了有关氮沉降增加植物叶氮含量的普遍观点,因此在未来相关研究中应当充分考虑到生态系统的氮状态和植物的适应性。
关于地下部分,主要是针对植物细根,但研究数量远少于对植物叶片的研究,且研究结论不统一。Mo等[56]对热带次生林的研究表明,氮添加增加了细根氮素含量,但对磷含量没有影响,进而导致了N/P比降低。Zhu等[57]在南亚热带成熟林、混交林和马尾松林的研究表明,为期5年的氮添加并没有改变细根氮和磷的含量。整合分析进一步表明,全球尺度上氮沉降显著增加了植物总根的生物量(20.2%),但是减少了细根的生物量(–12.8%),显著增加了植物细根的N含量(17.6%),降低了细根的C/N比,但事实没有显著改变细根的C含量[58]。
可以看出,不同生态系统植物元素化学对氮沉降的响应不同,主要原因有两个方面:首先,植物在长期生存进化过程中形成了不同的系统发育特征,导致其对于氮沉降响应的敏感程度因种而异, 此外,植物自我调整的适应性(acclimation),也会改变氮沉降对植物化学元素影响的方向[13]。其次,影响植物发育的外部环境(如气候、土壤发育特点等)会进一步决定植物的响应方式。中国北方温带地区生态系统一般认为氮限制,而南方热带亚热带生态系统相对更加富氮,常受磷或其他阳离子限制,这些特点决定了森林植物对氮沉降响应方式可能存在差异。
5.3 氮沉降对植物生长的影响
从全球尺度来看,氮添加对不同植物物种生长通常表现出刺激效应[59]。不同生态系统和功能类群对氮沉降响应的敏感程度不同,次生林树木的生长一般比原始林敏感[60],而草本植物比木本植物敏感[59,61]。同时,氮沉降对森林净初级生产力(NPP)或植物生长的影响也因氮沉降量而异,具有生态学中普遍的非线性关系[62]。中国东北地区森林中, 较低的氮添加(25 kg N hm–2a–1)导致最大的净生产力, 随着氮添加的增加(50 kg N hm–2a–1)正面效应降低,氮添加量最大时(75 kg N hm–2a–1)正面效应消失[63]。
目前,树木径向生长对氮沉降的响应在中国仅有少量研究报道。树木的生长响应因个体大小(如胸径和树高)或生长阶段而不同[64–66]。刘修元等[64]研究了模拟氮沉降对落叶松原始林树木胸径生长的影响,认为不同高度的树木对氮添加的响应有很大差异, 较低树木(树高<16.5 m)的生长对氮添加无显著响应,而较高(树高>16.5 m)的树木在中氮和高氮(50和100 kg N hm–2a–1)处理下胸径生长显著加速,但随着树木高度的进一步增加, 这种加速作用明显下降。在亚热带阔叶林,Tian等[65]报道3年的氮添加(50和100 kg N hm–2a–1)使甜栲()幼树和林下幼苗生长显著降低,但是大树的生长没有受到影响。7年的氮添加(40 kg N hm–2a–1)导致重庆马尾松()林土壤酸化和氮饱和,并使马尾松生长显著下降[42]。从长远看,氮沉降对森林群落树木结构的改变可能会引起物种组成和碳吸存能力的改变。
5.4 氮沉降对植物多样性的影响
大气氮沉降升高已成为全球多样性丧失的第三大驱动因素[67]。然而,中国仅有少量相关研究报道。与林下层植物相比,乔木植物对环境因素的响应较慢,所以已有研究主要集中在林下层植物多样性的响应上。长期氮沉降会改变林下层物种组成, 但改变的程度依赖于森林类型、功能类群以及氮状态等因素。在温带地区,Du[68]报道3年的氮添加试验对北方原始森林林下层植物的物种丰富度没有影响,但显著增加了禾草类植物的盖度,并降低了矮小灌木植物的盖度。在热带/亚热带原始林,Lu等[17]报道5年的高氮添加(>100 kg N hm–2a–1)显著降低了林下植物多样性(丰富度和多度),并首次提出负面效应主要与土壤酸化机制有关而不是传统上的竞争机制。Wu等[69]研究表明8年的高氮添加(>120 kg N hm–2a–1)可能通过降低土壤pH值和菌根真菌丰度来削弱亚热带森林林下植物丰富度。Huang等[42]通过对比NH4NO3和NaNO3处理在亚热带马尾松林的效应,认为林下主要物种多度的降低可能是氮饱和与土壤酸化共同作用的结果。与模拟氮沉降控制试验的结果基本一致,Huang等[70]报道在广州城乡氮沉降梯度上(30.1~43.3 kg N hm–2a–1),成熟林林下草本层植物多样性与氮沉降量呈负相关, 与土壤中的有效Ca2+和K+浓度呈正相关。此外,氮沉降效应也与土地利用方式有关,与原始林相比, 人工林或次生林中植物多样性对氮沉降的响应相对不敏感[71–72]。
5.5 氮沉降对土壤微生物和酶活性的影响
土壤微生物群落在森林生态系统中扮演着核心角色,其生长代谢能调控森林生态系统的物质循环和能量流动,影响土壤养分状态、有机质的数量及稳定性和土壤温室气体的排放等。氮沉降加剧会对土壤微生物群落的数量、组成和功能活性产生影响。
5.5.1 土壤微生物群落
野外试验表明,氮添加通常会减少微生物的生物量[30,51]。氮添加引起的土壤pH降低、可利用碳降低、土壤溶液毒害等是导致微生物量减少的主要原因[73–75]。但也有研究报道氮添加对土壤微生物量没有显著影响[76]。
氮添加对微生物量的影响还与添加量、氮添加类型、季节、微生物种类及林型有关。低氮添加(50 kg N hm–2a–1)没有显著改变温带油松()林土壤微生物生物量,但中氮和高氮添加(>100 kg N hm–2a–1)则显著降低了微生物生物量[77]。氨态氮添加(20~80 kg N hm–2a–1)增加了亚热带的冷杉()种植园细菌生物量,但硝态氮添加(20~80 kg N hm–2a–1)则降低了细菌生物量[78]。Wang等[79]报道氨态氮添加(120 kg N hm–2a–1)在非生长季显著降低了亚热带冷杉和松树种植园真菌生物量,在生长季则对真菌生物量影响不显著,但在两个季节对细菌生物量都没有显著影响。对鼎湖山3个南亚热带森林的研究表明,氮添加降低了季风常绿阔叶林的土壤微生物量,但对针阔混交林和针叶林的土壤微生物量则没有显著影响[80]。
氮添加可以改变土壤微生物的群落组成。首先,氮添加会改变真菌群落(真菌、丛枝菌根真菌和外生菌根真菌)的组成, 氮添加(70 kg N hm–2a–1)在春季会增加北方落叶松森林担子菌门的相对丰度, 但在夏季则会减少担子菌门的相对丰度[81]。氮添加(50~ 100 kg N hm–2a–1)会降低武夷山的亚热带常绿阔叶林丛枝菌根真菌的比例,但氮添加(150 kg N hm–2a–1)增加了鼎湖山季风常绿阔叶林丛枝菌根真菌的相对丰度[74,82]。氮添加(50~300 kg N hm–2a–1)能使亚热带湿地松()林对氮敏感的外生菌根真菌缺失[83]。其次,氮添加会影响细菌群落的组成, 基于磷脂脂肪酸分析技术,氮添加会降低革兰氏阴性细菌的相对丰度,增加革兰氏阳性细菌/革兰氏阴性细菌比[74]。基于高通量焦磷酸测序的研究也表明,氮添加会影响细菌群落的组成[81,84–85]。Nie等[85]报道,氮添加(105 kg N hm–2a–1)在干季会降低鼎湖山南亚热带常绿阔叶林酸杆菌门的相对丰度,但会增加变形菌门及放线菌门的相对丰度。第三,氮添加会影响真菌与细菌的比例。如氮添加增加了中国南部亚热带森林真菌的相对丰度,减少细菌的相对丰度,从而提高真菌/细菌比[74,86]。然而,氮添加降低了千烟洲的亚热带森林真菌/细菌比[73,79,87]。还有研究表明氮添加对真菌/细菌比没有显著影响[69,88]。
5.5.2 土壤酶活性
根据资源分配模型[89],氮添加可通过影响土壤养分的有效性来改变土壤酶活性。通常,氮添加会增加土壤有效氮的含量从而降低与微生物氮获取相关的酶活性(乙酰氨基葡糖酶、亮氨酸氨基肽酶、蛋白酶、脲酶等),但会增加与微生物碳(纤维素二糖水解酶、葡萄糖苷酶、木糖苷酶等)和磷(碱性磷酸酶、酸性磷酸单酯酶、酸性磷酸二酯酶等)获取相关的酶活性。然而,针对中国森林的研究表明,氮添加对酶活性的影响并不完全符合该理论框架[74,79,90]。如氮添加(100 kg N hm–2a–1)降低了北方落叶松森林土壤蛋白酶和脱氢酶活性,但对葡萄糖苷酶与酸性磷酸单酯酶的活性没有显著影响[76]。氮添加(50和100 kg N hm–2a–1)增加了千烟洲的亚热带冷杉种植园土壤乙酰氨基转移酶、葡萄糖苷酶和酸性磷酸单酯酶活性[91],但氨态氮和硝态氮添加(40 kg N hm–2a–1)降低了当地湿地松林土壤与微生物碳、氮、磷获取相关酶活性[73]。Fan等[76]报道氮添加(40和80 kg N hm–2a–1)增加了亚热带米槠()林表层土壤的乙酰氨基葡糖酶和酸性磷酸单酯酶活性,但降低了下层土壤氮乙酰氨基转移酶的活性。还有研究表明,氮添加对各类土壤酶活性都没有显著影响[92–93]。因此,氮添加对土壤酶活性的影响取决于氮添加的量、氮添加的类型、林型、酶的种类和土层等因素。
5.6 氮沉降对土壤动物的影响
土壤动物是指土壤中和落叶下生存的各种动物的总称。土壤动物作为生态系统中重要的分解者和消费者,对凋落物的分解、养分周转、微生物群落调控、以及生态系统结构和功能的维持均有重要作用。氮沉降的增加会对土壤动物的生物量、多样性和组成产生影响。
在中国,氮沉降对土壤动物的研究最早开展于鼎湖山自然保护区[94–95]。氮沉降对森林土壤动物影响的结论并不一致,施氮量和林型都是重要的影响因素。首先,施氮量可能存在阈值效应。Xu等[94]对南亚热带3种典型森林(季风常绿林、针阔混交林和针叶林)的研究表明,1年的氮处理并未对土壤动物生物量产生显著影响,但低氮处理(50 kg N hm–2a–1)各林分生物量都有不同程度的上升,而高氮处理(100 kg N hm–2a–1)均出现下降。Xu等[96]对鼎湖山森林苗圃地的研究进一步表明,氮沉降对土壤动物群落多样性的影响存在阈值效应(100 kg N hm–2a–1)。在北亚热带杨树人工林进行2年氮添加试验表明,中等浓度的氮添加对土壤动物群落有促进作用,高浓度则有抑制作用[97];氮添加4年后,低氮和高氮水平分别显著增加和降低了土壤动物总密度和植食性土壤动物密度,均表明氮添加对土壤动物的影响存在阈值作用[98]。其次,不同林型的氮沉降响应可能不同。南亚热带成熟林土壤动物密度、类群数等多样性指数随氮输入增加而降低,针叶林则相反,而针叶阔叶混交林则无明显响应[95]。亚热带人工林土壤线虫对氮输入的响应均不显著[99–101]。氮添加增加了温带针叶林土壤动物(跳虫和螨虫)的丰度[102]。
此外,不同类型的土壤动物对氮沉降的响应也可能不一致。对温带人工林[落叶松和水曲柳()]的研究表明,施肥改变了两林分不同食性土壤动物的密度,导致腐食性土壤动物数量降低,植食性土壤动物数量增加,但捕食性土壤动物数量变化不明显;这表明不同食性土壤动物对氮沉降的响应也不一致[103]。此外, 也有研究表明加氮对植食性线虫密度无显著影响,但可以改变外来生物(如蚯蚓)和植食性线虫之间的相互作用关系,从而潜在影响生态系统的功能[104]。
5.7 氮沉降对温室气体排放的影响
5.7.1 氮沉降对CO2排放的影响
森林土壤呼吸是CO2进入大气的重要过程,了解氮沉降对森林土壤呼吸及其组分(自养呼吸和异氧呼吸)的影响,对于理解森林碳源/汇功能和稳定性碳库改变、以及预测未来气候变化均非常重要。由于森林类型、环境条件和施氮持续时间不同,森林土壤呼吸对氮沉降的响应包括促进作用[74,105–106]、抑制作用[29,107]和无影响[108–110]。
