基于柔性苯二乙酸构筑的三个钴配合物的晶体结构和电化学性质
张美丽 郑艳金 刘 敏 任宜霞 王记江 崔华莉 刘 琳
(延安大学化学与化工学院,陕西省化学反应工程重点实验室,延安 716000)
0 Introduction
The rational design and synthesis of polymeric metal-organic hybrid complexes has been extensively studied in recent years,not only on their intriguing structural diversity[1], but also on their intrinsic aesthetic appeal and encouraging properties such as catalysis[2],luminescence[3-7],chemical sensing[8],energy storage conversion[9],and drug delivery[10-11].To date,it is still a challenge to predict the exact structure of assembly products in crystal engineering since the structure is mainly dependent by many factors such as the coordination geometries of the metal centers,the coordination behaviours ofthe organic building blocks,and guest solvent or counter ions[12].
Among the reported studies,organic aromatic polycarboxylate ligands have attracted intensive research interest due to their various coordination modes to metal ions,resulting from completely or partially deprotonated sites allowing for the large diversity of topologies[13].In contrast to those of rigid ligands,the backbone flexibility and conformation changeability afford these flexible ligands versatile bridging modes and make the structures of their coordination polymers more diversiform and more difficult to predict[14-15].However,it is still a challenge to understanding the control of structure and topology of coordination polymers.In our recent work,a series of coordination polymers based on flexible phenylenediacetate and different bis(imidazole)ligands have been successfully obtained[16-17],and we are systematically investigating the influence of the coordination chemistry of phenylenediacetate ligands with different coordination groups,conformations,and flexibility.As a continuation of our research,by employing the mixture of different flexible phenylenediacetate building blocks in the presence of bis(imidazole)ligand 1,3-bis(1-imidazoly)benzene(mbib),three new coordination polymers{[Co1.5(opda)1.5(mbib)2(H2O)2].H2O}n(1),{[Co(mpda)(mbib)].H2O}n(2)and{[Co(ppda)(mbib)].H2O}n(3)(H2opda=1,2-phenylenediacetic acid,H2mpda=1,3-phenylenediacetic acid,H2ppda=1,4-phenylenediacetic acid,showing structural diversities have been synthesized and structurally characterized.Furthermore,their thermal gravimetric analysis(TGA)and electrochemistry properties have also been i nvestigated.
1 Experimental
1.1 Materials and methods
Allthestartingreagentsand solventswere commercially available and used as received without further purification.The hydrothermal reaction was performed in a 25 mL Teflon-lined stainless steel autoclave under autogenous pressure. Elemental analyses for C,H,and N were carried out on a Flash 2000 elemental analyzer.Thermal gravimetric analysis(TGA)were carried out on a SDT Q600 thermogravimetric analyzer.A platinum pan was used for heating the sample with a heating rate of 10℃.min-1under a N2atmosphere.PowderX-raydiffraction (PXRD)measurementswereperformedonaBrukerD8-ADVANCE X-ray diffractometer with Cu Kα radiation(λ=0.154 2 nm),and the experimental test conditions were the voltage of 40 kV,the current of 30 mA,and the scanning range of 5°~50°.
1.2 Syntheses of the complexes
{[Co1.5(opda)1.5(mbib)2(H2O)2].H2O}n(1).A mixture of H2opda(0.009 6 g,0.05 mmol),Co(OAc)2.4H2O(0.024 9 g,0.1 mmol),(0.018 6 g,0.1 mmol)and KOH(5.6 mg,0.1 mmol)were added to water(12 mL)in a 25 mL Teflon-lined stainless steel vessel.The mixture was heated at 150℃for 72 h.After the reactive mixture was slowly cooled to room temperature,violet block crystals of 1 were obtained.Elemental analysis Calcd.for C78H76Co3N16O18(%):C 55.03,H 4.50,N 13.17;Found(%):C 55.37,H 4.25,N 13.53.
{[Co(mpda)(mbib)].H2O}n(2).Complex 2 was synthesized by a procedure similar to that of 1,except that H2mpda(0.009 6 g,0.05 mmol)replaced H2opda.Violet block crystals of 2 were obtained.Elemental analysis Calcd.for C44H40Co2N8O10(%):C 55.12,H 4.21,N 11.69;Found(%):C 54.89,H 4.35,N 11.37.
