水分胁迫对黍子幼苗生长和生理特性的影响*
韩志平,张海霞,张 巽,梁艳花
水分胁迫对黍子幼苗生长和生理特性的影响*
韩志平1,张海霞2,张 巽1,梁艳花1
(1.山西大同大学生命科学学院/设施农业技术研发中心,大同 037009;2.山西大同大学后勤管理处,大同 037009)
以“晋黍8号”黍子幼苗为材料,在大棚内采用盆栽砂培法浇灌营养液,设置重旱、轻旱、正常灌溉、轻涝和重涝5个处理,植株二叶一心时开始胁迫处理,于处理后20d测定植株形态指标、生物量和含水量、叶片质膜透性、光合色素、丙二醛、抗坏血酸、脯氨酸、可溶性糖和可溶性蛋白含量等指标,研究水分胁迫对黍子幼苗生长、膜脂过氧化和渗透调节的影响。结果表明,正常灌溉下黍子幼苗生长最好,株高、茎粗、茎节数、叶片数、最大叶面积及根系、茎叶、穗的鲜质量、干质量均最大,干旱和涝害下幼苗各形态指标和生物量均明显降低,且重旱和重涝下比轻旱和轻涝下降低更明显;根系、茎叶和穗的含水量在干旱下均明显降低,涝害下表现各不相同。叶片光合色素含量在干旱下显著降低而涝害下无明显变化,质膜透性、丙二醛、抗坏血酸和脯氨酸含量在干旱和涝害下明显增加,且重旱和重涝下比轻旱和轻涝下增加更明显;可溶性糖含量在干旱下明显增加而在涝害下明显降低,可溶性蛋白含量在干旱下显著降低而在涝害下无显著变化。研究说明,干旱和涝害均对黍子幼苗造成过氧化伤害,抗氧化物质和渗透调节物质含量随之增加,但是抗氧化物质的增加并不能完全消除胁迫导致的过氧化伤害,加上光合能力降低,使黍子植株生长显著抑制。在本试验条件下,干旱胁迫对黍子幼苗的伤害比涝害严重。
黍子;水分胁迫;生长;膜脂过氧化;渗透调节物质
中国季风气候显著,干旱和涝害经常发生,且分布地区广,是限制农业生产的主要因素[1]。近几十年来,气候不断恶化,旱涝灾害更加频繁,危害程度不断增强,对农作物生长发育的影响更加严重[2]。黍子(L.)为单子叶禾本科植物,籽粒脱壳后营养价值极高,可用于炸油糕和酿米酒[3]。大同、朔州、忻州、吕梁等市是山西省黍子种植和消费的主要地区,但这些地区均为干旱或半干旱地区[4−5],水资源缺乏,年降水量仅402~533mm,是华北地区缺水最严重的区域之一,每年夏秋雨季,还可能有突发性暴雨洪涝灾害发生[6−7]。虽然黍子较耐贫瘠和干旱,但降雨量不足和洪涝灾害都会严重影响黍子的生长发育、产量和品质。
然而,国内外对黍子水分胁迫抗性及其机理的研究很少。张美俊等[8]利用PEG-6000溶液模拟干旱胁迫,并采用隶属函数法对16个黍子品种萌发期的抗旱性进行了综合评价。王纶等[9]通过测定光合气体交换参数和离体叶片含水量,验证了反复干旱法鉴定和筛选黍子抗旱种质的准确性和可行性。张永清等[10]研究表明,拔节期或抽穗期干旱胁迫显著抑制黍子根系的生长,施肥可提高土壤水分利用效率,缓解干旱胁迫对根系生长的抑制。还有学者研究了黍子在干旱胁迫下生理生态特征的变化[11−12]。但以往研究并未完全阐明黍子的抗旱生理机制,涝害对黍子的影响更未见报道。为此,本研究以“晋黍8号”为材料,采用盆栽砂培浇灌营养液方法,研究干旱胁迫和涝害对黍子幼苗生长和生理指标的影响,以期为阐明黍子植株对水分胁迫的生理响应奠定基础,为同朔地区黍子的抗旱和抗涝栽培提供参考。
1 材料与方法
1.1 材料
实验于2017年5−7月在山西大同大学生命科学实验基地大棚内进行。供试品种“晋黍8号”由山西省农业科学院高寒区作物研究所提供。
1.2 方法
选取饱满、无病的种子,用清水浸种后播于以砂子:蛭石体积比3:1为基质的塑料盆中育苗,盆上口直径25cm,深20cm,盆下垫托盘。每盆播约180粒种子,每天浇水保持基质湿润。幼苗一叶一心后,每2d浇一次1/2剂量Hoagland配方营养液,逐渐使基质含水量保持在最大持水量的65%~70%,以充分浇水并自然渗漏2h后测得的基质含水量为最大持水量。植株二叶一心时开始胁迫处理,每日18:00按照基质最大持水量的百分比进行水分控制。共设5个处理:基质含水量65%~70%(正常灌溉,W3),通过称重法补充水分;干旱胁迫设2个水平,通过控制浇水和称重法使基质含水量降至15%~20%(重旱,W1)、40%~45%(轻旱,W2);涝害设2个水平,通过增加浇水和称重法使基质含水量达到90%~95%(轻涝,W4)、115%~120%(重涝,W5)。胁迫期间每5d按重旱胁迫灌水量浇一次1/2剂量Hoagland配方营养液,其它处理用清水补足灌水量,其余时间各处理仅浇清水。重涝胁迫处理将栽培盆置于水深5cm的盆中,每天浇水多次以保证基质含水量。处理前1d每盆定苗60株,实验重复3次,每重复3盆,随机排列。
