Effect of boron doping on waterproof and dielectric properties of polyborosiloxane coating on SiO2f/SiO2 composites
Long XIA, Siru LU, Bo ZHONG,*, Longnn HUANG, Hu YANG,To ZHANG, Hio HAN, Pn WANG, Li XIONG, Gungwu WEN
KEYWORDS
Abstract A hydrophobic coating of the silica fiber reinforced silica composites (SiO2f/SiO2) was synthesized by sol-gel method using methyltriethoxy-silane (MTES) and boric acid (B(OH)3) as raw materials.The relationship among boron doping,chemical structure of precursors and durability of hydrophobic coatings was discussed. The Si-O-B and methyl groups were successfully introduced in the gel precursors according to the FT-IR and XPS results. The resins were filled in the internal and surface holes of the SiO2f/SiO2 composites partially or completely, which is beneficial to reduce the physical adsorption of the moisture. In addition, hydroxyl groups of the SiO2f/SiO2 composites reacted with the resins and hydrophobic methyl groups were introduced, leading to the reduction of the chemical adsorption of water.Also,the boron doping was beneficial to enhancing the physical cross-linking between the coating and the SiO2f/SiO2 composites,and improved the adhesion of the coating to the substrate. The results show that the optimal hydrophobic coating with contact angle 130.33°, moisture absorption 0.33% and adhesion level 1 is obtained when the molar ratio of MTES to B(OH)3 is 10:4.The real permittivity of M10B4 is constant in the range of 2.32-2.51 and the dielectric tangent loss is constant in the range of 5.5×10-4-8.7×10-3. The hydrophobic coating has excellent dielectric properties.
1. Introduction
The radome is used to protect the entire microwave system and antenna including radar1and communication systems, and is generally made of inorganic materials. In order to ensure the accuracy of the receiving signal, the radome is required to use the electromagnetic(EM)wave transparent material which has distinctive functions such as thermal resistance2, loadbearing and good dielectric properties, and can withstand the harsh atmosphere.3
The SiO2f/SiO2composites are one kind of ideal materials to be used as radome due to the low density,low thermal conductivity, excellent thermal shock resistance, good corrosion resistance and dielectric properties.4-8However, the surface of the SiO2f/SiO2composites has hydrophilic siliconhydroxyl groups and porosities,which promote the absorption of water in air.9-11Thus, the dielectric properties are deteriorated, limiting application of the composites. On the other hand, moisture absorption can adversely affect the SiO2f/SiO2composites, resulting in a decline in mechanical properties.6,12,13Therefore, it is necessary to improve humidity resistance of the SiO2f/SiO2composites. Lei et al.10used polyvinylidene fluoride (PVDF) and SiO2micron particles to fabricate superhydrophobic coatings of inorganic materials,and the dielectric constant was close to 4 in the research. Wu et al.14fabricated ultralow dielectric constant and moistureresistant polyimide aerogels by incorporating trifluoromethyl pendent groups. Dielectric constants and loss tangents of the aerogels were constants in the range of 1.29-1.33 and 0.001-0.004, respectively.
Polysiloxane with good dielectric properties, thermal properties and low surface free energy values can maintain stable dielectric properties under wet conditions and has extensive application in hydrophobic coating.15-19However,the unmodified organosilicone resin has low adhesion,poor solvent resistance and long curing time.The bonding strength between the resin and the SiO2f/SiO2composites is so poor at high temperature that the film is easy to fall off.18,20The polyborosiloxane,which has more excellent dielectric properties than that of polysiloxane, can be synthesized by introducing boron in polysiloxane.21Li et al.15synthesized a hybrid polyborosiloxane,and the results showed that the interaction between boron and oxygen played an important role on the viscoelastic properties of polyborosiloxane and the effect of boron concentration on the Si-O: B weakened bonding of polyborosiloxane.Soraru et al.22synthesized polyborosiloxane directly through the sol-gel method by using an alkoxysilane to react with boric acid.The Si atom was used to connect different organic groups as silicon source. They found that ethoxy groups as organic groups had higher content of Si-O-B than that of methoxy groups. Wu and Chen23found that the boron atoms in the Si-O-B groups of polyborosiloxane could form boron/oxygen dative bonds with oxygen atoms.Many researchers have studied the thermal24-29and viscoelastic30,31properties of polyborosiloxane, but rarely have applied it to hydrophobic coating.