{[Co(ppda)(mbib)].H2O}n(3). Complex3was synthesized by a procedure similar to that of 1,except that H2ppda (0.009 6 g,0.05 mmol)replaced H2opda.Violet block crystals of 3 were obtained.Elemental analysis Calcd.for C22H20CoN4O5(%):C 55.12,H 4.21,N 11.69;Found(%):C 55.18,H 4.29,N 11.27.
1.3 Crystal structure determination
X-ray single-crystal diffraction data for complexes 1~3 were collected on a Bruker Smart 1000 CCD area-detector diffractometer with Mo Kα radiation(λ=0.071 073 nm)by ω scan mode.The crystal structure was solved by direct methods,using SHELXS-2014 and least-squares refined with SHELXL-2014[18].Nonhydrogen atomswere refined anisotropically and hydrogen atoms were placed in geometrically calculated positions.Further details for structural analysis are summarized in Table 1.Selected bond distances and bond angles are listed in Table S1.
CCDC:1856259,1;1856260,2;1856261,3.
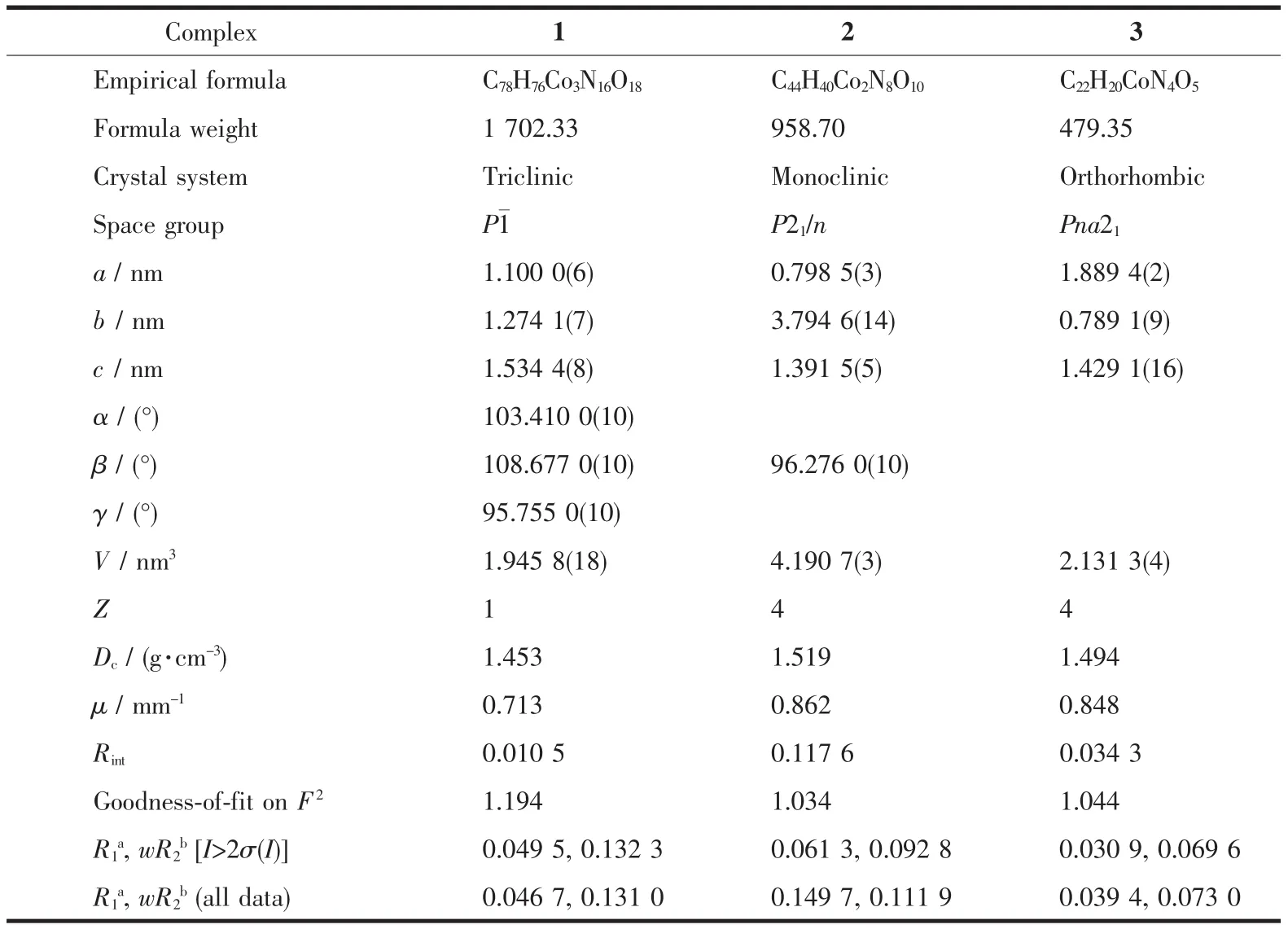
Table 1 Crystallographic data and refinement parameters for complexes 1~3
2 Results and discussion
2.1 Description of crystal structure
2.1.1 Crystal structure of{[Co1.5(opda)1.5(mbib)2(H2O)2].H2O}n(1)
Single-crystal X-ray diffraction analysis reveals that complex 1 crystallizes in the triclinic system,P1 space group,and features a 2D network.The asymmetric unit of 1 consists of one and a half of cobaltⅡions,one and a half of fully deprotonated opda2-ligands,two mbib ligands,two coordinated water molecules and one lattice water molecule.The central Co1 atom has a distorted octahedral coordination geometry,coordinated by three nitrogen atoms of three different mbib ligands(Co1-N1 0.208 3(3)nm,Co1-N4A 0.208 1(4)nm,Co1-N5 0.206 8(4)nm)and three oxygen atoms from two different opda ligands and one water molecule(Co1-O1 0.205 6(3)nm,Co1-O5 0.207 4(3)nm,Co1-O7 0.212 7(3)nm).While the Co2 centralatom issix-coordinated with a distorted octahedral coordination sphere,which is formed by two nitrogen atoms of two different mbib ligands(Co2-N8B/N8C 0.206 6(4)nm)and four oxygen atoms from three different opda2-ligands and one water molecule(Co2-O4/O4D 0.206 9(3)nm,Co2-O8/O8D 0.210 1(3)nm)(Fig.1a).
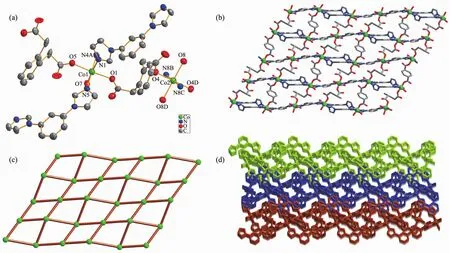