水分胁迫20d时每盆取10株幼苗测量相关生长指标,另取20株幼苗的叶片测定相关生理指标。
1.3 指标测定
1.3.1 生长指标
用直尺测量基部到植株最高处的长度为株高,游标卡尺测量基部以上2cm处茎的直径为茎粗,并统计地上部茎节数;以叶片完全展开及叶长超过5cm为标准统计叶片数,用Yaxin-1242叶面仪测量最大叶片的叶面积。取植株洗净并擦干后剪断分为根系、茎叶、穗3部分,分别称鲜质量;在105℃下杀青15min后降温至75℃下烘干到恒重,称干质量。
1.3.2 生理指标
参考沈伟其方法配制提取液,打孔取叶圆片提取后测定叶绿素a(Chla)、叶绿素b(Chlb)和类胡萝卜素(Car)含量[13];丙二醛(MDA)含量采用硫代巴比妥酸法[14],质膜透性采用电导仪法[15],以相对电导率表示,抗坏血酸(AsA)含量采用红菲罗啉法[16];脯氨酸含量采用水浴浸提法,可溶性糖含量采用蒽酮比色法[17],可溶性蛋白含量采用考马斯亮蓝染色法[18]。
1.4 数据处理
数据用DPS7.5软件进行方差分析,Duncan's新复极差法进行多重比较,Excel软件作图。
2 结果与分析
2.1 水分胁迫对黍子幼苗生长的影响
2.1.1 形态指标
由表1可见,控水处理20d后,所测幼苗各形态指标包括株高、茎粗、节数、叶片数、最大叶面积均表现出相同的变化特点,即:正常灌溉(W3,65%~70%)下植株形态生长最好,各项指标均最高;轻旱(W2,40%~45%)和轻涝(W4,90%~95%)处理下株高、茎粗、最大叶面积比正常灌溉下显著减小,对节数和叶片数影响不显著;重旱(W1,15%~20%)和重涝(W5,115%~120%)胁迫下株高、茎粗、最大叶面积比轻旱和轻涝下进一步显著减小,节数和叶片数减小不显著。除叶片数在重涝下减少不显著外,与正常灌溉下相比,重旱和重涝下各形态指标均显著降低,且重旱下降低幅度明显大于重涝下。可见,基质灌水量过多(涝害)或过少(干旱)均会抑制黍子植株的形态生长,导致幼苗弱小。
2.1.2 生物量和含水量
由表2可知,水分胁迫20d后,幼苗根系、茎叶、穗的鲜质量和干质量均在正常灌溉(W3)下最高,生长最好;轻旱(W2)和轻涝(W4)下各部分生物量比正常灌溉下均有不同程度降低,其中轻旱下穗鲜质量和干质量、轻涝下根系干质量降低达显著水平;重旱(W1)下所测各部分的鲜质量和干质量均比轻旱下进一步显著降低,重涝(W5)下仅茎叶鲜质量和干质量比轻涝下显著降低,根系和穗的鲜质量和干质量降低不显著。根系含水量随各处理的变化规律与生物量基本一致;茎叶含水量则在干旱下低于正常灌溉下,重旱下降低达显著水平,在涝害下高于正常灌溉下,重涝下升高达显著水平;穗含水量在干旱下低于正常灌溉下,重旱下降低达显著水平,涝害下与正常灌溉下基本一致。与正常灌溉下相比,重旱、轻旱、轻涝、重涝条件下,根系鲜质量和干质量分别降低69.89%、5.89%、5.63%、12.83%和47.77%、3.00%、7.48%、8.22%;茎叶鲜质量和干质量分别降低67.62%、5.16%、5.01%、30.63%和57.90%、4.06%、10.69%、39.74%;穗鲜质量和干质量分别降低65.63%、22.55%、6.53%、9.49%和60.80%、18.19%、6.26%、10.23%。结合形态指标结果可知,基质干旱或涝害均会明显抑制黍子幼苗的生长,且干旱对生长的抑制程度比涝害更大,其中重旱对幼苗生长的伤害最严重。

表1 不同水分处理20d时黍子幼苗形态指标的比较
注:W1、W2、W3、W4、W5分别表示重旱、轻旱、正常、轻涝、重涝5种水分处理,数据后小写字母表示处理间在0.05水平上的差异显著性,数据为平均值±标准差。下同。
Note:W1, W2, W3, W4, W5 indicate severe drought, slight drought, normal irrigation, slight flooding, severe flooding, respectively. Lowercase after data indicates the difference significance among treatments at 0.05 level. Data are mean±SD. The same as below.