Here, a hydrophobic coating structure with boron reinforcement was obtained as shown in Fig.1.Methyltriethoxysilane (MTES) and boric acid (B(OH)3) were used to prepare a polyborosiloxane by a sol-gel method in this work.The immersion method was adopted to get the hydrophobic coatings on the SiO2f/SiO2composites. Then the composite sheets were annealed at 200°C for 3 h. The synthetic process is schematically displayed in Fig. 2. The samples are denoted as M10Bx(x=0-5), corresponding to molar ratio of MTES to B(OH)3of 10:0, 10:1, 10:2, 10:3, 10:4 and 10:5, respectively.The compositions of the prepared sol-gel formulations and their properties are listed in Table 1. The effect of boron contents on the properties of polyborosiloxane resin as a hydrophobic coating of the SiO2f/SiO2composites is discussed.It shows that polyborosiloxane inherits the advantages of the excellent dielectric and weather resistance of polysiloxane,and improves the adhesion of polysiloxane,which has a bright future in aerospace field.
2. Experimental section
2.1. Materials
Methyltriethoxysilane (MTES) processes with the density of 0.8850±0.0050 g/cm3(C7H18O3Si, purity: 99%) were purchased from Qufu Yi Shun Chemical Co., Ltd. Boric acid (B(OH)3), ammonium hydroxide (NH3·H2O), hydrochloric acid(HCl) and absolute ethyl alcohol were purchased from Laiyang EDA Fine Chemical Plant and used without further purification. The 2.5D SiO2f/SiO2composites were purchased from Shandong Industrial Ceramic Research & Design institute Co., Ltd.
2.2. Synthesis of PBS sols
The M10B0 sol-gel was prepared at 70°C by stirring MTES(1 mol), distilled water (2 mol) and ethanol (2 mol) in a water bath, and 1 mL dilute hydrochloric acid (dilution ratio: 1:20,pH:1-2)was dropped into the solution.After 2 h,1 mL dilute ammonium hydroxide (dilution ratio: 1:5, pH: 12-13) was added into the solution to adjust pH to 8-9 and stirred for 2 h.The mixture was then heated to 90°C for 0.5 h.The liquid was allowed to cool down to room temperature.For the preparation of the M10Bx(x=1-5),MTES and H3BO3were added according to Table 1.The mixture was stirred well at 70°C for 4 h to form collosol. Then, the reaction temperature was increased to 90°C for 0.5 h. The sols were aged for 20 d to ensure that the reaction was carried out completely.
2.3. Preparation of hydrophobic coatings on SiO2f/SiO2
Firstly, the SiO2f/SiO2composite sheets were cut into suitable size and polished by ethyl alcohol, and dried at room temperature. Secondly, the pretreated samples were dipped in hydrophobic sol for about 30 min while the air bubbles are no longer produced. This procedure was repeated for another 2 times but for 10 min. After that, the composite sheets were aged for 24 h at room temperature and dried at 40°C for 3 h. Then the composite sheets were annealed at 200°C for 3 h and the final products were obtained.
2.4. Characterization
Viscosities of prepared sol were analyzed using a Bohlin Visco 88BV instrument.FT-IR spectra were collected using a Nicolet 380 FT-IR instrument (Nicolet, U.S.A). X-ray diffraction(XRD) measurements were carried out using a XD-2 with Cu-Kα radiation (acceleration voltage 30 kV, electric current 20 mA, scanning velocity 4 (°)/min, scan range 10°<2θ <18°). X-ray photoelectron spectroscopy (XPS)was investigated by a spectrometer with Al-Kα radiation.Thermogravimetric and differential analysis (TG-DTA) was performed with a thermal analysis mass spectrometry(Netzsch STAR449C)up to 1000°C with a heating rate of 10°C/min in air. The complex permittivity (ε) of the samples was measured using a network analyzer (VNA, N5245A, Agilent, U.S.A) at 2-18 GHz band. 50wt% of the as-prepared samples were mixed with paraffin and pressed into coaxial clapper in a dimension of outer diameter of 7 mm and inner diameter of 3 mm to carry on the measurement.