国内最早见于鼎湖山的研究报道,氮沉降显著抑制了我国南亚热带成熟森林土壤呼吸能力[27], 但对该区域内混交林和马尾松林土壤呼吸速率没有影响[108]。氮添加引起的细根生物量减少是抑制森林土壤自养呼吸(Ra)的直接原因,而氮添加导致的土壤酸化将减少微生物量及降低其活性是引起异氧呼吸(Rh)能力下降的根本原因[27,75]。一般而言,氮沉降引起的微生物生物量、凋落物量和土壤有机碳库的变化更易引起土壤异氧呼吸速率的改变[106]。整合分析表明,氮沉降总体降低了森林土壤呼吸速率的1.44%[106],其响应程度与氮添加的持续时间有关。试验早期,外源氮通过改变参与凋落物分解的微生物基因表达、改变其群落结构和活性,显著改变土壤异氧呼吸[79,111–112]。氮沉降对土壤自养呼吸的影响程度取决于其对细根生物量的影响,在“富氮”森林中,外源氮素输入可显著降低细根生物量,从而显著抑制森林土壤自养呼吸能力[27];而在相对“贫氮”系统中,氮添加刺激植物光合作用,增加细根生物量以获取更多的营养元素(如磷等)和水分,从而显著提高了该森林土壤自养呼吸能力[74]。此外,氮输入形式不同对森林土壤呼吸的影响也不相同,通常情况下,NH4NO3为氮源的模拟试验响应程度比较显著[113–114]。氮添加减少根际碳的输入,同时降低微生物量及活性,减少土壤有机质的分解速率[115],根系可能通过改变形态和分泌物来减轻氮输入对根际的影响。氮沉降可通过对细根生长和微生物结构及活性的影响来改变土壤呼吸的温度敏感性(Q10值)[27,116]。
5.7.2 氮沉降对N2O排放的影响
森林土壤是N2O的源,含氮底物(NH4+-N和NO3–-N)通过硝化及反硝化细菌等,将NH4+-N和NO3–-N在不同条件下(好氧或厌氧)发生硝化或反硝化作用[117],产生N2O并排放至大气中,其排放过程主要受土壤温度和湿度的调节。氮沉降背景下,森林土壤N2O排放量增加,尤其在热带/亚热带森林中(约增加739%)[118]。近年来国内虽相继开展了一系列研究,由于区域和林型不同,仍没有统一的响应结论报道。
国内最早的控制试验报道见于在我国鼎湖山(南亚热带)森林的研究[16],氮添加显著增加了南亚热带成熟林土壤无机氮(DON)含量,进而刺激了N2O排放[16,119–120],但对该区域的针阔混交林和马尾松林土壤N2O排放没有影响,人为干扰程度导致森林本身“氮状态”的不同引起响应差异性[16,120]。同一样地土壤进行室内培养试验也表明,外源氮输入显著降低了成熟林土壤15N自然丰度,增加其N2O排放,而对混交林和马尾松林土壤的N2O排放速率影响不明显[121]。南亚热带豆科固氮树种[大叶相思()]人工林土壤N2O排放速率在氮添加处理后显著增加,而非固氮树种[桉树(sp.)林]的响应不明显[122]。对亚热带千烟洲人工杉木林的研究表明,氮沉降显著促进了N2O排放速率, 提高了4~7倍[123],而且NH4+-N添加对土壤N2O排放的促进作用高于NO3–-N[124],可能是氮添加改变了氨氧化细菌的丰度(增加AOA、减少AOB)引起的[125]。来自重庆铁山坪(西南地区)森林的研究(15N示踪)表明,NH4NO3添加显著增加土壤N2O排放量,NH4+-N和NO3–-N对N2O的贡献率没有差异[126];然而对同一地区森林土壤15N示踪室内培养6 d,土壤N2O排放的71%~100%来源于NO3–-15N[127]。对海南尖峰岭长期氮添加森林土壤进行室内培养,结果表明N2O、N2排放及N2O/N2比例均没有明显改变(与对照比较)。在厌氧条件下,随着氮素输入,热带次生林土壤N2O排放量减少、N2排放量增加,随氮添加N2O/N2比例降低[128],进一步验证了反硝化过程可能是调控我国热带/亚热带森林土壤N2O产生的主要过程。
对我国北方温带森林的研究表明,氮添加引起长白山森林地表可溶性无机氮(DON)增加,加快土壤氮素循环速率,并增加土壤N2O的排放[129–130],这些促进作用主要集中在春季(冻融)和夏季(生长季节)[110]。氮沉降可明显增加反硝化速率,进而增加土壤N2O排放量[131];尿素添加增加了凋落物层和矿质土壤NO3–-N含量,从而引起N2O排放量呈指数增加[132]。与红松()林相比,北方阔叶林土壤中的有机质在氮添加下更容易被微生物分解、有机氮被矿化成无机氮,增加了硝化和反硝化作用底物,从而有更多的N2O排出[133]。对黑龙江凉水国家级自然保护区森林的研究也表明,随着氮处理水平的增加,早期高氮添加显著增加N2O排放能力[134],且对N2O排放促进作用主要发生在生长季节[135]。在北京西山林场的试验表明,模拟添加NH4NO3、(NH4)2SO4、NaNO3对N2O排放速率分别增加了356%、266%和188%[136]。这表明氮沉降对我国森林土壤N2O排放的影响取决于系统本身的氮状态、施氮量、施氮形态和实验周期等因素。
5.7.3 氮沉降对CH4吸收的影响
森林土壤一般认为是大气CH4的汇(CH4氧化与产生过程的综合)。已有研究表明,氮沉降对我国森林土壤CH4氧化及产生过程均有不同程度的影响。
国内有关森林土壤CH4吸收通量对氮沉降的响应研究最早见于南亚热带森林(鼎湖山)的报道[137],氮添加引起的土壤NH4+与CH4氧化菌的竞争以及酸化环境下Al3+的毒害作用可能是引起CH4吸收通量减少的主要原因[118,137]。不同N素形态(NH4+-N和NO3–-N)对CH4吸收能力的影响程度不同[125,138]。早期氮添加显著降低了我国南亚热带固氮树种(大叶相思)人工林土壤CH4吸收能力,而对非固氮树种(桉树林)人工林没有影响[139];氮添加对鼎湖山成熟林和次生林(针阔叶混交林)土壤CH4吸收通量显著抑制,但对该区域马尾松林没有显著影响[140], 氮沉降抑制森林土壤CH4吸收速率的程度与系统本身的“氮状态”呈正相关关系[139]。另外,氮沉降对土壤CH4吸收的影响与森林类型密切相关[132,141],阔叶林比针叶林更为敏感,原因是阔叶林中凋落物分解速率较快、土壤中留存了更多的有效氮[142]。
截止目前,氮沉降对我国森林土壤主要温室气体通量影响的微生物机制仍不清晰,亟需借助于基因(DNA)芯片技术及高通量测序等手段,解析调控不同温室气体通量的微生物丰度、群落结构及活性对氮沉降的响应,并综合考虑CO2、N2O及CH4温室效应潜能,才能更好地理解未来氮沉降增加背景下,森林生态系统对区域乃至全球气候变化的调控作用。
5.8 氮沉降对生态系统固氮能力的影响
生物固氮是自然生态系统重要的氮素来源之一。在工业化革命以前,生物固氮作为生态系统主要的氮素来源,在生态系统养分循环和生产力方面发挥不可替代的作用[143–144]。然而,随着工业的发展、人口增加和化石燃料的过度使用,大气氮沉降逐年加剧[145]。氮沉降的加剧已经对自然生态系统的固氮能力产生了影响。据估计,在过去15年间,陆地自然生态系统固氮速率已从100~290 Tg N a–1降低为44 Tg N a–1[145],而导致该现象的主要原因是人为活动排放的活性氮沉降[146]。
森林是固氮微生物分布比较广泛的生态系统,森林土壤、凋落物层、附生植物(苔藓和地衣)、豆科植物根瘤和植物叶片等都具有固氮微生物的分布[147]。美洲和欧洲等地区已开展了氮沉降(或氮添加处理)对森林固氮的影响研究,多数结果表明外源氮输入对森林固氮产生抑制作用[148–151]。由于经济的快速发展,中国已成为大气氮沉降较为严重的地区之一[152],但当前有关氮沉降对我国森林固氮影响的研究相对较少。Zheng等[153]的研究表明,氮添加降低了我国南亚热带鹤山大叶相思林土壤、凋落物和根瘤的固氮速率,但仅降低尾叶桉()林土壤的固氮速率。氮沉降对鹤山人工林固氮速率的抑制效应可以通过人为管理方式(如施加磷肥)得以缓解[90]。在我国南亚热带鼎湖山森林中,Zheng等[154]比较了不同演替阶段森林,表明氮沉降抑制了干扰林的固氮速率,但对恢复林的没有显著影响,这暗示加强森林保护可以有效增加森林的固氮潜力。此外,Zheng等[32]的研究表明,在鼎湖山土壤“氮饱和”成熟林中长期氮添加处理对森林总固氮速率的影响很小,这一定程度上验证了Hedin等[155]提出的热带森林“氮悖论”假设。Wang等[156]报道氮添加显著降低了江西杉木人工林土壤固氮微生物丰度和固氮速率。Tang等[157]报道鼎湖山和长白山森林土壤固氮菌对氮添加的响应存在差异,即长白山森林土壤固氮菌丰度对氮添加较为敏感(固氮菌丰度显著降低),而鼎湖山森林土壤固氮菌对氮添加的敏感性较低(固氮菌丰度没有显著变化)。因此,氮添加降低森林固氮的潜在机理是:(1)通过增加森林土壤有效氮的含量而减弱固氮菌的竞争优势[147]; (2)通过加剧土壤其他养分(如磷等)的流失进而制约生物固氮的过程[90]; (3)通过改变固氮附生植物和结瘤植物的氮素获取方式,从而减少对固氮菌的能量分配,进一步降低森林的固氮量[158]。
5.9 氮沉降对氮循环的影响
氮循环直接影响着土壤氮状况和土壤中氮的去向,是森林生态系统最重要的功能之一。森林生态系统氮循环可以分成外循环与内循环。外循环包括氮的输入(生物固氮、氮沉降)和氮的输出(淋溶、径流流失、反硝化与氨的挥发),内循环则是指氮在生态系统内部各氮库之间的周转,包括氮的矿化、硝化、植物和微生物对氮的固持等。氮沉降加剧会显著影响森林生态系统氮的输入和输出,还会改变氮在生态系统内部的周转[159]。
氮沉降加剧会增加中国森林生态系统氮的输入。在中国南部亚热带森林中,随着氮沉降量的增加,森林穿透雨中的氮含量也随之增加[40]。由于目前大部分氮沉降模拟实验都是在林下施加氮肥,因而氮添加对森林穿透雨中氮含量的影响并不能模拟氮沉降对穿透雨氮输入的影响。华南园在广东石门台和河南鸡公山开展的林冠模拟氮沉降的试验可能让我们更准确地了解氮沉降对森林穿透雨氮动态的影响[35]。
氮输入的增加还会加剧森林土壤氮素的淋失。氮是陆地生态系统最普遍的限制性元素之一。氮添加使土壤总氮在热带、亚热带、温带和北方森林分别增加26.9%、5.4%、4.5%和17.3%[51]。但是,过量的氮输入会改变森林生态系统的氮状况,使森林生态系统由“氮限制”状态变成“氮饱和”状态,导致氮的淋溶[20]。在重庆铁山坪亚热带混交林中,氨态氮(43 kg N hm–2a–1)和硝态氮(40 kg N hm–2a–1)添加都显著增加了土壤硝态氮的淋溶[160]。在鼎湖山南亚热带常绿阔叶林、针阔叶混交林、针叶林中,氮添加显著加剧了土壤无机氮和有机氮的淋溶[18,41,161]。最近的原位氮同位素示踪研究则表明,即使在“氮饱和”的森林土壤,新输入土壤的氮也不是直接从土壤中流失,而是先在生态系统内部进行重新分配和周转[162]。
氮输入增加可以改变土壤氮素转化过程,包括氮的矿化、硝化和氮固持。氮添加对我国温带和北方森林土壤氮的矿化和硝化过程通常表现为促进或不影响[88,163–165]。Gao等[164]报道氮添加(40 kg N hm–2a–1)增加了温带混交林土壤氮的净矿化速率,但对净硝化速率没有显著影响。氮添加则对赛罕乌拉的白桦()次生林同时增加了氮的净矿化速率和净硝化速率[163]。氮添加促进了长白山温带混交林总氮矿化速率,但对总硝化速率没有显著影响[166]。对中国热带和亚热带森林,氮添加对氮的矿化及硝化有促进和不影响[122]、或抑制作用[167]。氮添加降低了鼎湖山自然保护区和查湾自然保护区的常绿阔叶林土壤氮的净矿化和净硝化速率[167–168], 也降低了千烟洲亚热带混交林和松林土壤氮的总矿化速率[169–170]。总之,氮添加对中国森林土壤氮素转化过程的影响取决于氮添加的数量、种类和持续时间以及林型等因素。
目前,关于氮沉降对土壤氮素转化过程的了解大多基于净氮素转化速率,还鲜有研究揭示氮沉降背景下,土壤氮素转化速率的变化与相关的微生物群落变化之间的联系。未来需要更多地将总氮素转化速率与土壤微生物群落及酶活性综合考虑,以更加准确地揭示氮沉降影响土壤氮素转化速率机理。
5.10 氮沉降对磷循环的影响
森林磷循环包括磷素的输入(岩石风化或沉降输入)和输出(淋溶)、在生态系统各磷库中的固持(土壤磷的有效性、植物体的固持和微生物的固持)及在各磷库间的流转(如凋落物的分解和回收利用)等。目前国内有关氮沉降对对磷循环的研究极少。Zhou等[171]在鼎湖山长期高氮沉降背景下的淋溶试验表明,氮沉降显著降低了冠层穿透雨中的磷素,但对地表径流及土壤溶液的磷素淋溶没有显著影响,这可能与植物磷吸收、凋落物分解的抑制作用、土壤有效磷低和较强的生物固持作用有关。