表2 不同水分处理20d时黍子幼苗生物量和含水量的比较
2.2 水分胁迫对黍子幼苗叶片光合色素含量的影响
由图1可知,水分处理20d时,与正常灌溉(W3)下相比,干旱胁迫下幼苗的叶片光合色素含量显著降低,重旱(W1)比轻旱(W2)胁迫下降低幅度更大;涝害下叶片光合色素含量略有增加,但轻涝(W4)和重涝(W5)胁迫下均与正常灌溉下无显著差异。与正常灌溉条件下相比,重旱、轻旱处理下叶片叶绿素a、叶绿素b和类胡萝卜素含量分别降低30.02%、27.69%、23.62%和23.90%、21.20%、18.78%。结果说明,干旱胁迫使黍子幼苗叶片光合色素合成减少而分解加速,导致光合色素含量显著降低,涝害则对叶片光合色素的代谢无显著影响。
2.3 水分胁迫对黍子幼苗叶片膜脂过氧化的影响
图2显示,水分胁迫20d后,幼苗叶片质膜透性、MDA和AsA含量均以正常灌溉(W3)下最低;轻旱(W2)和轻涝(W4)处理下质膜透性和MDA含量比正常灌溉下显著增加,AsA含量在轻旱下显著增加而轻涝下增加不显著;重旱(W1)和重涝(W5)胁迫下质膜透性和MDA含量比轻旱和轻涝处理下进一步显著增加,AsA含量增加不显著。与正常灌溉下相比,重旱、轻旱、轻涝、重涝下,质膜透性、MDA和AsA含量分别增加80.79%、58.25%、21.94%,44.67%、31.90%、14.79%,31.98%、17.35%、10.72%和51.08%、44.19%、18.82%。说明基质干旱或涝害均会导致黍子幼苗膜脂过氧化伤害,使细胞膜透性增大,且干旱胁迫造成的过氧化伤害比涝害更严重,同时水分胁迫诱导幼苗体内AsA含量增加,有利于部分减轻胁迫造成的过氧化伤害,正常灌溉则有利于维持细胞膜的稳定性和完整性。
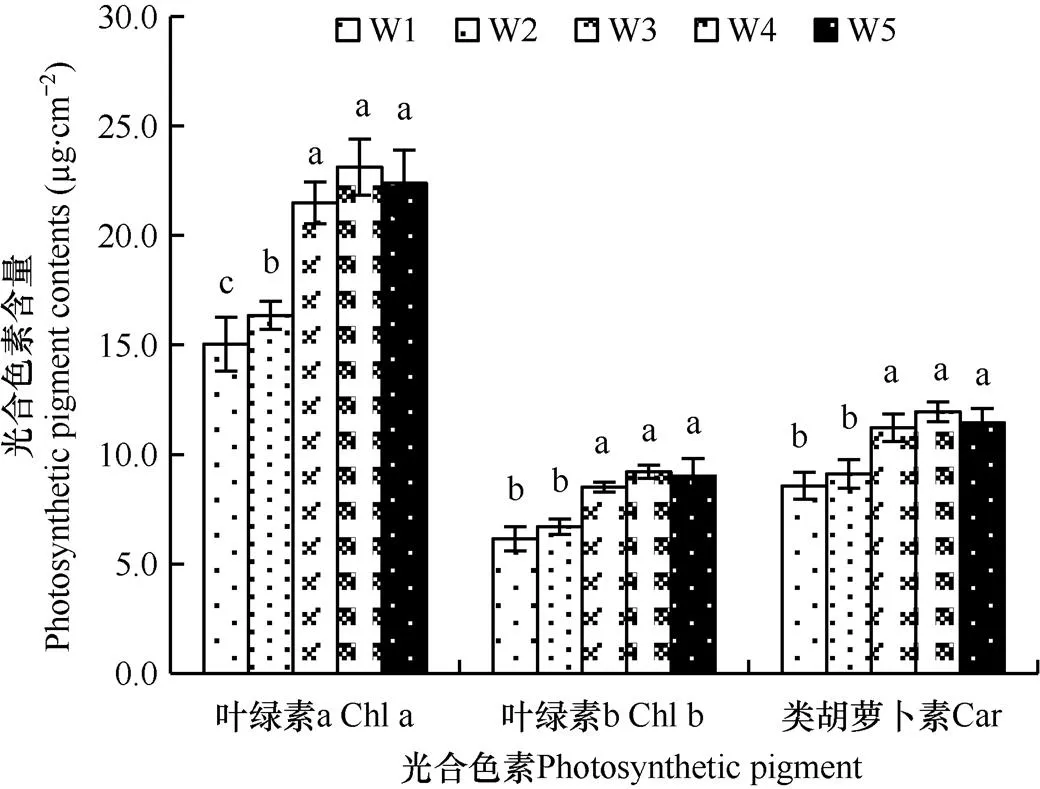
图1 不同水分处理20d时黍子幼苗叶片光合色素含量的比较
注:短线表示标准差。下同。
Note:The short bar is mean deviation. The same as below.