Fig. 1 Mechanism of hydrophobic coating.

Fig. 2 Schematic illustration of hydrophobic coating.

Table 1 Compositions of prepared polyborosiloxane formulations and their properties.
The water contact angle (WCA) of the coated fiber sheets was measured using a contact angle meter(Changzhou DEDU Precision Instruments CO., LTD. in China, JGW-360A). The volume of the reference water droplet is 2 μL, which drips out with a microsyringe.All the samples were measured for 5 times to obtain the average value. Scanning electron microscopy(SEM) images were recorded by a Tescan vega Ⅱmicroscope.The moisture absorption(MA)of samples was analyzed using an artificial wetting system by a GDS-100 instrument at 50°C and 96%for 72 h.And their weight changes were recorded.The value of MA was calculated by the following equation:

where m1and m2are the weight of composites before and after absorbing water, respectively.
The adhesions of coatings were confirmed by a lattice method: use a knife to scrape coatings at a constant pressure and rate; then rotate samples by 90°C and scrape coatings again to form cross grid; swipe the surface with a soft brush and stick tape on the substrate; peel out the tape and observe the scratch by a magnifying glass. The criteria of adhesion by lattice method are displayed in Table 2. Coating damage area(A) was qualified below level 1.
3. Results and discussion
Different boron contents have little effect on the appearance of the gel. A lot of floc appearing in M10B5 during the reaction process,which could be ascribed to the precipitation of superfluous boric acid, is hard for the adhesion of coatings on the composite sheets. So only the chemical structure and properties of the M10Bx (x=0-4) resins are discussed.

Table 2 Criteria of adhesion by lattice method (ISO 2409).
The hydroxyl groups connected with B(OH)3firstly react with the ethoxy groups of MTES to obtain a boric acid ester and a silanol structure. The silanol structure is further dehydrated and condensed with the B-OH groups to obtain the≡Si-O-B structure (2).32Meanwhile, the B-OH groups can directly react with the ethoxy groups in MTES as shown in reaction of (4).33In addition, the condensation reaction of(3).34provides the water required for the hydrolysis of ethyoxyl in reaction of(5).The ≡Si-O-Si ≡structure can also be further dehydrated by the silanol structure, as shown in(6).22
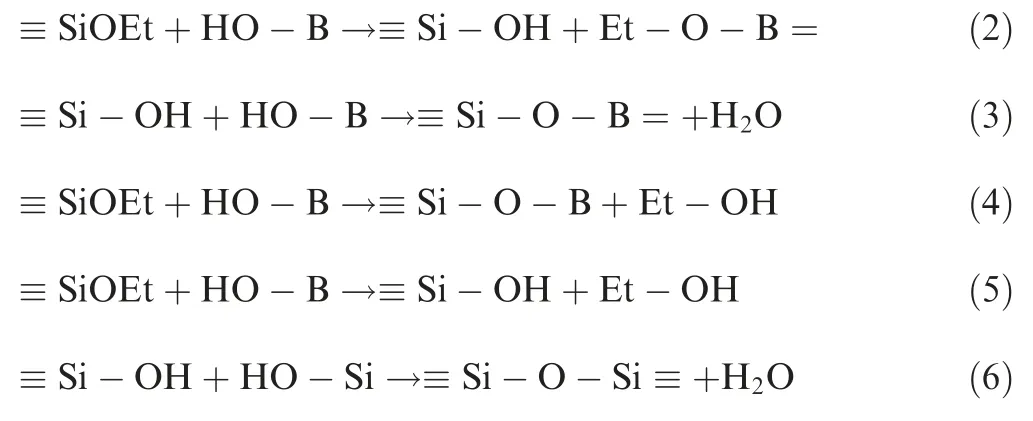

Fig. 3 FT-IR absorption spectra and XRD patterns of gel precursors.
FT-IR result (Fig. 3(a)) supports the existence of≡Si-O-Si ≡ and ≡Si-O-B= structures of the M10Bx (x=1-4) resin. In Fig. 3(a), the Si-O-Si group is found at 1026 cm-1,which confirms the condensation reaction between-Si-OH.The absorption peaks at 1268 cm-1(variable angle vibration)and 782 cm-1(stretching vibration)are due to the Si-CH3.35The introduction of the -CH3group could contribute to forming a hydrophobic surface. Compared to the spectrum of M10B0, the peaks at 1363 cm-1, 667 cm-1and 887 cm-1in spectra of other gel precursors correspond to the B-O stretching vibration, the B-O-Si shear vibration and the B-O-Si stretching vibration respectively, which represents the condensation reaction between B(OH)3and MTES. When the molar ratio of MTES to B(OH)3increases to 10:4,the peak of the B-OH group(3220 cm-1)shows that the B-OH of B(OH)3is successfully bonded to network structure.

