CRMP3在青少年皮质发育障碍型癫痫脑组织中的表达研究
覃璐 余瑞杭 蒋艳

[摘要] 目的 探讨CRMP3在青少年皮质发育障碍型癫痫脑组织中的表达情况。 方法 将收集的青少年皮质发育障碍型癫痫脑组织及脑外伤术后正常脑组织标本分为皮质发育障碍组(MCD组,n=15)和对照组(control组,n=11)。采用实时定量PCR方法检测CRMP3基因表达情况,采用Western-blot及免疫组化方法检测两组之间CRMP3的蛋白质表达水平。 结果 实时定量PCR结果提示CRMP3的mRNA表达水平在MCD组中表达增高,差异有统计学意义(P<0.05)。Western-blot及免疫组化结果提示在MCD组中CRMP3蛋白表达水平较control组显著上调,且CRMP3主要表达于神经元细胞质中(P<0.05)。 结论 CRMP3在青少年皮质发育障碍型癫痫脑组织中高表达,可能与皮质发育障碍型癫痫的发生发展具有相关性。
[关键词] 皮质发育障碍;癫痫;CRMP3;青少年
[中图分类号] R742.1 [文献标识码] A [文章编号] 1673-9701(2019)19-0018-04
[Abstract] Objective To investigate the expression of CRMP3 in brain tissues of adolescents with epilepsy induced by cortical developmental disorder. Methods The collected brain tissues of adolescents with epilepsy induced by cortical developmental disorder and normal specimens of brain tissue after the surgery of brain trauma were divided into cortical developmental disorder group (MCD group, n=15) and control group (control group, n=11). Real-time quantitative PCR was used to detect the expression of CRMP3 gene. The protein expression level of CRMP3 between the two groups was detected by Western-blot and immunohistochemistry. Results According to the real-time quantitative PCR results, the expression of CRMP3 gene was increased in the MCD group, and the difference was statistically significant(P<0.05). According to the Western-blot and immunohistochemistry results, the expression level of CRMP3 protein was significantly up-regulated in the MCD group compared with the control group, and CRMP3 was mainly expressed in neuronal cytoplasm(P<0.05). Conclusion CRMP3 is highly expressed in the brain tissues of adolescents with epilepsy induced by cortical developmental disorder, which may be related to the occurrence and development of epilepsy induced by cortical developmental disorder.
[Key words] Cortical developmental disorder; Epilepsy; CRMP3; Adolescent