图2 不同水分处理20d时黍子幼苗叶片膜脂过氧化的比较
2.4 水分胁迫对黍子幼苗叶片有机渗透调节物质含量的影响
图3表明,水分处理20d后,幼苗叶片脯氨酸含量在正常灌溉(W3)下最低,轻旱(W2)和轻涝(W4)处理下略有增加,重旱(W1)和重涝(W5)胁迫下显著增加;与正常灌溉下相比,可溶性糖含量在干旱胁迫下增加,其中重旱胁迫下增加达显著水平,涝害下降低,但轻涝和重涝下降低均不显著;可溶性蛋白含量在干旱胁迫下比正常灌溉下显著降低,涝害下则无显著变化。说明在干旱胁迫和涝害下,黍子幼苗可以通过促进脯氨酸的合成以调节植株体内的细胞渗透势,减轻胁迫造成的细胞水分亏缺;可溶性糖在干旱胁迫下可作为渗透调节物质起作用,在涝害下则被大量消耗,可溶性蛋白在干旱胁迫下被降解而在涝害下不起作用。
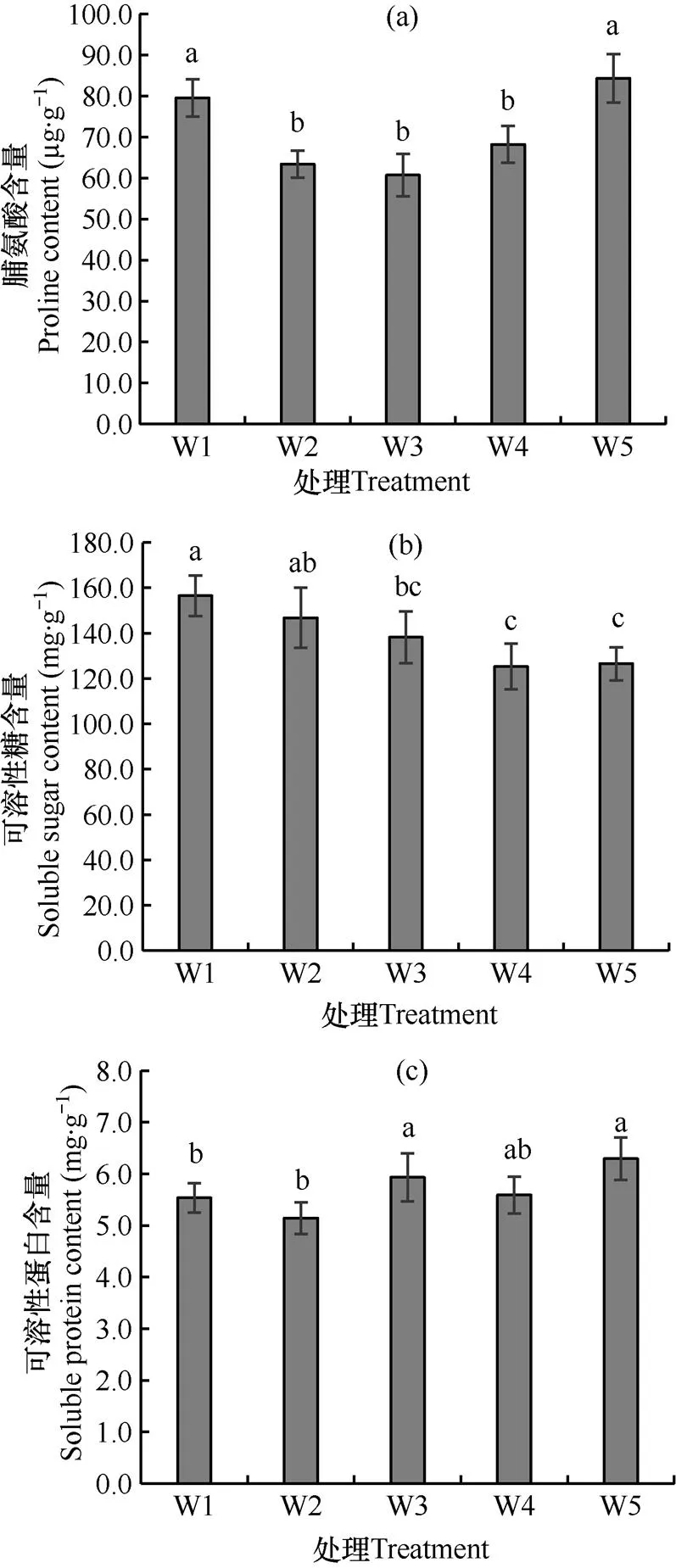
图3 不同水分处理20d时黍子幼苗叶片渗透调节物质含量的比较
3 结论与讨论
3.1 讨论
中国水资源分布极不均匀,西北和华北部分地区缺水严重,华中及华南地区降雨过多,因此作物的水分胁迫是农学和植物生理学研究的热点问题[19−21]。研究表明,水分胁迫下作物的生长发育显著抑制,产量品质明显降低,严重时导致植株死亡[22−24]。本研究中,黍子幼苗在正常灌溉下生长最好,干旱胁迫和涝害使黍子生长显著降低,且干旱胁迫对生长的抑制程度比涝害更大,这可能与实验所用栽培基质的持水性较弱、黍子根系吸收和运输水分的能力较强[25]有关,涝害下植株通过促进根系吸水使茎叶含水量增加而穗含水量稳定,有效减轻了涝害的影响。
光合作用是植物生长发育的基础,光合色素负责光合作用中光能的吸收、传递和转化,其含量直接反映植物的光合能力[21, 26]。水分胁迫不仅影响植物光合色素的合成,还会加速原有光合色素的分解[1, 27],同时抑制叶片生长、影响气孔开闭、损伤叶肉细胞、降低光合酶活性,使光合速率降低[28−30],最终使植物生长发育受阻,产量降低。本研究中,黍子叶片各光合色素含量均在干旱胁迫下显著降低而在涝害下略有增加,说明干旱胁迫促使叶片光合色素分解,抑制其合成,从而使植株的光合能力减弱,这是黍子植株对干旱的适应性反应,也是干旱胁迫下黍子生长降低的主要原因之一,与前人的研究结果[31−34]一致。涝害对黍子叶片光合色素的代谢几乎没有影响,可能是因为本实验所用基质的最大持水量较低(仅约30%),即使在重涝下基质含水量也仅为基质干重的39%~41%,虽然水分超过了基质的最大持水量,但基质表面并没有积水,仍具有一定的通气性,因此叶片光合色素仍能正常合成。
研究表明,水分胁迫下,细胞内自由基大量产生,导致膜脂中不饱和脂肪酸过氧化,使细胞膜结构破坏,质膜透性增加,电解质大量渗漏,膜脂过氧化的最终产物MDA含量增加[20, 24, 34−35]。AsA是植物体内的非酶自由基清除剂,能够减轻或消除膜脂过氧化对细胞造成的伤害[36]。本研究中,除AsA在轻涝下增加不显著外,黍子幼苗叶片质膜透性、MDA和AsA含量在干旱胁迫和涝害下均显著增加,说明根际水分状况与质膜的结构和功能关系密切,干旱胁迫和涝害均会引起活性氧大量产生,造成膜脂过氧化伤害,这与刘佳等[37−38]的研究结果一致。植株可通过促进AsA的合成来增强抗氧化能力,清除活性氧,缓解自由基大量产生造成的过氧化伤害,适应水分胁迫环境。但是水分胁迫下活性氧产生与清除的平衡已经破坏,AsA的增加并不能及时清除自由基,使膜脂过氧化伤害仍随胁迫程度增加而加重[39]。同时干旱胁迫对黍子的过氧化伤害比涝害更严重,这可能与本实验采用砂培并以基质最大持水量的百分比设置处理水平有关。正常灌溉下黍子细胞膜结构稳定,有利于细胞内生理生化代谢的正常进行。