Fig. 4 XPS spectra of gel precursors.
Fig.3(b)shows the XRD patterns recorded for different gel precursors. M10B0, M10B1, M10B2 and M10B3 just have very broad diffraction peaks at 2θ of 22.0°, suggesting that all of B(OH)3have completely reacted. In contrast, there are the diffraction peaks of B(OH)3in the XRD pattern of M10B4 because of superfluous B(OH)3. The XPS spectra(Fig. 4) demonstrate that B has been successfully doped into the resin structures. The existence of hydrophobic ethoxy groups is confirmed by XPS results(Tables 3 and 4).The main structures are ≡Si-O-Si ≡and ≡Si-O-B=structures.The R3SiO~(103.2-103.4 eV),R2SiO2~(102.6-102.8 eV)and RSiO3~groups(102.1-102.3 eV)can be found in XPS spectra of all gel precursors (Table 4). In particular, additional R4-Si ~groups occur in the M10B1,which is due to the unreacted MTES. The structure of RxSiO4-x~and specific reaction equation are shown in Fig. 5.
Fig. 6(a) and (b) show the TG and DTA profiles of hydrophobic coatings, respectively. Fig. 6(a) shows residual in mass of polyborosiloxane with different boron contents. It can be seen in Fig. 6(a) that the M10B0 resin gradually lost most of mass with only 34.91%weight retention ratio at a temperature of 1000°C. After boron doping, the M10Bx (x=1-4) resin retains a higher mass ratio above 75%.
It is also clear in Fig.6(a)and(b)that the thermal behavior of the M10Bx (x=1-4) resin is quite different from the M10B0 resin. For the M10B0 resin, the oxidative decomposition of methyl or other molecules occur in the temperature range of 250-340°C. The oxidative decomposition of residual ethoxy groups can be observed at a temperature between 340°C and 500°C, and the mass lost heavily up to 52.5%.After the introduction of B element, the decomposition temperature increases by about 100°C,indicating an enhancement of thermal stability by the introduction of B-O bond with high bonding energy (561 kJ/mol).21The Si-O-B network structure wraps small molecules such as methyl to prevent its oxidation and decomposition.

Table 3 Relative amount of atoms and ratio of Si/B at gel precursors determined by XPS.