Fig.1 (a)Coordination environments of CoⅡion in complex 1 with 30%probability displacement ellipsoids;(b)Ball-and-stick view of 2D layer in 1;(c)Schematic view of(4,4)net in 1;(d)View of 3D network of 1 extended by 2D layers in an offset stacking fashion
In the structure of 1,there are two kinds of mbib ligand showing non-planarconformation with the dihedral angle between benzene core and imidazole arms being 69.00°and 9.04°,23.09°and 4.88°,respectively.Thus,the two unique Co1 atoms are bridged by two mbib ligands to form a paddle-wheel[(Co1)2(mbib)2]loop.Adjacent ones are further connected through[Co2(mbib)2]motif to generate a 1D loop containing polymer chain (Fig.S1).The chains are further extended by opda2-in μ1-η1∶η0bis(monodentate)coordination modes to form a 2D(4,4)net(Fig.1b and 1c).In addition,viewed along c direction,the abovementioned adjacent 1D chains are further interlinked to extend into an overall 3D hydrogen-bonded network(Fig.1d)by the co-effects of the inter-layer C-H…π(C25-H25…π)stacking between benzene rings of mbib and opda2-ligands in an edge-to-face orientation with the C…π separations of 0.356 4 nm as well as CH…O hydrogen-bonding interactions between benzene rings and carboxylate O atoms of opda2-ligands(C24-H24…O5).
2.1.2 Crystal structure of{[Co(mpda)(mbib)].H2O}n
(2)
Utilizing the analogous bridging ligand mpda2-,instead of the opda2-,the 1D polymer chain of 2 was obtained.Single-crystalX-ray diffraction analysis reveals that complex 2 crystallizes in the monoclinic system,P21/n space group,and the asymmetric unit of 2 contains two crystallographically independent CoⅡcenters,each having a different coordination sphere.As shown in Fig.2a,Co1 centers adopt slightly distorted tetrahedral geometries,coordinated by two nitrogen atoms of two mbib ligands(Co1-N1 0.204 0(2)nm,Co1-N8A 0.200 6(3)nm)and two oxygen atoms from two mpda2-ligands(Co1-O1 0.195 7(2)nm,Co1-O8A 0.195 5(2)nm).Co2 has a slightly distorted octahedral coordination environment,formed by four carboxyl oxygen atoms from two different mpda2-ligands(Co2-O3 0.218 1(2)nm,Co2-O4 0.225(3)nm,Co2-O5 0.190 1(18)nm,Co2-O6 0.225 5(2)nm)and two nitrogen atoms from two different mbib molecules(Co2-N4 0.206 0(2)nm,Co2-N5 0.208 1(3)nm).