脯氨酸、可溶性糖和可溶性蛋白等植物体内重要的有机渗透调节物质,在环境胁迫下可迅速积累[24, 40−42],从而降低细胞渗透势,提高细胞吸水能力,保护代谢酶活性,维持细胞膜的完整性等[21, 43−45]。脯氨酸的积累还可以减轻氨毒害和保护生物大分子[46]。本研究中,黍子叶片脯氨酸含量在干旱胁迫和涝害下均明显增加,可溶性糖含量在干旱下增加而在涝害下降低,可溶性蛋白含量在干旱下显著降低而在涝害下无明显变化,说明干旱胁迫和涝害均可造成黍子幼苗渗透胁迫,干旱胁迫下植株可通过合成和积累脯氨酸和可溶性糖调节细胞渗透势,促进细胞吸水,维持细胞水分平衡,减轻水分亏缺,这与杜磊等[47]的研究结果一致。涝害下黍子幼苗主要利用脯氨酸进行渗透调节,可溶性糖可能由于无氧呼吸加强及同化产物运输受阻,为了维持生长被大量消耗,这有利于植株适应胁迫环境[24]。黍子幼苗可溶性蛋白质在干旱胁迫下被分解,这与水稻在臭氧胁迫下[48]、番茄在高温胁迫下[49]的变化相似,对涝害下的渗透调节则无贡献。
3.2 结论
基质干旱和涝害均会对幼苗造成过氧化伤害和渗透胁迫,使黍子生长被明显抑制,表现为株高降低、茎节数减少、叶面积减小、生物量降低。植株可通过提高抗氧化能力、积累有机渗透调节物质部分适应水分胁迫,但这种自我调节无法完全消除水分胁迫造成的伤害,特别是在干旱胁迫下植株的光合色素合成受阻、分解加快,光合能力降低,使黍子生长的抑制更加严重。在本研究条件下,干旱胁迫对黍子的影响比涝害更为严重,但幼苗即使在重旱下也未出现死亡,说明黍子植株的耐旱性很强,这可能与黍子根系较发达、吸水能力强、角质层较厚、蒸腾阻力大等形态结构特征有关[11],具体原因有待进一步研究。黍子对干旱和涝害的耐性和生理响应,特别是在光合色素代谢和渗透调节方面存在明显差异,有必要深入研究其生理和分子机制。
[1] 蔡庆生.植物生理学[M].北京:中国农业大学出版社,2014: 306-313.
Cai Q S.Plant physiology[M].Beijing:China Agricultural University Press,2014:306-313.(in Chinese)
[2] 董杰,贾学峰.全球气候变化对中国自然灾害的可能影响[J].聊城大学学报,2004,17(2):58-62.
Dong J,Jia X F.Possible impacts of global climate change on natural disasters of China[J].Journal of Liaocheng University, 2004,17(2):58-62.(in Chinese)
[3] 王纶,王星玉,温琪汾,等.中国黍稷种质资源研究与利用[J].植物遗传资源学报,2005,6(4):474-477.
Wang L,Wang X Y,Wen Q F,et al.Research and utilization of prosomillet germplasm resource in China[J].Journal of Plant Genetic Resources,2005,6(4):474-477.(in Chinese)
[4] 穆志新,郝晓鹏,秦慧彬,等.山西省干旱地区农作物种质资源普查与分析[J].植物遗传资源学报,2016,17(4):637-648.
Mu Z X,Hao X P,Qing H B,et al.General survey and analysis of crop germplasm resources in drought area of Shanxi province[J].Journal of Plant Genetic Resources,2016, 17(4): 637-648.(in Chinese)
[5] 周晋红.山西省干旱时空分布特征及形成机理研究[D].南京:南京信息工程大学,2010.
Zhou J H.Study on temporal and spatial distribution feature of drought and its formation mechanism in Shanxi province [D]. Nanjing:Nanjing University of Information Science & Technology,2010.(in Chinese)
[6] 庞鑫.山西省农业干旱时空变化特征及其与气象因子的响应研究[D].太原:太原理工大学,2017.