皮質发育障碍(malformations of cortical development,MCD)是中枢神经系统发育畸形而导致的一种疾病,包括局灶性皮质发育不良(focal cortical dysplasia,FCD)、结节性硬化(tuberous sclerosis complex,TSC)、无脑畸形等亚型[1,2]。MCD可导致癫痫发作,且是难治性癫痫的常见原因之一。研究认为MCD致痫的主要原因是异构神经元的过度兴奋及异常神经通路的形成[3,4]。
脑衰反应调节蛋白家族(CRMPs)控制着神经元树突、轴突的形态发展,神经元的极化以及神经干细胞的分化[5,6]。相关研究发现CRMP3能够促进神经元树突的生长,体内外实验证实敲除CRMP3可引起树突形态发育不良[7,8]。众多研究表明,CRMP3蛋白的异常表达与神经系统疾病具有相关性,如脑缺血[9]、边缘性脑炎[10]、副肿瘤综合征[11]等。但是,目前CRMP3与皮质发育障碍型癫痫的研究尚为空白。
本实验以青少年皮质发育障碍型癫痫脑组织及脑外伤术后正常脑组织为对象,运用实时定量PCR、Western Blot、免疫组化方法探索CRMP3在MCD型癫痫脑组织中的表达差异,从而初步探讨CRMP3与皮质发育障碍型癫痫的相关性以及其潜在的病理机制。现报道如下。
1 材料与方法
1.1材料来源
脑组织标本均由第三军医大学新桥医院提供,标本的采集得到了新桥医院伦理委员会的批准以及患者家属的同意。MCD型癫痫脑组织标本(MCD组)来源于MCD型癫痫患者手术,共15例,其中男8例,女7例,平均年龄(12.22±3.20)岁。正常脑组织标本(control组)由脑外伤后因颅内高压手术切除而来,共11例,其中男7例,女4例,平均年龄(13.10±3.70)岁,且所提供标本的患者既往均无神经系统疾病及癫痫病史。两组患者均为汉族,在性别、年龄方面差异均无统计学意义(P>0.05),具有可比性。
1.2主要试剂
TRIzol购自Invitrogen公司,蛋白质浓度测试剂购自Bradford公司,CRMP3引物购自Origene公司,总RNA提取试剂盒购自碧云天公司,CRMP3及GADPH抗体购自Abcam公司。
1.3 实时定量PCR
采用TRIzol试剂(1 mL/60 mg)提取脑组织中的总RNA。将所获得的RNA通过A3500逆转录仪使其反转录为cDNA。cDNA通过ABI 7500 Real-Time PCR仪对目的引物CRMP3进行PCR扩增。本实验以GADPH为内参,运用2-△△Ct方法分析CRMP3基因表达的相对定量,至少重复3次实验。
1.4 蛋白质免疫印迹
将脑组织标本匀浆后加入蛋白质裂解液从而提取其总蛋白质,用BCA方法测得浓度。蛋白变性后,于对应孔中分别加入40 μg蛋白,经SDS-PAGE电泳分离,并转至PVDF膜。4%明胶室温封闭1 h,一抗(兔多克隆CRMP3抗体,1:2000)4℃过夜。次日,加入辣根過氧化物酶标记的羊抗兔IgG(1:5000),室温孵育 1 h后,ECL发光显色。Quantity One软件分析目的条带光密度值(OD值),至少重复3次实验。
1.5 免疫组化
脑组织样本用多聚甲醛浸泡过夜,石蜡包埋。经脱蜡、水化后,切片经pH6.0的枸橼酸钠缓冲溶液加热,从而达到修复抗原作用。切片冷却后,加入H2O2灭活内源性过氧化物酶。山羊血清封闭液室温孵育30 min,CRMP3抗体(1:500稀释)4℃过夜。次日,二抗37℃孵育30 min,DAB显色。苏木精复染,再次脱水后用光学显微镜拍照。
该实验运用半定量方法分析蛋白质表达水平,免疫组化图片染色强度得分范围为0~3分,阳性细胞百分比范围0~100%,两者相乘得到结果范围为0~200[12,13]。通过得分比较可分析出CRMP3的表达情况。该实验至少进行3次。
1.6 统计学方法
采用SPSS19.0统计学软件进行分析,计量资料以均数±标准差(x±s)表示,采用独立样本t检验,P<0.05为差异有统计学意义。
2 结果
2.1 实时定量PCR检测CRMP3 mRNA的表达情况
MCD组中CRMP3的mRNA表达水平(2.23±0.14)显著高于control组(0.98±0.19),差异有统计学意义(P<0.05)(图1)。
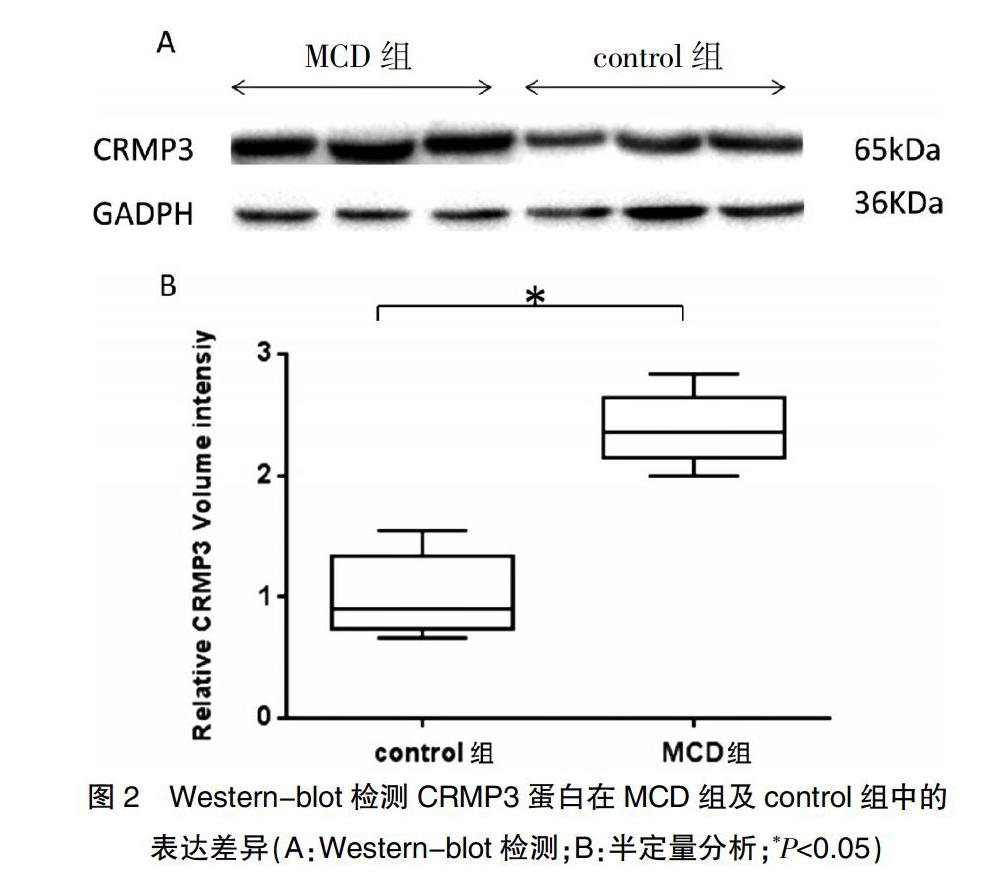
2.2 蛋白质免疫印迹检测CRMP3在各组中的表达情况
通过Western-blot方法发现:与control组(0.87±0.31)相比,CRMP3在MCD组中的表达(2.26±0.29)明显升高,差异有统计学意义(P<0.05)(图2)。
2.3 免疫组化检测CRMP3的表达情况及其分布
利用免疫组化方法检测CRMP3在青少年皮质发育障碍型癫痫脑组织中的表达情况。与control组(21.14±4.94)相比,CRMP3在MCD组(181.01±6.43)表达上调,差异有统计学意义(P<0.05),且主要表达于神经元细胞质中(封三图3)。