作为磷素循环的一部分,土壤有效磷对氮沉降响应的报道最常见,但相关结果并不一致。有研究表明,氮沉降降低了土壤磷的有效性,如中国东北帽儿山落叶林[172]。近年来的一些研究却表明,氮添加并没有降低甚至增加了土壤磷的有效性[173–175], Wang等[174]对桉树进行短期氮磷添加处理,高氮添加降低了亚热带森林土壤可溶性有机磷,同时有效磷含量上升,这些差异可能与地域间氮沉降量、土壤磷素含量、土壤钙铁铝离子含量、土壤pH、森林物种组成和土地利用方式不同有关[175]。
对生态系统而言,氮添加导致生态系统氮磷计量化学失衡(增加植物氮/磷比),并可能导致系统磷限制的发生[176–177]。然而,植物在磷限制压力下可以通过一系列的生理生态过程来缓解磷素的相对不足。首先,氮沉降促进植物对磷的吸收[49,178]。Deng等对华北落叶松的研究表明,氮添加通过改变丛枝菌根真菌与外生菌根的比例来促进对磷素的吸收,以保持体内氮磷比的稳定[49]。第二,氮输入会促进植物体对磷的重吸收[179–180],See等[181]对英国不同养分状态森林氮磷重吸收的研究表明,随着土壤总氮含量的上升,磷素的重吸收率随之升高。氮沉降还可以通过改变微生物学过程(如磷素的固持、酶的分泌等)来影响土壤磷素周转[54,74]。郑棉海等的研究表明,施加氮肥显著提高了鼎湖山季风林土壤的酸性磷酸酶活性[182]。此外,在氮/磷比较高的热带地区,森林生态系统倾向于闭合型循环,降低磷素的淋溶[171,183]。
5.11 氮沉降对凋落物分解的影响
凋落物是植物产生的枯落物或有机碎屑,植物体以产生凋落物的形式将营养返还到土壤表面,而凋落物在微生物分解的作用下,向环境中释放植物可吸收利用的营养。陆地生态系统中约90%的净初级生产量以凋落物的形式归还给土壤[184],养分再循环供给植物生长发育。因而,凋落物分解过程受环境因子、凋落物质量和生物因子等多因素共同调控。华南园率先开展了氮沉降增加对森林凋落物分解方面的研究,国内代表性的长期氮沉降样地有鼎湖山、长白山、大小兴安岭等,研究内容涵盖了凋落物的产量动态、分布、分解过程和影响因素等。
目前,氮沉降对凋落物分解速率的研究结果尚不一致,有促进[185–187]、抑制[188–189]和无显著影响[190–191]。同时也有学者提出,氮沉降对凋落物分解的影响具有阶段性[192–193],在初期促进其分解, 而后期反而受到高氮量的抑制作用。此外,凋落物分解过程还受到演替进程的影响。莫江明等[194]的研究表明,氮沉降对凋落物分解的影响随亚热带森林演替从正效应转向负效应。
中国学者对氮沉降与凋落物分解的研究多采用人工施加硝酸铵氮肥,其他无机氮或有机氮使用频次相对降低,且施氮研究时间都比较短,大都关注1、2年的凋落物动态,鲜少关注长期凋落物动态的研究。氮沉降对凋落物分解影响的研究结果虽然存在很大差异,但中国西南和南方等的亚热带森林,大多都表现出抑制凋落物分解的趋势[133,188–189,195]。然而北方温带阔叶林和针叶林等对氮添加的响应更为多样,氮添加对凋落物分解多具正负效应,且很多表现出阶段性的效应[185,187,193]。
5.12 氮沉降对植物源有机挥发物的影响
植物源挥发性有机物(biogenic volatile organic compounds, BVOCs)是指植物直接排放的沸点为50℃~260℃,室温下饱和蒸汽压超过133.322 Pa的易挥发性碳氢化合物,主要包括非甲烷烃类(烷烃、烯烃、芳香烃)和含氧原子的醛、酮、酚、醇、醚、酯等挥发性有机物。BVOCs来源于植物的光合碳同化物,是碳(C)素循环的一个重要组成部分,其排放与环境因子息息相关,并对胁迫环境有显著响应[196–197]。
国内氮沉降对森林植物BVOCs排放影响的报道极少,仅见于鼎湖山。黄娟等[198]提出了氮沉降对植物BVOCs影响的假说模型:在氮限制系统中,氮沉降的增加补充了系统所需的氮素,有利于植物的生长,大量BVOCs的排放会受到抑制;在氮素丰富或饱和的系统中,氮沉降导致系统氮素过饱和或富营养化,不利于植物的生长, 刺激BVOCs的排放增加。该假说模型在相对“氮限制”的鼎湖山苗圃地得到了验证,即为期一年半的氮添加(100 kg N hm–2a–1)显著抑制了海南红豆()和阴香()幼苗甲醛和乙醛的排放[199–200]。
5.13 氮沉降对生态系统碳吸存的影响
氮沉降通过影响森林生态系统主要碳库(植物生物量和土壤有机碳库)对生态系统碳吸存产生影响(图4)。氮沉降对生态系统碳吸存的影响包括对植被碳库(vegetation carbon pool)和土壤碳库(soil carbon pool)的影响。氮沉降通过改变植物的光合作用和呼吸作用的强弱来调节植物体作为生物碳库的净碳固存,同时又通过影响植物体产生凋落物的生物量大小及凋落物质量(包括地上部分凋落物及根系凋落物)来调节进入土壤碳库的碳输入量,此外,氮沉降还影响微生物群落与活性,从而控制着土壤碳库中各种碳组分间的转化(如有机碳的分解矿化与土壤呼吸),并与淋溶等其他生态过程共同调节着土壤碳库的碳储量。陆地生态系统通常受到氮素的限制[5],所以外源性氮添加会促进生态系统初级生产力[1–2,53]。Yue等[53]报道,氮添加后全球尺度上的陆地生态系统地上部分与地下部分的碳含量分别增加了25.65%和15.93%。Chen等[201]通过收集中国陆地生态系统数据并进行整合分析,义为中国的模拟氮沉降试验增加了地上植物碳库,但地下植物碳库显著减少。这种地上生物量的增加趋势与全球尺度上的变化趋势相似[2,53],而地下生物量的响应与全球尺度上的变化模式不同。Lu等[71]通过全球陆地生态系统土壤碳储存对氮沉降响应的Meta分析表明,尽管不同地区的地上植物碳库对氮沉降的响应强度不同,但总体上呈现正响应,氮添加处理下地上植物碳库增加了35.7%[71]。中国地上与地下生物量对氮沉降的响应与全球模式不同的原因可能在于中国氮沉降量非常高,因此氮沉降对根系的负效应可能更为强烈。模拟氮沉降处理降低了鼎湖山季风林的根系生物量,同时增加了死亡根系的生物量[57];这可能是氮添加通过影响土壤酸化而对根系产生了毒副作用。此外,过多的氮可以通过影响初级生产力来抑制碳的吸收。氮添加对森林植被,尤其是林下植被有显著的负效应,而对大树的影响还需要长期研究[17,65,202]。通常认为,“富氮”森林生态系统中的植物生长和生产力对长期氮添加没有明显响应[13]。
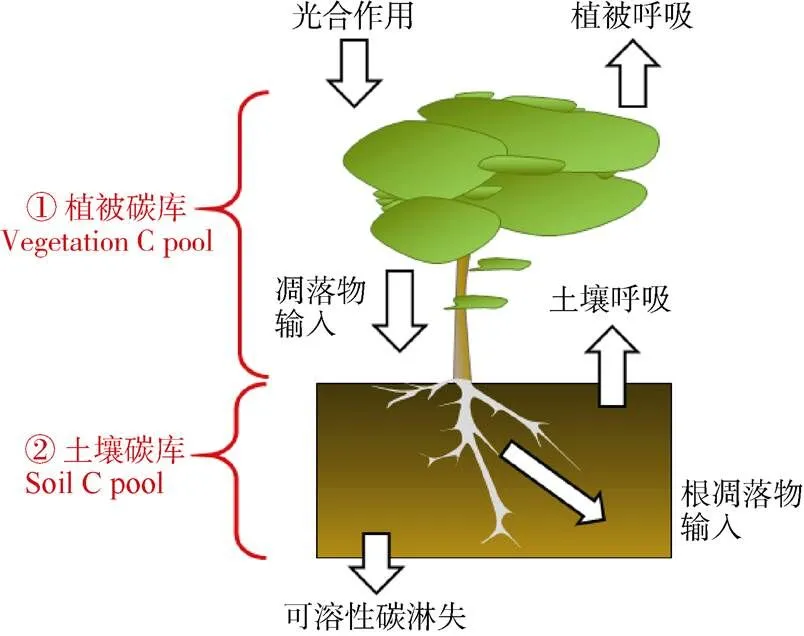
图4 森林生态系统碳吸存过程框架图
土壤碳库是陆地生态系统中重要的碳库。土壤碳库是碳通过枯叶(如叶,根和枝)输入和通过分解、淋溶输出的净储量。因此,土壤碳的分解过程是控制森林生态系统中土壤碳库量的关键,而包括底物质量、气候、水分及土壤化学性质等各种因素控制着底物的分解过程[203]。氮是控制有机物分解的重要因素。近几十年来由于人类活动的影响, 氮沉降量急剧增加,预计在热带亚热带地区将进一步增加[3]。因此,了解土壤有机质分解对高氮输入的响应是预测未来全球碳动态变化的关键。
许多研究表明,除分解的早期阶段外氮添加会降低有机物的分解,所以氮沉降可以提高森林生态系统中的土壤碳库[204]。在“氮饱和”的南亚热带原始森林,氮添加抑制了凋落物分解[1,26]。同时氮添加早期促进了人工林中的凋落物分解,这与氮相对较贫乏时的原因相同[26]。随后进行的凋落物分解研究与这一趋势相反,随着时间的推移,更多的氮输入生态系统中,导致凋落物分解受到抑制[205]。相应地,对鼎湖山成熟林的研究也表明,氮肥施加抑制了土壤呼吸[27]。因此,未来持续高氮沉降输入可能会通过抑制有机质分解来促进土壤碳吸存。
5.14 森林生态系统氮沉降去向研究
沉降到生态系统中的氮经过一系列的循环周转一般有4个去向:存留到植物和土壤的各个组分中,以气体形式(含N化合物的排放)或液体形式(淋溶流失)离开生态系统。15N示踪技术被认为是氮沉降背景下研究氮循环的有力工具,Templer等[206]对温带和北方森林生态系统的研究表明,15N添加后短期内(约1年),森林生态系统总的15N回收率维持在75%左右,且土壤(有机质层和表层矿质)是最大的氮汇,其次是植物。
近年来,我国陆续开展了基于15N示踪手段研究生态系统氮去向的相关试验。在热带(亚热带)区域的鼎湖山成熟林、重庆铁山坪的亚热带森林、海南热带山地原始林的研究(15NH415NO3、15NH4NO3或NH415NO3)表明,15N添加1年后生态系统15N回收率高达50%~70%以上,而土壤溶液中15N回收率为14%~33%[162,207–208]。因此,以NH4NO3为主要氮沉降形式的土壤和植物是最大的氮汇。然而,在铁山坪亚热带森林进行的Na15NO3示踪研究表明, 18个月内土壤溶液中15N回收率高达74%,这反映了生态系统在氮饱和情境下难以保持过量硝态氮的输入[160]。在长白山温带森林进行的15NH4NO3和NH415NO3示踪研究表明,1年后仍有80%15N存留在植物和土壤中,高于热带森林[207]。在亚热带常绿阔叶林和北方针叶林,Sheng等[209]利用(15NH4)2SO4和K15NO3两种15N进行示踪研究,15NH4+的回收率在两个森林中相差不大,15NO3–的回收率在针叶林高于亚热带阔叶林;和NH4+相比, NO3–更容易从生态系统中流失。这些表明,热带亚热带森林仍具有不可忽视的氮汇,而且沉降氮的形态将进一步决定氮素在生态系统中的命运。
6 结论和展望
通过上面综述,我们可以得出两个重要结论:首先,中国近三十年来大气氮沉降总量显著增加, 特别是NO3–氮沉降;其次,模拟氮沉降研究表明,持续高氮输入将会显著改变森林生态系统的结构和功能,特别是处于氮沉降热点区域的中国中南部。主要表现如下:(1)北方和温带森林仍存在氮素施肥效应,热带森林则趋向氮饱和、无明显的正生长效应;(2)过量氮沉降将会导致土壤酸化和养分失衡;(3)氮沉降增加促进了氮循环速率及其转化过程,但抑制了森林的固氮速率,并影响到了碳、磷等其他元素循环、凋落物分解进程和温室气体排放;(4)氮沉降降低了林下层植物,并改变了土壤微生物群落结构;(5)高氮沉降背景下热带亚热带森林仍具有不可忽视的氮汇;(6)森林生态系统的氮沉降效应依赖于系统的氮状态、土地利用历史、气候特征、林型和林龄等。
尽管目前的研究对深入理解和评估大气氮沉降增加对中国森林生态系统健康的影响做出了积极贡献,但是在全球变化背景下未来仍有以下几个方面值得深入探讨。
首先,在研究方式上应兼顾到以下3点:(1)需要追踪研究森林生态系统的长期氮沉降效应。目前的大部分氮沉降试验研究期限较短(<10年),研究结果的有效性和可靠性还有待于长期验证。(2)要加强各站点之间的联网研究,以更好地探究生态系统现象背后运转的规律,以期为森林生态系统的可持续发展提供理论基础和管理依据。(3)加强对我国不同气候和植被类型生态系统响应研究的评估,这主要是考虑到不同气候带的水、土、气、生等各方面均存在差异。
其次,在研究内容上有以下3个方面需要强化:(1)森林生态系统对长期氮沉降响应与适应的过程机制有待于深入研究,因为目前的研究更偏重于格局。(2)长期氮沉降对中国森林碳吸存与固氮潜力的影响,主要是考虑到地下碳氮循环过程仍存在巨大的不确定性。(3)需要加强与其他全球变化因子(如全球变暖、CO2浓度升高、降雨格局改变等)的耦合研究,以更好的评估生态系统未来的发展趋势。
[1] VITOUSEK P M, HOWARTH R W. Nitrogen limitation on land and in the sea: How can it occur? [J]. Biogeochemistry, 1991, 13(2): 87–115. doi: 10.1007/BF00002772.