Pang X.Spatial and temporal variation characteristics of agricultural drought and its response to meteorological factors in Shanxi province[D].Taiyuan:Taiyuan University of Technology, 2017.(in Chinese)
[7] 袁瑞强,龙西亭,王鹏,等.山西省降水量时空变化及预测[J].自然资源学报,2015,30(4):651-663. Yuan R Q,Long X T,Wang P,et al.Tempo-spatial variation and forecast of precipitation in Shanxi province[J].Journal of Natural Resources,2015,30(4):651-663.(in Chinese)
[8] 张美俊, 杨武德, 乔治军, 等.不同糜子品种萌发期对干旱胁迫的响应及抗旱性评价[J].草地学报,2013,21(2): 302-307.
Zhang M J,Yang W D,Qiao Z J,et al.Resistance evaluation and response of 16 millet varieties at germination stage to drought stress[J].Acta Agrestia Sinaca,2013,21(2):302-307. (in Chinese)
[9] 王纶,温琪汾,曹厉萍,等.黍稷抗旱种质筛选及抗旱机理研究[J].山西农业科学,2007,35(4):31-34.
Wang L,Wen Q F,Cao L P,et al.Drought-resistant germplasm screening and drought-resistance mechanism in proso millet[J].Journal of Shanxi Agricultural Sciences,2007,35(4): 31-34.(in Chinese)
[10] 张永清,苗果园.不同施肥水平下黍子根系对干旱胁迫的反应[J].作物学报,2006,32(4):601-606.
Zhang Y Q,Miao G Y.The biological response of broom corn millet root to drought stress with different fertilization levels[J].Acta Agronomica Sinica,2006,32(4):601-606.(in Chinese)
[11] 冯晓敏.不同黍稷品种耐旱性差异及生理生态特性研究[D].临汾:山西师范大学,2012.
Feng X M.Study on drought tolerance diversity and physiological characteristics of different prosoes[D].Linfen: Shanxi Normal University,2012.(in Chinese)
[12] 闫江艳,张永清,冯晓敏,等.干旱胁迫及复水对不同黍稷品种根系生理特性的影响[J].西北植物学报,2012,32(2): 348-354.
Yan J Y,Zhang Y Q,Feng X M,et al.Effect of drought stress and rewatering on physiological characteristics of roots in different prosomillet varieties[J].Acta Bot Boreal-Occident Sin,2012,32(2):348-354.(in Chinese)
[13] 王素平,郭世荣,胡晓辉,等.盐胁迫对黄瓜幼苗叶片光合色素含量的影响[J].江西农业大学学报,2006,28(2):32-38.
Wang S P,Guo S R,Hu X H,et al.Effects of NaCl stress on the content of photosynthetic pigments in the leaves of cucumber(L.)seedlings[J].Acta Agriculturae Universitatis Jiangxiensis,2006,28(2):32-38.(in Chinese)
[14] Madhava R K V,Sresty T V S.Antioxidative parameters in the seedlings of pigeonpea (L.) in response to Zn and Ni stresses[J].Plant Science,2000,157: 113-128.
[15] 汤绍虎,罗充.植物生理学实验教程[M].重庆:西南师范大学出版社,2012:199-202.
Tang S H,Luo C.Plant physiology experiment course[M]. Chongqing:Southwest Normal University Press, 2012: 199-202. (in Chinese)
[16] 中科院上海植物生理研究所、上海市植物生理学会.现代植物生理学实验指南[M].北京:科学出版社,1999:315-316.
Shanghai Institute of Plant Physiology,Chinese Academy of Sciences,Shanghai Society of Plant Physiology.Guidelines for modern plant physiology experiments[M].Beijing: Science Press,1999:315-316.(in Chinese)
[17] 李合生.植物生理生化实验原理与技术[M].北京:高等教育出版社,2002:195-197,258-260.
Li H S.Principle and technology of plant physiological and biochemical experiments[M].Beijing:Higher Education Press, 2002:195-197,258-260.(in Chinese)
[18] 王孝平,邢树礼.考马斯亮蓝法测定蛋白质含量的研究[J].天津化工,2009,23(3):88-90.
Wang X P,Xing S L.Determination of protein quantitation using the method of coomassie brilliant blue[J].Tianjin Chemical Industry,2009,23(3):88-90.(in Chinese)
[19] 闫瑞,钱春.无患子幼苗对水分胁迫的生理响应[J].西南大学学报(自然科学版),2014,36(4):29-33.
Yan R,Qian C.Physiological response ofGaertn.seedlings to water stress[J].Journal of Southwest University (Nat Sci Ed),2014,36(4):29-33.(in Chinese)
[20] 王三根,宗学凤.植物抗性生物学[M].重庆:西南师范大学出版社,2015:123-140.
Wang S G,Zong X F.Plant resistance biology[M].Chongqing: Southwest Normal University Press,2015:123-140.(in Chinese)
[21] 武维华.植物生理学(第三版)[M].北京:科学出版社,2018: 110-119,385-391.
Wu W H.Plant physiology(3rd Ed)[M].Beijing:Science Press, 2018:110-119,385-391.(in Chinese)
[22] Sharma M,Gupta S K,Majumder B,et al.Salicylic acid mediated growth,physiological and proteomic responses in two wheat varieties under drought stress[J].Journal of Proteomics,2017,163:28-51.
[23] 朱雨晴,杨再强.不同品种葡萄叶片光合特性对干旱胁迫的响应及旱后恢复过程[J].中国农业气象,2018,39(11): 739-750.