As same as 1,the mbib ligands in the structure of 2 also exhibit non-planar conformation with the dihedral angle between benzene core and imidazole arms of 37.36°and 15.85°,37.12°and 37.97°,respectively.In 1,the opda2-ligand has a transconformation with two carboxyl groups locating in two sides of the benzene ring plane,which can be viewed as a linear building block.Differently,the mpda2-ligand in 2 shows a cis-conformation with two carboxyl groups lying on the same sides of the benzene ring plane and acts as a "V"type building block.Thus,two kinds of such"V"type building block link the CoⅡions giving rise to a 1D loop containing polymeric ribbon running along b direction(Fig.2b).In addition,the adjacent ribbon motifs are arranged into a 2D plane parallel to the bc plane by the co-effects of the inter-layer C-H…π (C43-H43…π)stacking between benzene ring of mbib and methyl of mpda2-ligand in an edge-to-face orientation with the C…π separations of 0.407 3 nm as well as C-H…O hydrogen-bonding interactions between imidazole rings of mbib and carboxylate O atoms of mpda2-ligands (C24-H24…O1,C24…O1 0.321 5 nm).
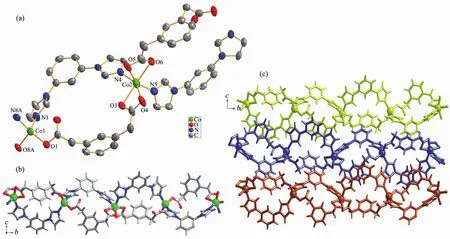
Fig.2 (a)Coordination environments of CoⅡion in complex 2 with 30%probability displacement ellipsoids;(b)Ball-and-stick view of 1D loop-containing chain along b direction in 2;(c)View of 2D extended supramolecular layer in 2
2.1.3 Crystal structure of{[Co(ppda)(mbib)].H2O}n
(3)
When the ppda2-building block was used,the similar 1D polymeric ribbon of 3 can also be isolated.Single crystal X-ray structure analysis reveals that the asymmetric unit of 3 contains one CoⅡion,one fully deprotonated ppda2-ligand,one mbib ligand and one lattice water molecule.As shown in Fig.3a,unlike in 1 and 2,each CoⅡion in 3 is penta-coordinated by two N-atom donors from two mbib ligands(Co1-N1 0.205 2(3)nm,Co1-N4A 0.204 7(3)nm)and three O atoms from two different ppda2-ligands(Co1-O1 0.220 4(18)nm,Co1-O3A 0.212 8(3)nm,Co1-O4A 0.204 7(3)nm).
In the structure of 3,the dihedral angle between two imidazole arms and benzene core is 32.91°and 14.22°,respectively.Two carboxyl groups of ppda2-in 3 adopt monodentate and chelate coordination mode and point to the same side of the benzene ring plane.Thus,the ppda2-ligand also has a cis-conformation and can be seen as a "V"type building block.CoⅡions are joined together to form a 1D loop containing polymeric ribbon running along a direction(Fig.3b)by the mixture of two"V"type building block:mbib and ppda2-ligands.The Co…Co separation through mbib ligand in 3(0.957 5 nm)is close to those in 2(0.950 3 and 0.969 3 nm)and shorter than that in 1(1.090 4 nm).The crystal packing diagram of 3 shows that the adjacent 1D polymeric ribbon motif are bound together by strong intermolecular hydrogen bonds to create a 2D supramolecular layer(Fig.S2).The hydrogen bonding system in 3 consists of the C-H…O (C2…O4 0.363 2 nm,∠C2-H2B…O4=163.18°)hydrogen bonding interactions between methyl of ppda2-from ribbon and carboxylate O atoms from the other ppda2-.In addition,the extended 3D porous supramolecular network (Fig.3c)is formed through π … π interactions(centroid-centroid distance 0.397 5 nm)between benzene rings of ppda2-and mbib ligands with a faceto-face orientation.The lattice water molecules are fixed in the channels through C-H…O (C13…O5 0.348 4 nm, ∠C13-H13…O5=170.49°;C17…O5 0.336 1 nm, ∠C17-H17…O5=163.1843°;C23…O5 0.352 6 nm,∠C23-H123…O5=153.95°)and O-H…O(O5…O2 0.294 4 nm,∠O5-H5A…2=151.73°;O5…O3 0.338 3 nm,∠O5-H5B…O3=146.26°)hydrogen bonding interactions.There is no doubt that these strong hydrogen-bonding interactions play an important role in the formation ofthe 3D supramolecular architecture.