[2] LEBAUER D S, TRESEDER K K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed [J]. Ecology, 2008, 89(2): 371–379. doi: 10.1890/06-2057.1.
[3] GALLOWAY J N, DENTENER F J, CAPONE D G, et al. Nitrogen cycles: Past, present, and future [J]. Biogeochemistry, 2004, 70(2): 153–226. doi: 10.1007/s10533-004-0370-0.
[4] CUI S H, SHI Y L, GROFFMAN P M, et al. Centennial-scale analysis of the creation and fate of reactive nitrogen in China (1910–2010) [J]. Proc Natl Acad Sci USA, 2013, 110(6): 2052–2057. doi: 10.1073/pnas. 1221638110.
[5] LÜ C Q, TIAN H Q. Spatial and temporal patterns of nitrogen deposition in China: Synthesis of observational data [J]. J Geophys Res-Atmos, 2007, 112(D22): D22S05. doi: 10.1029/2006JD007990.
[6] GU B J, JU X T, CHANG J, et al. Integrated reactive nitrogen budgets and future trends in China [J]. Proc Natl Acad Sci USA, 2015, 112(28): 8792–8797. doi: 10.1073/pnas.1510211112.
[7] XU W, LUO X S, PAN Y P, et al. Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China [J]. Atmos Chem Phys, 2015, 15(21): 12345–12360. doi: 10.5194/acp-15- 12345-2015.
[8] JIA Y L, YU G R, HE N P, et al. Spatial and decadal variations in inorganic nitrogen wet deposition in China induced by human activity [J]. Sci Rep, 2014, 4: 3763. doi: 10.1038/srep03763.
[9] BOBBINK R, HICKS K, GALLOWAY J, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis [J]. Ecol Appl, 2010, 20(1): 30–59. doi: 10.1890/08-1140.1.
[10] YU G R, JIA Y L, HE N P, et al. Stabilization of atmospheric nitrogen deposition in China over the past decade [J]. Nat Geosci, 2019, 12(6): 424–429. doi: 10.1038/s41561-019-0352-4.
[11] YU G R, GAO Y, WANG Q F et al. Discussion on the key processes of carbon-nitrogen-water coupling cycles and biological regulation mechanisms in terrestrial ecosystem [J]. Chin J Eco-Agric, 2013, 21(1): 1–13. doi: 10.3724/SP.J.1011.2013.00001.于贵瑞, 高扬, 王秋凤, 等. 陆地生态系统碳-氮-水循环的关键耦合过程及其生物调控机制探讨 [J]. 中国生态农业学报, 2013, 21(1): 1–13. doi: 10.3724/SP.J.1011.2013.00001.
[12] GRUBER N, GALLOWAY J N. An Earth-system perspective of the global nitrogen cycle [J]. Nature, 2008, 451(7176): 293–296. doi: 10. 1038/nature06592.
[13] LU X K, VITOUSEK P M, MAO Q G, et al. Plant acclimation to long-term high nitrogen deposition in an N-rich tropical forest [J]. Proc Natl Acad Sci USA, 2018, 115(20): 5187–5192. doi: 10.1073/pnas. 1720777115.
[14] PHOENIX G K, HICKS W K, CINDERBY S, et al. Atmospheric nitrogen deposition in world biodiversity hotspots: The need for a greater global perspective in assessing N deposition impacts [J]. Glob Change Biol, 2006, 12(3): 470–476. doi: 10.1111/j.1365-2486.2006. 01104.x.
[15] BOWMAN W D, CLEVELAND C C, HALADA Ĺ, et al. Negative impact of nitrogen deposition on soil buffering capacity [J]. Nat Geosci, 2008, 1(11): 767–770. doi: 10.1038/ngeo339.
[16] ZHANG W, MO J M, YU G R, et al. Emissions of nitrous oxide from three tropical forests in southern China in response to simulated nitrogen deposition [J]. Plant Soil, 2008, 306(1/2): 221–236. doi: 10.1007/ s11104-008-9575-7.
[17] LU X K, MO J M, GILLIAM F S, et al. Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest [J]. Glob Change Biol, 2010, 16(10): 2688–2700. doi: 10.1111/j.1365- 2486.2010.02174.x.
[18] LU X K, MAO Q G, GILLIAM F S, et al. Nitrogen deposition contri- butes to soil acidification in tropical ecosystems [J]. Glob Change Biol, 2014, 20(12): 3790–3801. doi: 10.1111/gcb.12665.
[19] MIDOLO G, ALKEMADE R, SCHIPPER A M, et al. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis [J]. Glob Ecol Biogeogr, 2019, 28(3): 398–413. doi: 10. 1111/geb.12856.
[20] ABER J, McDOWELL W, NADELHOFFER K, et al. Nitrogen satu- ration in temperate forest ecosystems [J]. BioScience, 1998, 48(11): 921–934. doi: 10.2307/1313296.
[21] ROCKSTRÖM J, STEFFEN W, NOONE K, et al. A safe operating space for humanity [J]. Nature, 2009, 461(7263): 472–475. doi: 10.1038/ 461472a.
[22] WRIGHT R F, RASMUSSEN L. Introduction to the NITREX and EXMAN projects [J]. For Ecol Manage, 1998, 101(1/2/3): 1–7. doi: 10. 1016/S0378-1127(97)00120-5.
[23] MAGILL A H, ABER J D, CURRIE W S, et al. Ecosystem response to 15 years of chronic nitrogen additions at the Harvard Forest LTER, Massachusetts, USA [J]. For Ecol Manage, 2004, 196(1): 7–28. doi: 10. 1016/j.foreco.2004.03.033.
[24] RUSTAD L E. The response of terrestrial ecosystems to global climate change: Towards an integrated approach [J]. Sci Total Environ, 2008, 404(2/3): 222–235. doi: 10.1016/j.scitotenv.2008.04.050.
[25] ZHAO D W, SUN B Z. Air pollution and acid rain in China [J]. Ambio, 1986, 15(1): 2–5.
[26] MO J M, BROWN S, XUE J H, et al. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China [J]. Plant Soil, 2006, 282(1/2): 135–151. doi: 10. 1007/s11104-005-5446-7.
[27] MO J M, ZHANG W, ZHU W X, et al. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China [J]. Glob Change Biol, 2008, 14(2): 403–412. doi: 10.1111/j.1365-2486.2007. 01503.x.
[28] DU E Z, ZHOU Z, LI P, et al. NEECF: A project of nutrient enrichment experiments in China’s forests [J]. J Plant Ecol, 2013, 6(5): 428–435. doi: 10.1093/jpe/rtt008.
[29] LIU X J, DUAN L, MO J M, et al. Nitrogen deposition and its ecological impact in China: An overview [J]. Environ Pollut, 2011, 159(10): 2251–2264. doi: 10.1016/j.envpol.2010.08.002.
[30] FU Z, NIU S L, DUKES J S. What have we learned from global change manipulative experiments in China? A meta-analysis [J]. Sci Rep, 2015, 5: 12344. doi: 10.1038/srep12344.
[31] ZHU J X, WANG Q F, HE N P, et al. Imbalanced atmospheric nitrogen and phosphorus depositions in China: Implications for nutrient limitation [J]. J Geophys Res-Biogeosci, 2016, 121(6): 1605–1616. doi: 10.1002/ 2016JG003393.
[32] ZHENG M H, ZHANG W, LUO Y Q, et al. Stoichiometry controls asymbiotic nitrogen fixation and its response to nitrogen inputs in a nitrogen-saturated forest [J]. Ecology, 2018, 99(9): 2037–2046. doi: 10. 1002/ecy.2416.
[33] KOU L, LI S G, WANG H M, et al. Unaltered phenology but increased production of ectomycorrhizal roots ofunder 4 years of nitrogen addition [J]. New Phytol, 2019, 221(4): 2228–2238. doi: 10. 1111/nph.15542.
[34] XING A J, XU L C, SHEN H H, et al. Long term effect of nitrogen addition on understory community in a Chinese boreal forest [J]. Sci Total Environ, 2019, 646: 989–995. doi: 10.1016/j.scitotenv.2018.07. 350.
[35] ZHANG W, SHEN W J, ZHU S D, et al. Can canopy addition of nitrogen better illustrate the effect of atmospheric nitrogen deposition on forest ecosystem? [J]. Sci Rep, 2015, 5(1): 11245. doi: 10.1038/srep 11245.
[36] FANG Y T, WANG X M, ZHU F F, et al. Three-decade changes in chemical composition of precipitation in Guangzhou City, southern China: Has precipitation recovered from acidification following sulphur dioxide emission control? [J]. Tellus B, 2013, 65: 20213. doi: 10.3402/tellusb.v65i0.20213.
[37] DU E, BE VRIES W, LIU X, et al. Spatial boundary of urban ‘acid islands’ in China [J]. Sci Rep, 2015, 5: 12625. doi: 10. 1038/srep12625.
[38] ULRICH B. Natural and anthropogenic components of soil acidify- cation [J]. Z Pflanz Bodenkunde, 1986, 149(6): 702–717. doi: 10.1002/ jpln.19861490607.
[39] DU E Z, JIANG Y, FANG J Y, et al. Inorganic nitrogen deposition in China’s forests: Status and characteristics [J]. Atmos Environ, 2014, 98: 474–482. doi: 10.1016/j.atmosenv.2014.09.005.
[40] FANG Y T, YOH M, KOBA K, et al. Nitrogen deposition and forest nitrogen cycling along an urban-rural transect in southern China [J]. Global Change Biol, 2011, 17(2): 872–885. doi: 10.1111/j.1365-2486. 2010.02283.x.
[41] LU X K, MAO Q G, MO J M, et al. Divergent responses of soil buffering capacity to long-term N deposition in three typical tropical forests with different land-use history [J]. Environ Sci Technol, 2015, 49(7): 4072–4080. doi: 10.1021/es5047233.
[42] HUANG Y M, KANG R H, MULDER J, et al. Nitrogen saturation, soil acidification, and ecological effects in a subtropical pine forest on acid soil in southwest China [J]. J Geophys Res-Biogeo, 2015, 120(11): 2457–2472. doi: 10.1002/2015JG003048.
[43] MAO Q G, LU X K, MO H, et al. Effects of simulated N deposition on foliar nutrient status, N metabolism and photosynthetic capacity of three dominant understory plant species in a mature tropical forest [J]. Sci Total Environ, 2018, 610–611: 555–562. doi: 10.1016/j.scitotenv. 2017.08.087.
[44] HE X J, HOU E Q, LIU Y, et al. Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China [J]. Sci Rep, 2016, 6: 24261. doi: 10.1038/srep24261.
[45] GILLIAM F S, MAY J D, ADAMS M B. Response of foliar nutrients ofto nutrient amendments in a central Appala- chian hardwood forest [J]. For Ecol Manage, 2018, 411: 101–107. doi: 10.1016/j.foreco.2018.01.022.
[46] LI D J, MO J M, FANG Y T, et al. Effects of simulated nitrogen deposition on biomass production and allocation inandseedlings in subtropical China [J]. Acta Phytoecol Sin, 2005, 29(4): 543–549. doi: 10.17521/cjpe.2005.0073. 李德军, 莫江明, 方运霆, 等. 模拟氮沉降对南亚热带两种乔木幼苗生物量及其分配的影响 [J]. 植物生态学报, 2005, 29(4): 543–549. doi: 10.17521/cjpe.2005.0073.