Zhu Y Q,Yang Z Q.Photosynthetic responses of different grape cultivars to drought stress and their recovery after drought[J].Chinese Journal of Agrometeorology,2018,39 (11):739-750.(in Chinese)
[24] 张立军,刘新.植物生理学(第二版)[M].北京:科学出版社,2011:307-315.
Zhang L J,Liu X.Plant physiology(2nd Ed)[M].Beijing: Science Press,2011:307-315.(in Chinese)
[25] 裴荣信.黍子的高产抗灾优势[J].山西农业,1998,(6):9-10.
Pei R X.High yield and disaster resistance advantages of millet[J].Shanxi Agriculture,1998,(6):9-10.(in Chinese)
[26] Nijs I,Ferris R,Blum H.Stomatal regulation in a changing climate:field study using free air temperature increase(FATI) and free air CO2enrichment[J].Plant Cell and Environment, 1997,42:1041-1050.
[27] 王嘉楠,李小艳,魏石美,等.5-ALA对干旱胁迫下小麦幼苗光合作用及D1蛋白的调节作用[J].作物杂志,2018,(5): 121-126.
Wang J N,Li X Y,Wei S M,et al.Regulation of exogenous 5-aminolevulinic acid on photosynthesis and D1 protein of wheat seedlings under drought stress[J].Crops,2018,(5): 121-126. (in Chinese)
[28] Lawlor D W,Cornic G.Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants[J].Plant,Cell and Environment,2002,25:275-294.
[29] 韩瑞宏,卢欣石,高桂娟,等.紫花苜蓿()对干旱胁迫的光合生理响应[J].生态学报,2007,27(12): 5230-5236.
Han R H,Lu X S,Gao G J,et al.Photosynthetic physiological response of alfalfa () to drought stress[J]. Acta Ecologica Sinica,2007,27(12):5230-5236.(in Chinese)
[30] 郑海,陈小华,黎健龙,等.水分胁迫对皇竹草形态及叶片光合特性的影响[J].广东农业科学,2014,(13):33-36.
Zheng H,Chen X H,Li J L,et al.Effects of water stress on morphology and photosynthetic characteristics of hybrid giant napier()[J].Guangdong Agricultural Sciences,2014,(13):33-36.(in Chinese)
[31] Zhang H T,Li J J,Yoo J H,et al.Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development[J].Plant Molecular Biology,2006,62(3):325-337.
[32] 李倩,王明,王雯雯,等.华山新麦草光合特性对干旱胁迫的响应[J].生态学报,2013,32(13):4278-4284.
Li Q,Wang M,Wang W W,et al.Response of photosynthetic characteristic ofHuashanica Keng to drought stress[J].Acta Ecologica Sinica,2013,32(13): 4278-4284.(in Chinese)
[33] Ghotbi-Ravandi A A,Shahbazi M,Shariati M,et al.Effects of mild and severe drought stress on photosynthetic efficiency in tolerant and susceptible barley(L) genotypes[J].Journal of Agronomy and Crop Science,2014, 200(6):403-415.
[34] 张曼义,杨再强,侯梦媛.土壤水分胁迫对设施黄瓜叶片光合及抗氧化酶系统的影响[J].中国农业气象,2017,38(1): 21-30.
Zhang M Y,Yang Z Q,Hou M Y.Effects of soil water stress on photosynthetic characteristics and antioxidant enzyme system of cucumber leaves in greenhouse[J].Chinese Journal of Agrometeorology,2017,38(1):21-30.(in Chinese)
[35] 阎秀峰,李晶,祖元刚.干旱胁迫对红松幼苗保护酶活性及脂质过氧化作用的影响[J].生态学报,1999,19(6):850-854.
Yan X F,Li J,Zu Y G.Effect of drought stress on activity of cell defense enzymes and lipid peroxidation inpine seedlings[J].Acta Ecologica Sinica,1999,19(6):850-854.(in Chinese)
[36] 刘旻霞,马建组.逆境胁迫下加工番茄种子萌发及抗氧化酶系统的影响[J].石河子大学学报(自然科学版),2010,28 (4): 422-426.
Liu M X,Ma J Z.Effects of stress on germination and antioxidant enzyme system of processing tomato seeds[J]. Journal of Shihezi University (Nat.Sci.Ed.),2010,28(4): 422-426.(in Chinese)
[37] 刘佳,郁继华,徐秉良,等.干旱气候条件下水分胁迫对辣椒叶片生理特性的影响[J].核农学报,2012,26(8):1197-1203.
Liu J,Yu J H,Xu B L,et al.Effects of water stress on the physiological characteristics of pepper leaves in arid climates[J].Journal of Nuclear Agricultural Sciences,2012, 26(8):1197-1203.(in Chinese)
[38] 杨再强,刘朝霞,韩秀君,等.水分胁迫对番茄保护酶活性及果实产量的影响[J].东北农业大学学报,2014,45(3):40-45.
Yang Z Q,Liu Z X,Han X J,et al.Effects of water stress on protective enzyme activity and yield of tomato[J].Journal of Northeast Agricultural University,2014,45(3):40-45.(in Chinese)
[39] 张旭东,王智威,韩清芳,等.玉米早期根系构型及其生理特性对土壤水分的响应[J].生态学报,2016,36(10):2969-2977.
Zhang X D,Wang Z W,Han Q F,et al.Effects of water stress on the root structure and physiological characteristics of early-stage maize[J].Acta Ecologica Sinica,2016,36(10): 2969-2977.(in Chinese)
[40] 赵坤.干旱胁迫条件下春大豆生理生化特性研究[D].哈尔滨:东北农业大学,2011.