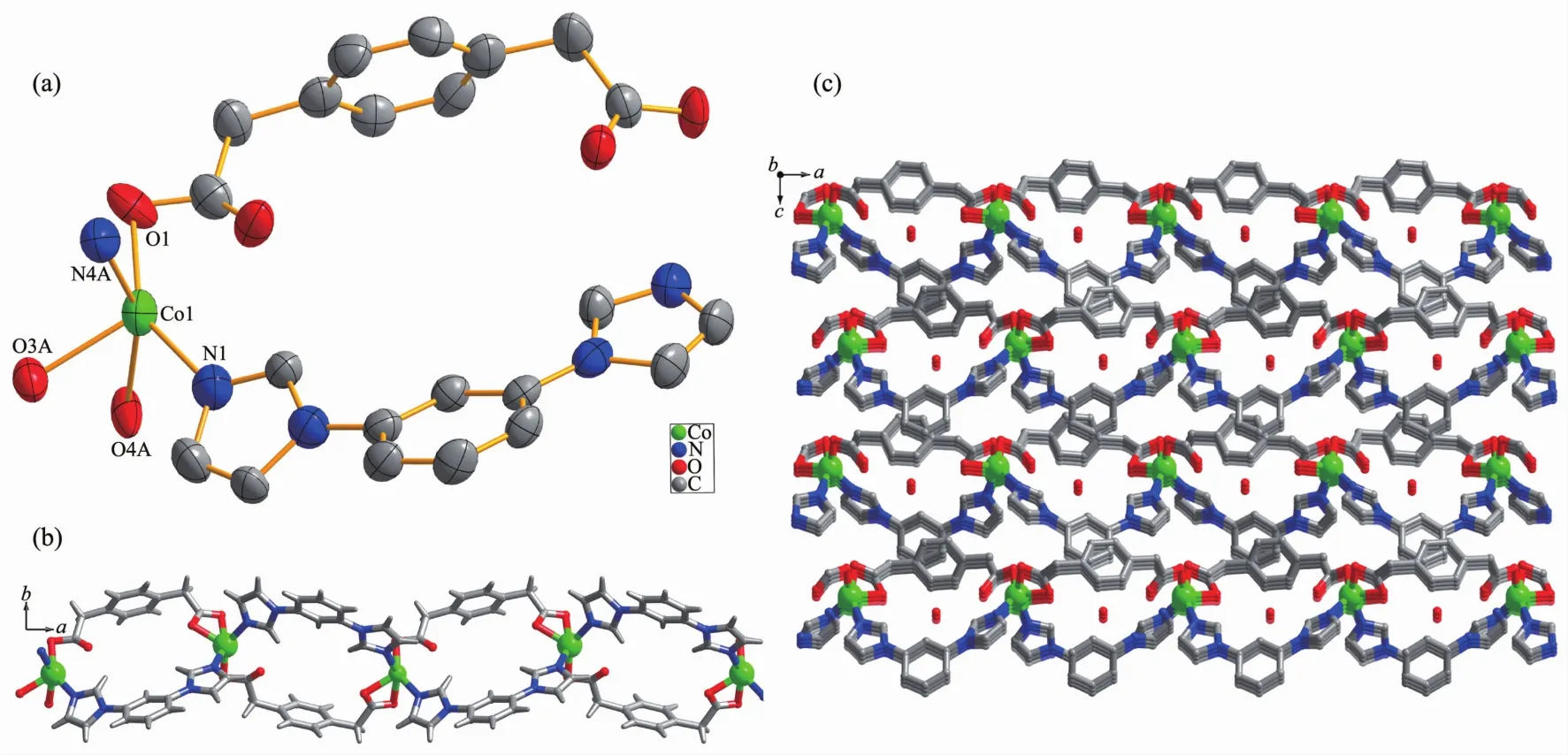
Fig.3 (a)Coordination environments of CoⅡion in complex 3 with 30%probability displacement ellipsoids;(b)Ball-and-stick view of 1D loop-containing chain along a direction in 3;(c)View of 3D porous supramolecular network in 3 with lattice molecules in the channel
2.2 PXRD results and thermal analysis of
complexes 1~3
In order to confirm the phase purity of the bulk materials,powder X-ray diffraction(PXRD)patterns of complexes 1~3 were recorded at room temperature.As shown in Fig.S3, the peak positions of the experimental PXRD and computer-simulated patterns are in agreement with each other,which confirms its phase purity.The difference in intensity of some diffraction peaks may be attributed to the preferred orientation of the crystalline powder samples.To examine the stability of these complexes,thermal gravimetric analysis(TGA)experiment was performed.As shown in Fig.4,the TGA curves exhibit a weight loss of 5.9%(95~208℃)for 1,3.3%(80~130℃)for 2,and 3.5% (78~135℃)for 3,corresponding to the release of coordinated water molecules and/or free water molecules(Calcd.6.1%,3.7%,and 3.7%for 1~3,respectively).The skeleton of 1~3 can be stable up to 267,300 and 311℃,respectively.