Table 4 Deconvoluted peak parameters and relative amount of different bonds at sample surface determined by XPS.
To determine an appropriate heat treatment temperature,the FT-IR absorption spectra of the M10B0 and M10B4 treated at different temperatures (200°C, 400°C and 500°C) are shown in Fig.6(c)and(d).For both coatings at different temperatures, the characteristic peaks of the methyl group at 1268 cm-1and 782 cm-1become weaker from 200°C to 400°C and disappear at 500°C,leaving only the Si-O-Si characteristic peaks. In conclusion, after the treatment at 400°C and 500°C, the methyl groups in coatings are oxidized into small molecules such as CO2and H2O,which is not conducive to the formation of hydrophobic surfaces. In conclusion,200°C is the best heat treatment temperature. And the lowtemperature heat treatment has little effect on the mechanical properties of the SiO2f/SiO2composites.36
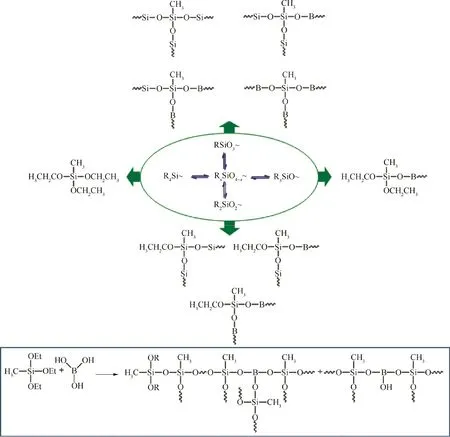
Fig. 5 Structure of RxSiO4-x ~and reaction equation.
The WCA of hydrophobic coatings treated at 200°C for 3 h is shown in Fig.7.The WCA can be influenced by the following factors: (1) Methyl content: The addition of non-polar methyl groups reduces the surface energy of the coating and enhances the hydrophobicity of the coating.37(2) Hydroxyl content: The polar hydroxyl easily bonds with hydrogen of the water in air.38(3) The microstructure of coating surface:The microstructure of the coating surface directly affects the surface energy of the system by varying the roughness and texture.39(4) B element content: Oxygen atoms with unshared electron pairs in the water absorbed from air attack B atoms with empty p orbit, which leads to deliquesce.40
Uncoated SiO2f/SiO2composite sheets are infiltrated by water.5,41It is obvious that the coatings have significant effect on the hydrophobicity of the SiO2f/SiO2composites.
For the M10B1, the molar ratio of MTES to boric acid is 10:1, and there are ten MTES molecules distributed around one boric acid molecule under stirring, where only partially effective MTES molecules collide with boric acid molecules to obtain a ≡Si-OH structure. Compared to themselves,the ≡Si-OH groups are more easily condensed with the unreacted hydroxyl groups of boric acid or the ethoxy groups of MTES, which can form a network structure centered on BO3.Although the MTES reacts incompletely and the product possesses a small average molecular weight,the RSiO3~structure is dominant. A network structure that the B atoms are surrounded by the Si-CH3groups is formed. Obviously, the hydrophilic groups (-OH) are replaced by the hydrophobic groups (-CH3) to reduce surface energy of coatings,42which results in the high WCA of 141.84°.
With the increase of boric acid amount, MTES completely reacts.The contents of B in M10Bx(x=2-4)increase and the actual Si/B radio decreases. The M10B1 and M10B2 show much higher actual than theoretical radio of Si/B, indicating high contents of the ≡Si-O-Si ≡structure and low contents of ≡Si-O-B= structure in M10Bx (x=1-2). The Si-OH groups are obtained by the self-hydrolysis or esterification process with B-OH groups.Under the repulsion of methyl group, the electron cloud is pushed to the Si atom to increase the electron cloud density in the vicinity of the Si atom,so that the polarity of the entire molecule is weakened.The condensation between the Si-OH and B-OH groups is more difficult than that between the B-OH groups.43Therefore, in the case of sufficient boric acid, the self-polycondensation of silanol is inhibited, and the formation of ≡Si-O-B= structure is more conductive.The WCA has a slight downward trend with the increase of boric acid. This can be explained by the fact that the B atom, which cannot be completely wrapped by the network, is easily connected with the water droplets through van der Waals forces.
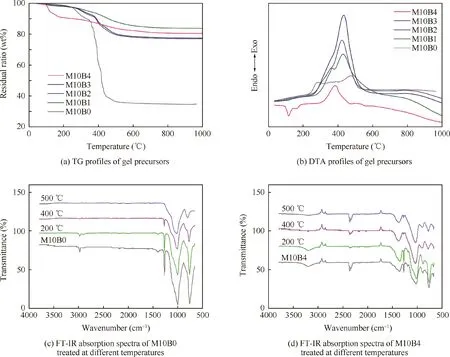
Fig.6 TG and DTA profiles of gel precursors, and FT-IR absorption spectra of M10B0 and M10B4 treated at different temperatures.

Fig. 7 WCAs of different hydrophobic coatings treated at 200°C for 3 h.

Fig. 8 Moisture absorption and adhesion of SiO2f/SiO2 treated with different sol-gel coatings at 200°C for 3 h.
With the amount of boric acid further increasing to 0.4 mol,the B-OH groups provide enough crosslinking points to form the ≡Si-O-B= structure. As a consequence, the SiO2f/SiO2composites coated with M10B4 possess a lower WCA of 130.33° than M10B1 and M10B2.

Fig. 9 Surface morphology of SiO2f/SiO2 composites treated with M10B1, M10B2 , M10B3 and M10B4 at 200°C for 3 h.
The WCA only implies the relationship between the static water droplets and the coating surface. Nevertheless, the moisture-proof performance cannot be formulated completely.42The MA and adhesion of SiO2f/SiO2composites coated with different sol-gel coatings after heat treatment at 200°C for 3 h are shown in Fig.8.The SiO2f/SiO2composites coated with M10B4 possess the lowest MA about 0.33%and a WCA of 130.33°. The adhesion of M10B4 reaches to level 1,which is higher than that of other samples.
The SEM images of the SiO2f/SiO2composite sheets coated with different sol-gel coatings at 200°C for 3 h are shown in Fig. 9. Due to the low viscosities of M10B1 and M10B2 resin(Table 1), only a few fine granular protrusions adhere to the surface of the SiO2f/SiO2fibers (red arrows in Fig. 9). The composites with M10Bx (x=1-2) coating exhibit good hydrophobicity, attributing to the methyl groups.
The M10B1 and M10B2 resins are not fully formed inside the composites when the ethanol is released (yellow arrows in Fig. 9(a)-(d)), and the internal pores of the composites could not be effectively filled. Some SiO2fibers in the SiO2f/SiO2composites contact with the water in the air, due to the existence of pores in the coating.44Therefore, the MA of the SiO2f/SiO2composite sheets coated with M10B1 or M10B2 is not optimal.