[47] MO J M, LI D J, GUNDERSEN P. Seedling growth response of two tropical tree species to nitrogen deposition in southern China [J]. Eur J For Res, 2008, 127(4): 275–283. doi: 10.1007/s10342-008-0203-0.
[48] YUAN Z Y, CHEN H Y H. Negative effects of fertilization on plant nutrient resorption [J]. Ecology, 2015, 96(2): 373–380. doi: 10.1890/ 14-0140.1.
[49] DENG M F, LIU L L, SUN Z Z, et al. Increased phosphate uptake but not resorption alleviates phosphorus deficiency induced by nitrogen deposition in temperateplantations [J]. New Phytol, 2016, 212(4): 1019–1029. doi: 10.1111/nph.14083.
[50] ZHAO Q, LIU X Y, HU Y L, et al. Effects of nitrogen addition on nutrient allocation and nutrient resorption efficiency in[J]. Sci Silv Sin, 2010, 46(5): 14–19. doi: 10.11707/j.1001-7488.2010 0503.赵琼, 刘兴宇, 胡亚林, 等. 氮添加对兴安落叶松养分分配和再吸收效率的影响[J]. 林业科学, 2010, 46(5): 14–19. doi: 10.11707/j. 1001-7488.20100503.
[51] TIAN D, DU E Z, JIANG L, et al. Responses of forest ecosystems to increasing N deposition in China: A critical review [J]. Environ Pollut, 2018, 243: 75–86. doi: 10.1016/j.envpol.2018.08.010.
[52] YUE K, YANG W Q, PENG Y, et al. Individual and combined effects of multiple global change drivers on terrestrial phosphorus pools: A meta-analysis [J]. Sci Total Environ, 2018, 630: 181–188. doi: 10. 1016/j.scitotenv.2018.02.213.
[53] YUE K, Peng Y, PENG C H, et al. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A meta-analysis [J]. Sci Rep, 2016, 6: 19895. doi: 10.1038/srep19895.
[54] DENG Q, HUI D F, DENNIS S, et al. Responses of terrestrial ecosystem phosphorus cycling to nitrogen addition: A meta-analysis [J]. Glob Ecol Biogeogr, 2017, 26(6): 713–728. doi: 10.1111/geb.12576.
[55] YUE K, FORNARA D A, YANG W Q, et al. Effects of three global change drivers on terrestrial C∶N∶P stoichiometry: A global synthesis [J]. Glob Change Biol, 2017, 23(6): 2450–2463. doi: 10.1111/gcb. 13569.
[56] MO Q F, ZOU B, LI Y W, et al. Response of plant nutrient stoichio- metry to fertilization varied with plant tissues in a tropical forest [J]. Sci Rep, 2015, 5: 14605. doi: 10.1038/srep14605.
[57] ZHU F F, YOH M, GILLIAM F S, et al. Nutrient limitation in three lowland tropical forests in southern China receiving high nitrogen deposition: Insights from fine root responses to nutrient additions [J]. PLoS One, 2013, 8(12): e82661. doi: 10.1371/journal.pone.0082661.
[58] LI W B, JIN C J, GUAN D X, et al. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis [J]. Soil Biol Biochem, 2015, 82: 112–118. doi: 10.1016/j.soilbio.2015.01.001.
[59] XIA J Y, WAN S Q. Global response patterns of terrestrial plant species to nitrogen addition [J]. New Phytol, 2008, 179(2): 428–439. doi: 10. 1111/j.1469-8137.2008.02488.x.
[60] WRIGHT S J, TURNER B L, YAVITT J B, et al. Plant responses to fertilization experiments in lowland, species-rich, tropical forests [J]. Ecology, 2018, 99(5): 1129–1138. doi: 10.1002/ecy.2193.
[61] SIDDIQUE I, VIEIRA I C G, SCHMIDT S, et al. Nitrogen and phosphorus additions negatively affect tree species diversity in tropical forest regrowth trajectories [J]. Ecology, 2010, 91(7): 2121–2131. doi: 10.1890/09-0636.1.
[62] de VRIES W, DU E Z, BUTTERBACH-BAHL K. Short and long-term impacts of nitrogen deposition on carbon sequestration by forest eco- systems [J]. Curr Opin Environ Sust, 2014, 9–10: 90–104. doi: 10. 1016/j.cosust.2014.09.001.
[63] YAN G Y, XING Y J, WANG J Y, et al. Sequestration of atmospheric CO2in boreal forest carbon pools in northeastern China: Effects of nitrogen deposition [J]. Agric For Meteorol, 2018, 248: 70–81. doi: 10. 1016/j.agrformet.2017.09.015.
[64] LIU X Y, DU E Z, XU L C, et al. Response of tree growth to nitrogen addition in aprimitive forest [J]. Chin J Plant Ecol, 2015, 39(5): 433–441. doi: 10.17521/cjpe.2015.0042.刘修元, 杜恩在, 徐龙超, 等. 落叶松原始林树木生长对氮添加的响应 [J]. 植物生态学报, 2015, 39(5): 433–441. doi: 10.17521/cjpe. 2015.0042.
[65] TIAN D, LI P, FANG W J, et al. Growth responses of trees and understory plants to nitrogen fertilization in a subtropical forest in China [J]. Biogeosciences, 2017, 14: 3461–3469. doi: 10.5194/bg-14- 3461-2017.
[66] JIANG L, TIAN D, MA S H, et al. The response of tree growth to nitrogen and phosphorus additions in a tropical montane rainforest [J]. Sci Total Environ, 2018, 618: 1064–1070. doi: 10.1016/j.scitotenv.2017. 09.099.
[67] SALA O E, CHAPIN F S III, ARMESTO J J, et al. Global biodiversity scenarios for the year 2100 [J]. Science, 2000, 287(5459): 1770–1774. doi: 10.1126/science.287.5459.1770.
[68] DU E Z. Integrating species composition and leaf nitrogen content to indicate effects of nitrogen deposition [J]. Environ Pollut, 2017, 221: 392–397. doi: 10.1016/j.envpol.2016.12.001.
[69] WU J P, LIU W F, FAN H B, et al. Asynchronous responses of soil microbial community and understory plant community to simulated nitrogen deposition in a subtropical forest [J]. Ecol Evol, 2013, 3(11): 3895–3905. doi: 10.1002/ece3.750.
[70] HUANG L J, ZHU W X, REN H, et al. Impact of atmospheric nitrogen deposition on soil properties and herb-layer diversity in remnant forests along an urban-rural gradient in Guangzhou, southern China [J]. Plant Ecol, 2012, 213(7): 1187–1202. doi: 10.1007/s11258-012-0080-y.
[71] LU X K, MO J M, GILLIAM F S, et al. Effects of experimental nitrogen additions on plant diversity in tropical forests of contrasting disturbance regimes in southern China [J]. Environ Pollut, 2011, 159: 2228–2235. doi: 10.1016/j.envpol.2010.10.037.
[72] LI H S, WANG J S, LIU X, et al. Effect of simulation N deposition on herbaceous vegetation community in the plantation and natural forests ofin the Taiyue Mountain [J]. Acta Ecol Sin, 2015, 35(11): 3910–3721. doi: 10.5846/stxb201307141892.李化山, 汪金松, 刘星, 等. 模拟N沉降对太岳山油松人工林和天然林草本群落的影响 [J]. 生态学报, 2015, 35(11): 3910–3721. doi: 10. 5846/stxb201307141892.
[73] ZHANG C, ZHANG X Y, ZOU H T, et al. Contrasting effects of ammonium and nitrate additions on the biomass of soil microbial communities and enzyme activities in subtropical China [J]. Biogeo- sciences, 2017, 14: 4815–4827. doi: 10.5194/bg-14-4815-2017.
[74] WANG C, LU X K, MORI T, et al. Responses of soil microbial community to continuous experimental nitrogen additions for 13 years in a nitrogen-rich tropical forest [J]. Soil Biol Biochem, 2018, 121: 103–112. doi: 10.1016/j.soilbio.2018.03.009.
[75] LIANG L Z, CHEN F, HAN H R, et al. Pathways regulating decreased soil respiration with nitrogen addition in a subtropical forest in China [J]. Water Air Soil Pollut, 2019, 230: 91. doi: 10.1007/s11270-019- 4144-7.
[76] FAN Y X, LIN F, YANG L M, et al. Decreased soil organic P fraction associated with ectomycorrhizal fungal activity to meet increased P demand under N application in a subtropical forest ecosystem [J]. Biol Fert Soils, 2018, 54(1): 149–161. doi: 10.1007/s00374-017-1251-8.
[77] ZHAO B, GENG Y, CAO J, et al. Contrasting responses of soil respi- ration components in response to five-year nitrogen addition in aforest in northern China [J]. Forests, 2018, 9: 544. doi: 10.3390/f9090544.
[78] LIU C X, DONG Y H, SUN Q W, et al. Soil bacterial community response to short-term manipulation of the nitrogen deposition form and dose in a Chinese fir plantation in southern China [J]. Water Air Soil Pollut, 2016, 227: 447. doi: 10.1007/s11270-016-3152-0.
[79] WANG Y S, CHENG S L, FANG H J, et al. Contrasting effects of ammonium and nitrate inputs on soil CO2emission in a subtropical coniferous plantation of southern China [J]. Biol Fert Soils, 2015, 51(7): 815–825. doi: 10.1007/s00374-015-1028-x.
[80] CHEN X M, LI Y L, MO J M, et al. Effects of nitrogen deposition on soil organic carbon fractions in the subtropical forest ecosystems of S China [J]. J Plant Nutr Soil Sci, 2012, 175(6): 947–953. doi: 10.1002/ jpln.201100059.
[81] YAN G Y, XING Y J, Xu L J, et al. Effects of different nitrogen additions on soil microbial communities in different seasons in a boreal forest [J]. Ecosphere, 2017, 8(7): e01879. doi: 10.1002/ecs2.1879.
[82] TIAN D, JIANG L, MA S H, et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China [J]. Sci Total Environ, 2017, 607–608: 1367–1375. doi: 10.1016/ j.scitotenv.2017.06.057.
[83] NING C, MUELLER G M, EGERTON-WARBURTON L M, et al. Diversity and enzyme activity of ectomycorrhizal fungal communities following nitrogen fertilization in an urban-adjacent pine plantation [J]. Forests, 2018, 9(3): 99. doi: 10.3390/f9030099.
[84] CUI J, WANG J J, XU J, et al. Changes in soil bacterial communities in an evergreen broad-leaved forest in east China following 4 years of nitrogen addition [J]. J Soil Sediment, 2017, 17(8): 2156–2164. doi: 10.1007/s11368-017-1671-y.
[85] NIE Y X, WANG M C, ZHANG W, et al. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment [J]. Sci Total Environ, 2018, 624: 407–415. doi: 10.1016/j.scitotenv.2017.12.142.
[86] LIU L, ZHANG T, GILLIAM F S, et al. Interactive effects of nitrogen and phosphorus on soil microbial communities in a tropical forest [J]. PLoS One, 2013, 8(4): e61188. doi: 10.1371/journal.pone.0061188.
[87] KOU L, ZHANG X Y, WANG H M, et al. Nitrogen additions inhibit nitrification in acidic soils in a subtropical pine plantation: Effects of soil pH and compositional shifts in microbial groups [J]. J For Res, 2019, 30(2): 669–678. doi: 10.1007/s11676-018-0645-2.
[88] WANG X Y, WEN T. Effects of simulated nitrogen deposition on soil nitrogen transformation in artificial Korean pine of Xiaoxing’anling region [J]. Chin J Soil Sci, 2017, 48(3): 604–610. doi: 10.19336/j.cnki. trtb.2017.03.14.王小云, 温腾. 模拟氮沉降对小兴安岭地区人工红松林土壤氮转化的影响 [J]. 土壤通报, 2017, 48(3): 604–610. doi: 10.19336/j.cnki. trtb.2017.03.14.
[89] ALLISON S D, VITOUSEK P M. Responses of extracellular enzymes to simple and complex nutrient inputs [J]. Soil Biol Biochem, 2005, 37(5): 937–944. doi: 10.1016/j.soilbio.2004.09.014.
[90] ZHENG M H, LI D J, LU X, et al. Effects of phosphorus addition with and without nitrogen addition on biological nitrogen fixation in tropical legume and non-legume tree plantations [J]. Biogeochemistry, 2016, 131: 65–76. doi: 10.1007/s10533-016-0265-x.
[91] DONG W Y, ZHANG X Y, LIU X Y, et al. Responses of soil microbial communities and enzyme activities to nitrogen and phosphorus additions in Chinese fir plantations of subtropical China [J]. Biogeosciences, 2015, 12: 5537–5546. doi: 10.5194/bg-12-5537-2015.
[92] JING X, CHEN X, TANG M, et al. Nitrogen deposition has minor effect on soil extracellular enzyme activities in six Chinese forests [J]. Sci Total Environ, 2017, 607–608: 806–815. doi: 10.1016/j.scitotenv. 2017.07.060.