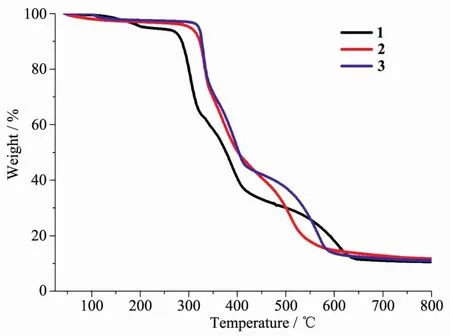
Fig.4 Thermal gravimetric analysis(TGA)curves of complexes 1~3
2.3 Electrochemistry properties
In the cyclic voltammetry (CV)measurement of complexes,we employed a conventional three-electrodes system where a saturated calomel electrode(SCE)as the reference electrode,platinum electrodes as auxiliary electrode,and foam nickel electrode was chosen as the working electrode.Water was used as solvent,and the supporting electrolyte was 1 mol.L-1KOH solution.Cyclic voltammograms of complexes 1~3 are shown in Fig.5.During scanning from-0.5 to 0.5 V in a rate of 100 mV.s-1,the cyclic voltammogram curves had an oxidation-reduction peak,which corresponds to CoⅡ/Coガ redox process[19-21].The cyclic voltammograms of complexes 1,2 and 3 all showed a reversible redox wave,and the observed potential were as follows:E1/2=0.258 V(ΔEp=0.089 V)for 1,E1/2=0.202 V (ΔEp=0.091 V)for 2,E1/2=0.178 V (ΔEp=0.105V)for 3.For complexes 1~3,their electrochemical behaviors are similar except for some slight potential shifts,due to the similarity of the different ligands that make up the three complexes.
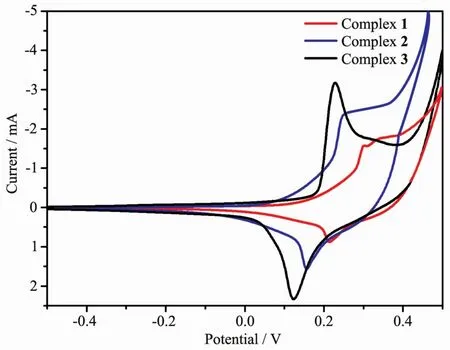
Fig.5 Cyclic voltammograms of complexes 1~3
3 Conclusions
In summary,three new coordination polymers{[Co1.5(opda)1.5(mbib)2(H2O)2].H2O}n(1), {[Co(mpda)(mbib)].H2O}n(2)and{[Co(ppda)(mbib)].H2O}n(3)have been synthesized under hydrothermal conditions by the reaction of CoⅡsalt and bis(imidazole)ligand in the presence of different flexible phenylenediacetate blocks.In these complexes,the central CoⅡions show different coordination geometries,the opda2-ligand exhibits trans-conformation and acts as bridging building block to extend 1D loop containing(Co-mbib)polymer chain into a 2D network in 1.While mpda2-and ppda2-ligands in 2 and 3 possesses cis-conformation and can be seen as "V"type building block,linking CoⅡions to form 1D loop containing chain together with mbib ligand.The structural diversities of these complexes are significantly affected by the coordination geometries of the central metal ions and the coordination modes,conformations of the organic ligands.All of three complexes are electrochemically active.This study is significant for extending the metalorganic complexes containing electronic conjugated system,exploring the interrelation between structure and property to develop potential electrochemical functional materials.
Supporting information is available at http://www.wjhxxb.cn