Fig. 10 Real permittivity, imagery permittivity and dielectric loss of M10Bx (x=1-4) gel precursors treated at 200°C for 3 h.
The viscosities of M10B3 and M10B4 resins are higher than those of M10B1 and M10B2. The internal pores of the SiO2f/SiO2composites are filled relatively by M10B3 and M10B4 resins in time,which are created by the evaporation of ethanol and water during dipping process. Therefore, the physical adsorption of water on SiO2f/SiO2composites is reduced.However,shrinkage effect during the process of heat treatment results in the formation of large-size cracks (blue arrow in Fig. 9(f)) on the surface of the SiO2f/SiO2composites coated with M10B3, which leads to the water absorption. Consequently, the MA increases significantly. The surface of M10B4 coating is no longer a continuous whole, but is a surface with micro cracks and holes of network structure (blue circle in Fig. 9(g) and (h)), which can capture more air to reduce the contact area with water. The contact type between water droplets and surface of the SiO2f/SiO2composites hardly turns to Wenzel model45,46and water droplets are difficult to wet the surface. As a result, the M10B4 coating demonstrates the best hydrophobic performance.
The Si-OH or B-OH groups on the resin coating react with the Si-OH groups on the SiO2f/SiO2composite fibers to obtain the Si-O-Si and Si-O-B groups, which decrease the surface activity of the SiO2f/SiO2composites by consuming the -OH groups of fibers and introducing hydrophobic methyl groups.The B atoms with empty p orbit could form intermolecular van der Waals forces with the O atoms of Si-O-Si groups on the fibers (Fig. 1).47
To reveal EM wave permeability of coatings treated at 200°C, the complex permittivity and dielectric tangent loss of M10Bx (x=1-4) are measured (Fig. 10). The permittivity represents the ability of microwave to reflect and refract at the interface between air and EM wave transparent material. The dielectric tangent loss represents the conversion of waves through the radome to themosteresis. Therefore, in order to achieve excellent dielectric properties of radome material, the coating must possess the permittivity and dielectric tangent loss as low as possible. Generally, the real permittivity of an excellent radome material is required to be between 1 and 4 and the dielectric tangent loss should be less than 10-2.48It can be seen from Fig. 10 that in the frequency range of 2-8 GHz, the real and imaginary part of complex permittivity of M10B4 are stable within the change of frequency. The real and imaginary permittivity of M10B4 are constant in the range of 2.32-2.51 and 0.001-0.002, respectively. And the dielectric tangent loss is constant in the range of 5.5×10-4-8.7×10-3. As a consequence, it is clearly that the M10B4 coating possesses more excellent EM wave permeability than other resins, and has little effect on dielectric properties of the SiO2f/SiO2composites.
4. Conclusions
In summary, the SiO2f/SiO2composites are converted from a completely wetted surface to a hydrophobic surface after being coated. The boron element can be combined with negative oxygen atoms in the SiO2f/SiO2composites to improve adhesion of coatings. With boron doping increasing, the adhesion of the coating improves from level 3 to level 1.The best performance of the hydrophobic coating M10B4 is obtained with contact angle 130.33°, moisture absorption 0.33% and adhesion level 1, which also exhibits excellent dielectric properties.
Acknowledgments
This work was supported by the Taishan Scholar Project(No.ts201511080), the National Natural Science Foundation of China (Nos. 51672059, 51172050, 51102060 and 51302050),and the Natural Scientific Research Innovation Foundation in Harbin Institute of Technology(No.HIT.NSRIF.2014129).
 CHINESE JOURNAL OF AERONAUTICS2019年8期
CHINESE JOURNAL OF AERONAUTICS2019年8期
- CHINESE JOURNAL OF AERONAUTICS的其它文章
- Special Column of BWB Civil Aircraft Technology
- Assessment on critical technologies for conceptual design of blended-wing-body civil aircraft
- Exploration and implementation of commonality valuation method in commercial aircraft family design
- Effects of stability margin and thrust specific fuel consumption constrains on multi-disciplinary optimization for blended-wing-body design
- Nacelle-airframe integration design method for blended-wing-body transport with podded engines
- On developing data-driven turbulence model for DG solution of RANS