[93] WANG S H, MORI T, MO J M, et al. The responses of carbon- and nitrogen-acquiring enzymes to nitrogen and phosphorus additions in two plantations in southern China [J]. J For Res, 2019. doi: 10.1007/ s11676-019-00905-0.
[94] XU G L, MO J M, ZHOU G Y. Responses of soil fauna biomass to N deposition in three forests in subtropical China [J]. Zool Res, 2005, 26 (6): 609–615. doi: 10.3321/j.issn:0254-5853.2005.06.006.徐国良, 莫江明, 周国逸. 氮沉降对三种林型土壤动物群落生物量的影响 [J]. 动物学研究, 2005, 26(6): 609–615. doi: 10.3321/j.issn: 0254-5853.2005.06.006.
[95] XU G L, MO J M, ZHOU G Y, et al. Preliminary response of soil fauna to simulated N deposition in three typical subtropical forests [J]. Pedo- sphere, 2006, 16(5): 596–601. doi: 10.1016/S1002-0160(06)60093-3.
[96] XU G L, MO J M, FU S L, et al. Response of soil fauna to simulated nitrogen deposition: A nursery experiment in subtropical China [J]. J Environ Sci, 2007, 19(5): 603–609. doi: 10.1016/S1001-0742(07)60 100-4.
[97] ZHOU D Y, BU D R, GE Z W, et al. Effects of nitrogen addition on soil fauna in poplar plantation with different ages in a coastal area of eastern China [J]. Chin J Ecol, 2015, 34(9): 2553–2560. 周丹燕, 卜丹蓉, 葛之葳, 等. 氮添加对沿海不同林龄杨树人工林土壤动物群落的影响 [J]. 生态学杂志, 2015, 34(9): 2553–2560.
[98] BIAN H X, GENG Q H, XIAO H R, et al. Fine root biomass mediates soil fauna community in response to nitrogen addition in poplar planta- tions () on the east coast of China [J]. Forests, 2019, 10: 122. doi: 10.3390/f10020122.
[99] ZHAO J, WANG F M, LI J, et al. Effects of experimental nitrogen and/ or phosphorus additions on soil nematode communities in a secondary tropical forest [J]. Soil Biol Biochem, 2014, 75: 1–10. doi: 10.1016/j. soilbio.2014.03.019.
[100] FU X L, GUO D L, WANG H M, et al. Differentiating between root- and leaf-litter controls on the structure and stability of soil micro- food webs [J]. Soil Biol Biochem, 2017, 113: 192–200. doi: 10.1016/j. soilbio.2017.06.013.
[101] CHENG Y Y, SUN T, WANG Q K, et al. Effects of simulated nitrogen deposition on temperate forest soil nematode communities and their metabolic footprints [J]. Acta Ecol Sin, 2018, 38(2): 475– 484. doi: 10. 5846/stxb201606231225.程云云, 孙涛, 王清奎, 等. 模拟氮沉降对温带森林土壤线虫群落组成和代谢足迹的影响 [J]. 生态学报, 2018, 38(2): 475–484. doi: 10.5846/stxb201606231225.
[102] LIN H, HE Z H, HAO J W, et al. Effect of N addition on home-field advantage of litter decomposition in subtropical forests [J]. For Ecol Manage, 2017, 398: 216–225. doi: 10.1016/j.foreco.2017.05.015.
[103] ZHUANG H F, SUN Y, GU J C, et al. Effects of nitrogen addition on soil fauna communities inandplantations [J]. Biodiv Sci, 2010, 18(4): 390–397. doi: 10.3724/SP.J. 1003.2010.390.庄海峰, 孙玥, 谷加存, 等. 施氮肥对落叶松和水曲柳人工林土壤动物群落的影响 [J]. 生物多样性, 2010, 18(4): 390–397. doi: 10. 3724/SP.J.1003.2010.390.
[104] SHAO Y H, ZHANG W X, EISENHAUER N, et al. Nitrogen depo- sition cancels out exotic earthworm effects on plant-feeding nematode communities [J]. J Anim Ecol, 2017, 86(4): 708–717. doi: 10.1111/ 1365-2656.12660.
[105] GAO Q, HASSELQUIST N J, PALMROTH S, et al. Short-term response of soil respiration to nitrogen fertilization in a subtropical evergreen forest [J]. Soil Biol Biochem, 2014, 76: 297–300. doi: 10. 1016/j.soilbio.2014.04.020.
[106] ZHOU L Y, ZHOU X H, ZHANG B C, et al. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis [J]. Glob Change Biol, 2014, 20(7): 2332–2343. doi: 10.1111/gcb.12490.
[107] FAN H B, WU J P, LIU W F, et al. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest [J]. Plant Soil, 2014, 379(1/2): 361–371. doi: 10. 1007/s11104-014-2076-y.
[108] MO J M, ZHANG W, ZHU W X, et al. Response of soil respiration to simulated N deposition in a disturbed and a rehabilitated tropical forest in southern China [J]. Plant Soil, 2007, 296(1/2): 125–135. doi: 10. 1007/s11104-007-9303-8.
[109] ZHONG Y Q W, YAN W M, SHANGGUAN Z P. The effects of nitrogen enrichment on soil CO2fluxes depending on temperature and soil properties [J]. Glob Ecol Biogeogr, 2016, 25(4): 475–488. doi: 10.1111/geb.12430.
[110] TIAN P, ZHANG J B, CAI Z C, et al. Different response of CO2and N2O fluxes to N deposition with seasons in a temperate forest in northeastern China [J]. J Soil Sediment, 2018, 18(5): 1821–1831. doi: 10.1007/s11368-018-1919-1.
[111] LU M, ZHOU X H, LUO Y Q, et al. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis [J]. Agric Ecosyst Environ, 2011, 140(1/2): 234–244. doi: 10.1016/j.agee.2010.12.010.
[112] WANG Q K, ZHANG W D, SUN T, et al. N and P fertilization reduced soil autotrophic and heterotrophic respiration in a youngforest [J]. Agric For Meteorol, 2017, 232: 66–73. doi: 10.1016/j.agrformet.2016.08.007.
[113] SUN Z Z, LIU L L, MA Y C, et al. The effect of nitrogen addition on soil respiration from a nitrogen-limited forest soil [J]. Agric For Meteorol, 2014, 197: 103–110. doi: 10.1016/j.agrformet.2014.06.010.
[114] DU Y H, GUO P, LIU J Q, et al. Different types of nitrogen deposi- tion show variable effects on the soil carbon cycle process of temperate forests [J]. Glob Change Biol, 2014, 20(10): 3222–3228. doi: 10.1111/ gcb.12555.
[115] PENG Y, CHEN G S, CHEN G T, et al. Soil biochemical responses to nitrogen addition in a secondary evergreen broad-leaved forest ecosystem [J]. Sci Rep, 2017, 7: 2783. doi: 10.1038/s41598-017-03 044-w.
[116] DENG Q, ZHOU G, LIU J, et al. Responses of soil respiration to elevated carbon dioxide and nitrogen addition in young subtropical forest ecosystems in China [J]. Biogeosciences, 2010, 7: 315–328. doi: 10.5194/bg-7-315-2010.
[117] DALAL R C, WANG W J, ROBERTSON G P, et al. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review [J]. Aust J Soil Res, 2003, 41(2): 165–195. doi: 10.1071/SR02 064.
[118] LIU L L, GREAVER T L. A review of nitrogen enrichment effects on three biogenic GHGs: The CO2sink may be largely offset by stimu- lated N2O and CH4emission [J]. Ecol Lett, 2009, 12(10): 1103–1117. doi: 10.1111/j.1461-0248.2009.01351.x.
[119] CHEN H, GURMESA G A, ZHANG W, et al. Nitrogen saturation in humid tropical forests after 6 years of nitrogen and phosphorus addition: Hypothesis testing [J]. Funct Ecol, 2016, 30(2): 305–313. doi: 10.1111/1365-2435.12475.
[120] ZHENG M H, ZHANG T, LIU L, et al. Effects of nitrogen and phosphorus additions on nitrous oxide emission in a nitrogen-rich and two nitrogen-limited tropical forests [J]. Biogeosciences, 2016, 13: 3503–3517. doi: 10.5194/bg-13-3503-2016.
[121] FANG H J, YU G R, CHENG S L, et al. Nitrogen-15 signals of leaf- litter-soil continuum as a possible indicator of ecosystem nitrogen saturation by forest succession and N loads [J]. Biogeochemistry, 2011, 102(1/2/3): 251–263. doi: 10.1007/s10533-010-9438-1.
[122] ZHANG W, ZHU X, LUO Y, et al. Responses of nitrous oxide emissions to nitrogen and phosphorus additions in two tropical plantations with N-fixing vs. non-N-fixing tree species [J]. Biogeo- sciences, 2014, 11: 4941–4951. doi: 10.5194/bg-11-4941-2014.
[123] WANG Y S, CHENG S L, FANG H J, et al. Simulated nitrogen deposition reduces CH4uptake and increases N2O emission from a subtropical plantation forest soil in southern China [J]. PLoS One, 2014, 9(4): e93571. doi: 10.1371/journal.pone.0093571.
[124] LI X Y, CHENG S L, FANG H J, et al. The contrasting effects of deposited NH4+and NO3–on soil CO2, CH4and N2O fluxes in a sub- tropical plantation, southern China [J]. Ecol Eng, 2015, 85: 317–327. doi: 10.1016/j.ecoleng.2015.10.003.
[125] ZHANG W, ZHU X M, LIU L, et al. Large difference of inhibitive effect of nitrogen deposition on soil methane oxidation between plantations with N-fixing tree species and non-N-fixing tree species [J]. J Geophys Res: Biogeosci, 2012, 117: G00N16. doi: 10.1029/2012JG 002094.
[126] XIE D N, SI G Y, ZHANG T, et al. Nitrogen deposition increases N2O emission from an N-saturated subtropical forest in southwest China [J]. Environ Pollut, 2018, 243: 1818–1824. doi: 10.1016/j.envpol.2018.09. 113.
[127] ZHU J, MULDER J, BAKKEN L, et al. The importance of denitri- fication for N2O emissions from an N-saturated forest in SW China: Results from in situ15N labeling experiments [J]. Biogeochemistry, 2013, 116(1/2/3): 103–117. doi: 10.1007/s10533-013-9883-8.
[128] TANG W G, CHEN D X, Phillips O L, et al. Effects of long-term increased N deposition on tropical montane forest soil N2and N2O emissions [J]. Soil Biol Biochem, 2018, 126: 194–203. doi: 10.1016/j. soilbio.2018.08.027.
[129] XU X K, HAN L, LUO X B, et al. Effects of nitrogen addition on dissolved N2O and CO2, dissolved organic matter, and inorganic nitrogen in soil solution under a temperate old-growth forest [J]. Geoderma, 2009, 151(3/4): 370–377. doi: 10.1016/j.geoderma.2009. 05.008.
[130] GENG S C, CHEN Z J, HAN S J, et al. Rainfall reduction amplifies the stimulatory effect of nitrogen addition on N2O emissions from a temperate forest soil [J]. Sci Rep, 2017, 7: 43329. doi: 10.1038/srep 43329.
[131] BAI E, LI W, LI S L, et al. Pulse increase of soil N2O emission in response to N addition in a temperate forest on Mt Changbai, north- east China [J]. PLoS One, 2014, 9(7): e102765. doi: 10.1371/journal. one.0102765.
[132] CHENG S L, WANG L, FANG H J, et al. Nonlinear responses of soil nitrous oxide emission to multi-level nitrogen enrichment in a tempe- rate needle-broadleaved mixed forest in northeast China [J]. Catena, 2016, 147: 556–563. doi:10.1016/j.catena.2016.08.010.
[133] CHEN Z J, SETÄLÄ H, GENG S C, et al. Nitrogen addition impacts on the emissions of greenhouse gases depending on the forest type: A case study in Changbai Mountain, northeast China [J]. J Soil Sedi- ment, 2017, 17(1): 23–34.doi: 10.1007/s11368-016-1481-7.
[134] SONG L, TIAN P, ZHANG J B, et al. Effects of three years of simulated nitrogen deposition on soil nitrogen dynamics and green- house gas emissions in a Korean pine plantation of northeast China [J]. Sci Total Environ, 2017, 609: 1303–1311. doi: 10.1016/j.scitotenv. 2017.08.017.
[135] SONG L, ZHANG J B, MÜLLER C, et al. Responses of soil N trans- formations and N loss to three years of simulated N deposition in a temperate Korean pine plantation in northeast China [J]. Appl Soil Ecol, 2019, 137: 49–56. doi: 10.1016/j.apsoil.2019.01.008.
[136] XU K, WANG C M, YANG X T. Five-year study of the effects of simulated nitrogen deposition levels and forms on soil nitrous oxide emissions from a temperate forest in northern China [J]. PLoS One, 2017, 12(12): e0189831. doi: 10.1371/journal.pone.0189831.
[137] ZHANG W, MO J M, ZHOU G Y, et al. Methane uptake responses to nitrogen deposition in three tropical forests in southern China [J]. J Geophys Res Atmos, 2008, 113: D11116. Doi: 10.1029/2007JD009195.