3讨论
皮质发育障碍型癫痫为药物难治型癫痫种类之一,目前主要通过手术切除致痫灶方式加以治疗[14]。但是,外科手术不仅加重了患者的经济负担,而且手术本身也是一种创伤。目前普遍研究认为,MCD是由于神经元异常增殖凋亡、神经元迁移及皮层重构异常导致[15]。MCD病理学常表现为大量异形及巨形神经元、气球样细胞及巨细胞,这些细胞又称为异构神经元。研究发现这些异构神经元具有高度兴奋性,可自发产生痫样放电[16]。
树突的异常生长、突触的畸形重组不仅会导致神经元及皮层重构异常产生MCD,而且通过新形成的神经环路,导致痫样放电[17]。CRMPs是在1995年通过Sema 3A信号通路而被大众所认识,共包括5个成员(即CRMP1~5)[18-20]。强有力的证据表明CRMPs主要通过微管聚合、微丝运动、肌动蛋白网络形成从而调控细胞骨架,参与神经细胞分化、迁徙及神经突起的生长发育[21,22]。CRMP1的表达能够抑制神经突起的生长及分支形成[23]。2012年研究发现CRMP1在成年人颞叶难治性癫痫脑组织中表达下调,证实CRMP1与颞叶难治性癫痫具有相关性[24]。CRMP3作为 CRMPs家族成员之一,Quach TT等[7,8]发现CRMP3在树突及轴突形态发育上起着重要作用,在小鼠体内敲除CRMP3可导致树突形态发育不良,在体外实验发现CRMP3可通过调节离子门控通道从而影响树突形态发育。Aylsworth A等[25]发现CRMP3通过阻止微管蛋白的聚合从而调控神经元的生长。2013年,有报道提出CRMP3参与调节神经元死亡[26]。因此,我们推测CRMP3通过树突及神经元的异常延伸,参与皮质发育障碍型癫痫的发生机制。
通过本实验,发现在MCD组中,CRMP3的mRNA水平表达较conrtol组明显升高。在MCD型癫痫脑组织中CRMP3的蛋白水平表达同样高于正常脑组织。研究结果证实CRMP3的高表达与青少年皮质发育障碍型癫痫的发生发展具有相关性。但是,本研究只是一种现象存在,CRMP3是否引起MCD型癫痫的发生,尚需进一步研究证实。
綜上所述,本实验研究了CRMP3在青少年皮质发育障碍型癫痫脑组织中的表达情况,结果提示CRMP3与皮质发育障碍型癫痫具有相关性,该结果为进一步研究皮质发育障碍型癫痫的机制奠定了基础,为新药物的研究提供了一个新的方向。
[参考文献]
[1] Ho CSH,Dubeau F,Seguin R,et al. Prevalence of neuropsychiatric symptoms associated with malformations of cortical development[J]. Epilepsy & Behavior:E&B,2019,92:306-310.
[2] Juric-Sekhar G,Hevner RF. Malformations of cerebral cortex development:Molecules and mechanisms[J]. Annual Review of Pathology,2019,14:293-318.
[3] Cepeda C,Andre VM,Wu N,et al. Immature neurons and GABA networks may contribute to epileptogenesis in pediatric cortical dysplasia[J]. Epilepsia,2007,48( Suppl 5):79-85.
[4] Barkovich AJ,Dobyns WB,Guerrini R. Malformations of cortical development and epilepsy[J]. Cold Spring Harbor Perspectives in Medicine,2015,5(5):a022392.
[5] Lin PC,Chan PM,Hall C,et al. Collapsin response mediator proteins(CRMPs) are a new class of microtubule-associated protein(MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction[J]. The Journal of Biological Chemistry,2011, 286(48):41466-41478.