[138] XU X K, HAN L, LUO X B, et al. Synergistic effects of nitrogen amendments and ethylene on atmospheric methane uptake under a temperate old-growth forest [J]. Adv Atmos Sci, 2011, 28(4): 843– 854. doi: 10.1007/s00376-010-0071-7.
[139] WANG Y S, CHENG S L, FANG H J, et al. Relationships between ammonia-oxidizing communities, soil methane uptake and nitrous oxide fluxes in a subtropical plantation soil with nitrogen enrichment [J]. Eur J Soil Biol, 2016, 73: 84–92. doi: 10.1016/j.ejsobi.2016.01.008.
[140] ZHENG M H, ZHANG T, LIU L, et al. Effects of nitrogen and phosphorus additions on soil methane uptake in disturbed forests [J]. J Geophys Res Biogeosci, 2016, 121(12): 3089–3100. doi: 10.1002/ 2016JG003476.
[141] YANG X T, WANG C M, XU K. Response of soil CH4fluxes to stimulated nitrogen deposition in a temperate deciduous forest in northern China: A 5-year nitrogen addition experiment [J]. Eur J Soil Biol, 2017, 82: 43–49. doi: 10.1016/j.ejsobi.2017.08.004.
[142] GENG J, CHENG S L, FANG H J, et al. Soil nitrate accumulation explains the nonlinear responses of soil CO2and CH4fluxes to nitrogen addition in a temperate needle-broadleaved mixed forest [J]. Ecol Indic, 2017, 79: 28–36. doi: 10.1016/j.ecolind.2017.03.054.
[143] CLEVELAND C C, TOWNSEND A R, SCHIMEL D S, et al. Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems [J]. Glob Biogeochem Cy, 1999, 13(2): 623–645. doi: 10. 1029/1999GB900014.
[144] VITOUSEK P M, MENGE D N L, REED S C, et al. Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems [J]. Philos Trans R Soc Lond B Biol Sci, 2013, 368 (1621): 20130119. doi: 10.1098/rstb.2013.0119.
[145] GALLOWAY J N, TOWNSEND A R, ERISMAN J W, et al. Trans- formation of the nitrogen cycle: Recent trends, questions, and potential solutions [J]. Science, 2008, 320(5878): 889–892. doi: 10.1126/ science.1136674.
[146] SULLIVAN B W, SMITH W K, TOWNSEND A R, et al. Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle [J]. Proc Natl Acad Sci USA, 2014, 111(22): 8101–8106. doi: 10.1073/pnas.1320646111.
[147] REED S C, CLEVELAND C C, TOWNSEND A R. Functional ecology of free-living nitrogen fixation: A contemporary perspective [J]. Annu Rev Ecol Evol Syst, 2011, 42(1): 489–512. doi: 10.1146/annurev- ecolsys-102710-145034.
[148] CREW T E, FARRIONTON H, VITOUSEK P M. Changes in asymbiotic, heterotrophic nitrogen fixation on leaf litter of metrosi- deros polymorpha with long-term ecosystem development in Hawaii [J]. Ecosystems, 2000, 3(4): 386–395. doi: 10.1007/s100210000034.
[149] ZECKRISSON O, DELUCA T H, NILSSON M C, et al. Nitrogen fixation increases with successional age in boreal forests [J]. Ecology, 2004, 85(12): 3327–3334. doi: 10.1890/04-0461.
[150] BARRON A R, WURZBURGER N, BELLENGER J P, et al. Molyb- denum limitation of asymbiotic nitrogen fixation in tropical forest soils [J]. Nat Geosci, 2009, 2(1): 42–45. doi: 10.1038/ngeo366.
[151] CUSACK D F, SILVER W, MICOWELL W H. Biological nitrogen fixation in two tropical forests: Ecosystem-level patterns and effects of nitrogen fertilization [J]. Ecosystems, 2009, 12(8): 1299–1315. doi: 10. 1007/s10021-009-9290-0.
[152] LIU X J, ZHANG Y, HAN W X, et al. Enhanced nitrogen deposition over China [J]. Nature, 2013, 494(7438): 459–462. doi: 10.1038/ nature11917.
[153] ZHENG M H, CHEN H, LI D J, et al. Biological nitrogen fixation and its response to nitrogen input in two mature tropical plantations with and without legume trees [J]. Biol Fert Soils, 2016, 52(5): 665–674. doi: 10.1007/s00374-016-1109-5.
[154] ZHENG M H, ZHANG W, LUO Y Q, et al. Different responses of asymbiotic nitrogen fixation to nitrogen addition between disturbed and rehabilitated subtropical forests [J]. Sci Total Environ, 2017, 601– 602: 1505–1512. doi: 10.1016/j.scitotenv.2017.06.036.
[155] HEDIN L O, BROOKSHIRE E N J, MENGE D N L, et al. The nitrogen paradox in tropical forest ecosystems [J]. Annu Rev Ecol Evol Syst, 2009, 40(1): 613–635. doi: 10.1146/annurev.ecolsys.37.091305.110246.
[156] WANG Q, WANG J L, LI Y Z, et al. Influence of nitrogen and pho- sphorus additions on N2-fixation activity, abundance, and composition of diazotrophic communities in a Chinese fir plantation [J]. Sci Total Environ, 2018, 619–620: 1530–1537. doi: 10.1016/j.scitotenv.2017. 10.064.
[157] TANG Y Q, YU G R, ZHANG X Y, et al. Different strategies for regulating free-living N2fixation in nutrient-amended subtropical and temperate forest soils [J]. Appl Soil Ecol, 2019, 136: 21–29. doi: 10. 1016/j.apsoil.2018.12.014.
[158] MARKHAM J H, ZEKVELD C. Nitrogen fixation makes biomass allocation to roots independent of soil nitrogen supply [J]. Can J Bot, 2007, 85(9): 787–793. doi: 10.1139/B07-075.
[159] LU M, YANG Y H, LUO Y Q, et al. Responses of ecosystem nitrogen cycle to nitrogen addition: A meta-analysis [J]. New Phytol, 2011, 189 (4): 1040–1050. doi: 10.1111/j.1469-8137.2010.03563.x.
[160] LIU W J, YU L F, ZHANG T, et al.15N labeling experiment reveals different long-term responses to ammonium and nitrate inputs in N-saturated subtropical forest [J]. J Geophys Res Biogeosci, 2017, 122(9): 2251–2264. doi: 10.1002/2017JG003963.
[161] FANG Y T, ZHU W X, GUNDERSEN P, et al. Large loss of dissolved organic nitrogen from nitrogen-saturated forests in subtropical China [J]. Ecosystems, 2009, 12(1): 33–45. doi: 10.1007/s10021-008-9203-7.
[162] GURMESA G A, LU X K, GUNDERSEN P, et al. High retention of15N-labeled nitrogen deposition in a nitrogen saturated old-growth tropical forest [J]. Glob Change Biol, 2016, 22(11): 3608–3620. doi: 10.1111/gcb.13327.
[163] BAO X, BAO X X, LIU X C. Effects of nitrogen deposition on soil nitrogen mineralization offorest in Daxing’an Mountains [J]. J NE For Univ, 2015, 43(7): 78–83. doi: 10.3969/j.issn. 1000-5382.2015.07.018.包翔, 包秀霞, 刘星岑. 施氮量对大兴安岭白桦次生林土壤氮矿化的影响[J]. 东北林业大学学报, 2015, 43(7): 78–83. doi: 10.3969/j. issn.1000-5382.2015.07.018.
[164] GAO W L, KOU L, ZHANG J B, et al. Ammonium fertilization causes a decoupling of ammonium cycling in a boreal forest [J]. Soil Biol Biochem, 2016, 101: 114–123. doi: 10.1016/j.soilbio.2016.07.007.
[165] TIAN P, ZHANG J B, MÜLLER C, et al. Effects of six years of simulated N deposition on gross soil N transformation rates in an old- growth temperate forest [J]. J For Res, 2018, 29(3): 647–656. doi: 10. 1007/s11676-017-0484-6.
[166] SUN J F, PENG B, LI W, et al. Effects of nitrogen addition on potential soil nitrogen-cycling processes in a temperate forest eco- system [J]. Soil Sci, 2016, 181(1): 29–38. doi: 10.1097/SS.00000000 00000134.
[167] ZHAO Y, ZHANG C, ZHAO H F, et al. Effects of N and P addition on soil nitrogen mineralization in a subtropical evergreen broad-leaved forest [J]. Chin J Ecol, 2013, 32(7): 1690–1697. 赵阳, 张驰, 赵竑绯, 等. 氮磷添加对亚热带常绿阔叶林土壤氮素矿化的影响 [J]. 生态学杂志, 2013, 32(7): 1690–1697.
[168] CHEN H, ZHANG W, GURMESA G A, et al. Phosphorus addition affects soil nitrogen dynamics in a nitrogen-saturated and two nitrogen- limited forests [J]. Eur J Soil Sci, 2017, 68(4): 472–479. doi: 10.1111/ ejss.12428.
[169] GAO W L, KOU L, YANG H, et al. Are nitrate production and retention processes in subtropical acidic forest soils responsive to ammonium deposition? [J]. Soil Biol Biochem, 2016, 100: 102–109. doi: 10.1016/j.soilbio.2016.06.002.
[170] GAO W L, KOU L, ZHANG J B, et al. Enhanced deposition of nitrate alters microbial cycling of N in a subtropical forest soil [J]. Biol Fert Soils, 2016, 52(7): 977–986. doi: 10.1007/s00374-016-1134-4.
[171] ZHOU K J, LU X K, MORI T, et al. Effects of long-term nitrogen deposition on phosphorus leaching dynamics in a mature tropical forest [J]. Biogeochemistry, 2018, 138(2): 215–224. doi: 10.1007/ s10533-018-0442-1.
[172] YANG K, ZHU J J, GU J C, et al. Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in aplantation [J]. Ann For Sci, 2015, 72(4): 435–442. doi: 10. 1007/s13595-014-0444-7.
[173] FAN Y X, ZHONG X J, LIN F, et al. Responses of soil phosphorus fractions after nitrogen addition in a subtropical forest ecosystem: Insights from decreased Fe and Al oxides and increased plant roots [J]. Geoderma, 2019, 337: 246–255. doi: 10.1016/j.geoderma.2018.09.028.
[174] WANG Q K, WANG S L, LIU Y X. Responses to N and P fertili- zation in a youngplantation: Microbial properties, enzyme activities and dissolved organic matter [J]. Appl Soil Ecol, 2008, 40(3): 484–490. doi: 10.1016/j.apsoil.2008.07.003.
[175] LU X K, MO J M, GILLIAM F S, et al. Nitrogen addition shapes soil phosphorus availability in two reforested tropical forests in southern China [J]. Biotropica, 2012, 44(3): 302–311. doi: 10.1111/j.1744-7429. 2011.00831.x.
[176] LI Y, NIU S L, YU G R. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis [J]. Glob Change Biol, 2016, 22(2): 934–943. doi: 10.1111/gcb.13125.
[177] BRAUN S, THOMAS V F D, QUIRING R, et al. Does nitrogen deposition increase forest production? The role of phosphorus [J]. Environ Pollut, 2010, 158(6): 2043–2052. doi: 10.1016/j.envpol.2009. 11.030.
[178] LONG M, WU H H, SMITH M D, et al. Nitrogen deposition promotes phosphorus uptake of plants in a semi-arid temperate grassland [J]. Plant Soil, 2016, 408(1/2): 475–484. doi: 10.1007/s11104-016-3022-y.
[179] WANG M, MEAGHAN T M, MOORE T R. Nutrient resorption of two evergreen shrubs in response to long-term fertilization in a bog [J]. Oecologia, 2014, 174(2): 365–377. doi: 10.1007/s00442-013-2784-7.
[180] GONZALES K, RUTH Y. Nitrogen-phosphorous interactions in young northern hardwoods indicate P limitation: Foliar concentrations and resorption in a factorial N by P addition experiment [J]. Oecologia, 2019, 189(3): 829–840. doi: 10.1007/s00442-019-04350-y.
[181] SEE C R, YANAI R D, FISK M C, et al. Soil nitrogen affects phosphorus recycling: Foliar resorption and plant-soil feedbacks in a northern hardwood forest [J]. Ecology, 2015, 96(9): 2488–2498. doi: 10.1890/15-0188.1.
[182] ZHENG M H, HUANG J, CHEN H, et al. Effects of nitrogen and phosphorus addition on soil phosphatase activity in different forest types [J]. Acta Ecol Sin, 2015, 35(20): 6703–6710. doi: 10.5846/stxb 201405120970.郑棉海, 黄娟, 陈浩, 等. 氮、磷添加对不同林型土壤磷酸酶活性的影响 [J]. 生态学报, 2015, 35(20): 6703–6710. doi: 10.5846/stxb 201405120970.
[183] PARRON L M, BUSTAMANTE M M C, MARKEWITZ D. Fluxes of nitrogen and phosphorus in a gallery forest in the Cerrado of central Brazil [J]. Biogeochemistry, 2011, 105(1/2/3): 89–104. doi: 10.1007/ s10533-010-9537-z.
[184] SMITH S W, WOODIN S J, PAKEMAN R J, et al. Root traits predict decomposition across a landscape-scale grazing experiment [J]. New Phytol, 2014, 203(3): 851–862. doi: 10.1111/nph.12845.
[185] ZHANG Y T, LI J M, LI X, et al. Effects of simulated nitrogen deposition on decomposition and nutrient release of leaf litter of[J]. Arid Zone Res, 2016, 33(5): 966–973. doi: 10.13866/ j.azr.2016.05.08.张毓涛, 李吉玫, 李翔, 等. 模拟氮沉降对天山云杉凋落叶分解及其养分释放的影响 [J]. 干旱区研究, 2016, 33(5): 966–973. doi: 10. 13866/j.azr.2016.05.08.
[186] YAO X. Effects of nitrogen addition on litter decomposition in artificialforests [D]. Yangling: Northwest Agriculture and Forest University, 2017: 8. 姚旭. 氮添加对人工油松林叶凋落物分解的影响 [D]. 杨凌: 西北农林科技大学, 2017: 8.
[187] LI X F, ZHENG X B, HAN S J, et al. Effects of nitrogen additions on nitrogen resorption and use efficiencies and foliar litterfall of six tree species in a mixed birch and poplar forest, northeastern China [J]. Can J For Res, 2010, 40(11): 2256–2261. doi: 10.1139/X10-167.
[188] ZHOU S X, XIAO Y X, XIANG Y B, et al. Effects of simulated nitrogen deposition on the substrate quality of foliar litter in a natural evergreen broad-leaved forest in the Rainy Area of western China [J]. Acta Ecol Sin, 2016, 36(22): 7428–7435. doi: 10.5846/stxb2016010 80054.周世兴, 肖永翔, 向元彬, 等. 模拟氮沉降对华西雨屏区天然常绿阔叶林凋落叶分解过程中基质质量的影响 [J]. 生态学报, 2016, 36(22): 7428–7435. doi: 10.5846/stxb201601080054.
[189] ZHANG W D, CHAO L, YANG Q P, et al. Litter quality mediated nitrogen effect on plant litter decomposition regardless of soil fauna presence [J]. Ecology, 2016, 97(10): 2834–2843. doi: 10.1002/ecy. 1515.
[190] FANG X, LIU J X, ZHANG D Q, et al. Effects of precipitation change and nitrogen addition on organic carbon mineralization and soil microbial carbon of the forest soils in Dinghushan, southeastern China [J]. Chin J Appl Environ Biol, 2012, 18(4): 531–538. doi: 10. 3724/SP.J.1145.2012.00531.方熊, 刘菊秀, 张德强, 等. 降水变化、氮添加对鼎湖山主要森林土壤有机碳矿化和土壤微生物碳的影响 [J]. 应用与环境生物学报, 2012, 18(4): 531–538. doi: 10.3724/SP.J.1145.2012.00531.
[191] TIE L H, FU R, ZHANG S B, et al. Effects of simulated nitrogen and sulfur deposition on litter decomposition rate in an evergreen broad- leaved forest in the rainy area of western China [J]. Chin J Appl Ecol, 2018, 29(7): 2243–2250. doi: 10.13287/j.1001-9332.201807.012.铁烈华, 符饶, 张仕斌, 等. 模拟氮、硫沉降对华西雨屏区常绿阔叶林凋落叶分解速率的影响 [J]. 应用生态学报, 2018, 29(7): 2243–2250. doi: 10.13287/j.1001-9332.201807.012.
[192] LI Y Y, WANG Z W, SUN T. Response of fine root decomposition to long-term nitrogen addition in the temperate forest [J]. Bull Bot Res, 2017, 37(6): 848–854. doi: 10.7525/j.issn.1673-5102.2017.06.007.李媛媛, 王正文, 孙涛. 氮添加对温带森林细根长期分解的影响 [J]. 植物研究, 2017, 37(6): 848–854. doi: 10.7525/j.issn.1673-5102. 2017.06.007.
[193] CHEN X, ZHOU M, WEI J S, et al. Effects of simulated nitrogen deposition on litter decomposition inforest [J]. Ecol Environ Sci, 2013, 22(9): 1496–1503. doi: 10.3969/j.issn.1674-5906. 2013.09.007.陈翔, 周梅, 魏江生, 等. 模拟氮沉降对兴安落叶松林凋落物分解的影响 [J]. 生态环境学报, 2013, 22(9): 1496–1503. doi: 10.3969/ j.issn.1674-5906.2013.09.007.
[194] MO J M, XUE J H, FANG Y T. Litter decomposition and its responses to simulated N deposition for the major plants of Dinghushan forests in subtropical China [J]. Acta Ecol Sin, 2004, 24 (7): 1413–1420. doi: 10.3321/j.issn:1000-0933.2004.07.015.莫江明, 薛璟花, 方运霆. 鼎湖山主要森林植物凋落物分解及其对N沉降的响应 [J]. 生态学报, 2004, 24(7): 1413–1420. doi: 10.3321/ j.issn:1000-0933.2004.07.015.
[195] ZHOU S X, HUANG C D, XIANG Y B, et al. Effects of simulated nitrogen deposition on lignin and cellulose degradation of foliar litter in natural evergreen broad-leaved forest in Rainy Area of western China [J]. Chin J Appl Ecol, 2016, 27(5): 1368–1374. doi: 10.13287/j. 1001-9332.201605.004.周世兴, 黄从德, 向元彬, 等. 模拟氮沉降对华西雨屏区天然常绿阔叶林凋落物木质素和纤维素降解的影响 [J]. 应用生态学报, 2016, 27(5): 1368–1374. doi: 10.13287/j.1001-9332.201605.004.
[196] HOLOPAINEN J K, GERSHENZON J. Multiple stress factors and the emission of plant VOCs [J]. Trends Plant Sci, 2010, 15(3): 176– 184. doi: 10.1016/j.tplants.2010.01.006.
[197] LORETO F, SCHNITZLER J P. Abiotic stresses and induced BVOCs [J]. Trends Plant Sci, 2010, 15(3): 154–166. doi: 10.1016/j.tplants. 2009.12.006.
[198] HUANG J, MO J M, KONG G H, et al. Research perspective for the effects of nitrogen deposition on biogenic volatile organic compounds [J]. Acta Ecol Sin, 2011, 31(21): 6616–6623. 黄娟, 莫江明, 孔国辉, 等. 植物源挥发性有机物对氮沉降响应研究展望 [J]. 生态学报, 2011, 31(21), 6616–6623.
[199] HUANG J, LU X K, MO J M, et al. Effects of simulated N deposition on carbonyl compounds released by nursery plants [C]// Proceedings of the Annual Conference of Chinese Society of Environmental Sciences. Chengdu: China Environment Press, 2014: 6867–6873. 黄娟, 鲁显楷, 莫江明, 等. 模拟N沉降对苗圃植物排放羰基化合物的影响 [C]// 中国环境科学学会学术年会论文集. 成都: 中国环境出版社, 2014: 6867–6873.
[200] HUANG J, MO J M, LU X K, et al. The effect of simulated nitrogen deposition on the emission of carbonyl compounds fromand[J/OL]. Expert Opin Environ Biol, 2016(S1): 1–7. doi: 10.4172/2325-9655.S1-004.
[201] CHEN H, LI D J, GURMESA G A, et al. Effects of nitrogen deposi- tion on carbon cycle in terrestrial ecosystems of China: A meta-analysis [J]. Environ Pollut, 2015, 206: 352–360. doi: 10.1016/j.envpol.2015. 07.033.
[202] RAINEY S M, NADELHOFFER K J, SILVER W L, et al. Effects of chronic nitrogen additions on understory species in a red pine plantation [J]. Ecol Appl, 1999, 9(3): 949–957. doi: 10.1890/1051-0761(1999) 009[0949:EOCNAO]2.0.CO;2.
[203] BERG B, MATZNER E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems [J]. Environ Rev, 1997, 5(1): 1–25. doi: 10.1139/a96-017.
[204] JANSSENS I A, DIELEMAN W, LUYSSAERT S, et al. Reduction of forest soil respiration in response to nitrogen deposition [J]. Nat Geosci, 2010, 3(5): 315–322. doi: 10.1038/ngeo844.
[205] FANG H, MO J M, PENG S L, et al. Cumulative effects of nitrogen additions on litter decomposition in three tropical forests in southern China [J]. Plant Soil, 2007, 297(1/2): 233–242. doi: 10.1007/s11104- 007-9339-9.
[206] TEMPLER P H, MACK M C, CHAPIN F S III, et al. Sinks for nitrogen inputs in terrestrial ecosystems: A meta-analysis of15N tracer field studies [J]. Ecology, 2012, 93(8): 1816–1829. doi: 10.1890/11- 1146.1.
[207] LIU J, PENG B, XIA Z W, et al. Different fates of deposited NH4+and NO3–in a temperate forest in northeast China: A15N tracer study [J]. Glob Change Biol, 2017, 23(6): 2441–2449. doi: 10.1111/gcb.13533.
[208] WANG A, ZHU W X, GUNDERSEN P, et al. Fates of atmospheric deposited nitrogen in an Asian tropical primary forest [J]. For Ecol Manage, 2018, 411: 213–222. doi: 10.1016/j.foreco.2018.01.029.
[209] SHENG W P, YU G R, FANG H J, et al. Sinks for inorganic nitrogen deposition in forest ecosystems with low and high nitrogen deposition in China [J]. PLoS One, 2014, 9(2): e89322. doi: 10.1371/journal.pone. 0089322.
Effects of Simulated Atmospheric Nitrogen Deposition on Forest Ecosystems in China: An Overview
LU Xian-kai1, MO Jiang-ming1, ZHANG Wei1, MAO Qing-gong1, LIU Rong-zhen1,2, WANG Cong1,2, ZHENG Mian-hai1, WANG Sen-hao1,2, MORI Taiki1, MAO Jin-hua1,2, ZHANG Yong-qun1,2, WANG Yu-fang1,2, HUANG Juan1
(1. Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystem, Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China; 2. University of Chinese Academy of Sciences, Beijing 100049, China)
Human activities, such as combustion of fossil fuel, production and application of nitrogenous fertilizer, and intensive livestock production, have been accelerating the production and emission of reactive nitrogen (e.g., NH4+, NO3–), leading to elevated nitrogen (N) deposition at regional and global scales. Human interference with N cycle has gone beyond the safe operating space for humanity. China is one of the three regions with the highest N deposition in the world. High N deposition has threatened the health and safety of terrestrial ecosystems, which should be addressed urgently during the process of ecological civilization construction. The research history on simulated N deposition in China and world was reviewed, focused on how simulated N deposition affects forest ecosystems in China, including soil acidification, plant element chemistry, plant growth and diversity, soil microbial community and enzyme activities, soil fauna, greenhouse gas emissions, ecosystem N and phosphorus cycles, soil N transformation, ecosystem N fixation, litter decomposition, and ecosystem carbon sequestration. The atmospheric N deposition has been concerned since 2000s. In 2002, the first long-term forest ecosystem N manipulative experiments were established by South China Botanical Garden (SCBG) of the Chinese Academy of Sciences, which is playing a leading role in the field of nitrogen deposition and forest ecosystems in China. In 2013, SCBG, for the first time, designed a novel experiment with canopy addition of N (CAN) vs. understory addition of N (UAN) in China. Results from N manipulative experiments across China showed that continuing high N deposition greatly altered forest structure and functioning, threatening ecosystem health, especially in the south-central China. The main results are as follows: (1) There is a fertilization effect of N deposition in temperate and boreal forests, but there seem no positive effects on plant growth in N-rich tropical forests because of N saturation. (2) Excess N deposition can lead to soil acidification and nutrient imbalance. (3) Elevated N deposition has accelerated N cycling rate and its transformation process, but depressed ecosystem N fixation rate, and altered ecosystem P availability and cycling, litter decomposition process and greenhouse gas emissions. (4) High N deposition reduced understory plants diversity and changed the structure of soil microbial community. (5) Nitrogen deposition generally simulates aboveground vegetation C sequestration across China, but there remains uncertain on belowground soil C sequestration. (6) Tropical and subtropical forest ecosystems are non-ignorable N sinks, depending on the forms and fates of added N. (7) The effects of N deposition on forest ecosystems are variable, depending on ecosystem N status, land-use history, climate, and forest types and ages. Considering that there remain uncertainties on the long-term effects of N deposition in China, it is suggested that it is necessary to continue the present studies in a longer term, and to expand ajointly consider multiple global change factors (e.g., climate warming, CO2enrichment, changes in precipitation patterns), all of which are important for forest management and sustainable development in the future.
Nitrogen deposition; Global change; Forest ecosystem; Nitrogen saturation; Nitrogen limitation; Nitrogen biogeochemical cycle; Biodiversity; Carbon sequestration
10.11926/jtsb.4113
2019–06–21
2019–08–20
国家自然科学基金项目(41731176, 31700422); 中国科学院青年创新促会基金项目(2015287)资助
This work was supported by the National Natural Science Foundation of China (Grant No. 41731176, 31700422); and the Project for Youth Innovation Promotion of Chinese Academy of Science (Grant No. 2015287).
鲁显楷, 研究员, 主要研究方向为全球变化生态学,氮素生物地球化学。E-mail: luxiankai@scbg.ac.cn