[6] Quach TT,Honnorat J,Kolattukudy PE,et al. CRMPs:Critical molecules for neurite morphogenesis and neuropsychiatric diseases[J]. Molecular Psychiatry,2015,20(9):1037-1045.
[7] Quach TT,Auvergnon N,Khanna R,et al. Opposing morphogenetic defects on dendrites and mossy fibers of dentate granular neurons in CRMP3-deficient mice[J]. Brain Sci,2018,8(11):E196.
[8] Quach TT,Wilson SM,Rogemond V,et al. Mapping CRMP3 domains involved in dendrite morphogenesis and voltage-gated calcium channel regulation[J]. Journal of Cell Science,2013,126(Pt 18):4262-4273.
[9] Jiang SX,Kappler J,Zurakowski B,et al. Calpain cleavage of collapsin response mediator proteins in ischemic mouse brain[J]. The European Journal of Neuroscience,2007,26(4):801-809.
[10] Knudsen A,Bredholt G,Storstein A,et al. Antibodies to CRMP3-4 associated with limbic encephalitis and thymoma[J]. Clinical and Experimental Immunology,2007, 149(1):16-22.
[11] Honnorat J,Byk T,Kusters I,et al. Ulip/CRMP proteins are recognized by autoantibodies in paraneoplastic neurological syndromes[J]. The European Journal of Neuroscience,1999,11(12):4226-4232.
[12] Ho J,Kong JW,Choong LY,et al. Novel breast cancer metastasis-associated proteins[J]. Journal of Proteome Research,2009,8(2):583-594.
[13] Tong SW,Yang YX,Hu HD,et al. HSPB1 is an intracellular antiviral factor against hepatitis B virus[J]. Journal of Cellular Biochemistry,2013,114(1):162-173.
[14] Martinez-Lizana E,Fauser S,Brandt A,et al. Long-term seizure outcome in pediatric patients with focal cortical dysplasia undergoing tailored and standard surgical resections[J]. Seizure,2018,62:66-73.
[15] Omidi A,Akbari M,Mortezaee K,et al. Prenatal transplantation of epidermal neural crest stem cells in malformation of cortical development mouse model[J]. Microscopy Research and Technique,2017,80(4):394-405.
[16] Wong M. Mammalian target of rapamycin(mTOR) activation in focal cortical dysplasia and related focal cortical malformations[J]. Experimental Neurology,2013,244:22-26.
[17] Stouffer MA,Golden JA,Francis F. Neuronal migration disorders:Focus on the cytoskeleton and epilepsy[J]. Neurobiology of Disease,2016,92(Pt A):18-45.
[18] Tang Y,Ye Z,Wei Y,et al. Vertebrate paralogous CRMPs in nervous system:Evolutionary,structural,and functional interplay[J]. Journal of Molecular Neuroscience:MN,2015, 55(2):324-334.
[19] Takaya R,Nagai J,Piao W,et al. CRMP1 and CRMP4 are required for proper orientation of dendrites of cerebral pyramidal neurons in the developing mouse brain[J]. Brain Research,2017,1655:161-167.
[20] Nagai J,Baba R,Ohshima T. CRMPs function in neurons and glial cells:Potential therapeutic targets for neurodegenerative diseases and CNS injury[J]. Molecular Neurobiology,2017,54(6):4243-4256.
[21] Fukata Y,Kimura T,Kaibuchi K. Axon specification in hippocampal neurons[J]. Neuroscience Research,2002,43(4):305-315.
[22] Arimura N,Kaibuchi K. Neuronal polarity:From extracellular signals to intracellular mechanisms[J]. Nature Reviews Neuroscience,2007,8(3):194-205.
[23] Yao L,Liu YH,Li X,et al. CRMP1 interacted with Spy1 during the collapse of growth cones induced by sema3A and acted on regeneration after sciatic nerve crush[J]. Molecular Neurobiology,2016,53(2):879-893.
[24] Luo J,Zeng K,Zhang C,et al. Down-regulation of CRMP-1 in patients with epilepsy and a rat model[J]. Neurochemical Research,2012,37(7):1381-1391.
[25] Aylsworth A,Jiang SX,Desbois A,et al. Characterization of the role of full-length CRMP3 and its calpain-cleaved product in inhibiting microtubule polymerization and neurite outgrowth[J]. Experimental Cell Research,2009, 315(16):2856-2868.
[26] Hou ST,Jiang SX,Aylsworth A,et al. Collapsin response mediator protein 3 deacetylates histone H4 to mediate nuclear condensation and neuronal death[J]. Scientific Reports,2013,3:1350.
(收稿日期:2019-02-26)