Zhao K.Research on physiological and biochemical characteristics of spring soybean under drought stress [D]. Harbin:Northeast Agricultural University,2011.(in Chinese)
[41] Azymi S,Sofalian O,Jahanbakhsh G S,et al.Effect of chilling stress on soluble protein,sugar and proline accumulation in cotton (L.) genotypes[J].Intl J Agri Crop Sci,2011,4:825-830.
[42] 李钱峰,鲁军,余佳雯,等.油菜素内酯与脱落酸互作调控植物生长与抗逆的分子机制研究进展[J].植物生理学报, 2018,54(3):370-378.
Li Q F,Lu J,Yu J W,et al.Advances in molecular mechanisms of brassinosteroid-abscisic acid crosstalk coordinating plant growth and stress tolerances[J].Plant Physiology Journal, 2018,54(3):370-378.(in Chinese)
[43] Parida A K,Das A B.Salt tolerance and salinity effects on plants:a review[J].Ecotoxicology and Environmental Safety, 2005,60:324-349.
[44] 刘景辉,赵海超,任永峰,等.土壤水分胁迫对燕麦叶片渗透调节物质含量的影响[J].西北植物学报,2009,29(7): 1432-1436.
Liu J H,Zhao H C,Ren Y F,et al.Change of osmotica in oat leaf under soil moisture stress[J].Acta Bot Boreal-Occident Sin,2009,29(7):1432-1436.(in Chinese)
[45] 张保青,杨丽涛,李杨瑞.自然条件下甘蔗品种抗寒生理生化特性的比较[J].作物学报,2011,37(3):496-505.
Zhang B Q,Yang L T,Li Y R.Comparison of physiological and biochemical characteristics related to cold resistance in sugarcane under field conditions[J].Acta Agronomica Sinica,2011,37(3):496-505.(in Chinese)
[46] Sonja D,Pignocchi C,Noctor G,et al.Low ascorbic acid in themutant of arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system[J].Plant Physiology,2001,127:426-435.
[47] 杜磊,赵尊练,巩振辉,等.水分胁迫对线辣椒叶片渗透调节作用的影响[J].干旱地区农业研究,2010,28(3):188-198.
Du L,Zhao Z L,Gong Z H,et al.Effects of water stress on the osmotic regulation of pepper leaves[J].Agricultural Research in Arid Regions,2010,28(3):188-198.(in Chinese)
[48] 张巍巍,郑飞翔,王效科,等.臭氧对水稻根系活力、可溶性蛋白含量与抗氧化系统的影响[J].植物生态学报,2009, 33(3):425-432.
Zhang W W,Zheng F X,Wang X K,et al.Effects of ozone on root viability,soluble protein content and antioxidant system in rice[J].Chinese Journal of Plant Ecology,2009,33(3): 425-432.(in Chinese)
[49] 王琳,杨再强,王明田,等.空气相对湿度对高温下番茄幼苗营养物质含量及干物质分配的影响[J].中国农业气象, 2018,39(5):304-313.
Wang L,Yang Z Q,Wang M T,et al.Effect of air humidity on nutrient content and dry matter distribution of tomato seedlings under high temperature[J].Chinese Journal of Agrometeorology,2018,39(5):304-313.(in Chinese)
Effects of Water Stress on Growth and Physiological Properties of Millet Seedlings
HAN Zhi-ping1, ZHANG Hai-xia2, ZHANG Xun1, LIANG Yan-hua1
(1. School of Life Science/Protected Agricultural Technology Development Center, Shanxi Datong University, Datong 037009, China; 2. Department of Logistics, Shanxi Datong University, Datong 037009)
In order to study the effects of water stress on the growth, membrane lipid peroxidation and osmotic adjustment of broom corn millet seedlings, the experiment was conducted 5 treatments: severe drought, slight drought, normal irrigation control, slight flooding and severe flooding. ‘Jinshu No. 8’ millet as material was grown in sand culture and irrigated with nutrient solution, and indicators including the morphological indicators, biomass, water content of plant, and the membrane permeability, the contents of photosynthetic pigments, MDA, AsA, proline, soluble sugar and soluble protein in leaves were determined on 20th day after treatment. The results showed that the millet seedling grew best under normal irrigation condition, with the largest values of plant height, stem diameter, nodule number, leaf number, maximum leaf area, fresh mass and dry mass of root, stem and leaf, and panicle. All morphological indicators and biomasses were obviously decreased under drought and flooding treatments, and the extent of decrease under severe drought and severe flooding were more obvious than those under slight drought and slight flooding. The water contents of root, stem and leaf, panicle were clearly decreased under drought, but showed different tendency under flooding treatments. The contents of photosynthetic pigments were significantly decreased under drought, while relatively stable under flooding. The membrane permeability, the contents of MDA, AsA and proline were obviously increased under drought and flooding, and the extents of increase under severe drought and severe flooding were more obvious than those under slight drought and slight flooding. The soluble sugar content was clearly increased under drought and was clearly decreased under flooding, the soluble protein content was significantly reduced under drought and was relatively stable under flooding. The research illustrated that the drought and flooding caused the peroxidation injury to the millet seedlings, and the contents of antioxidants and osmotic adjusting materials were increased. But the increase of antioxidants could not completely eliminate the peroxidation injury caused by stress. In addition, the photosynthetic ability was decreased under stress, which caused the inhibition of the growth of millet seedlings. Under the experimental condition, drought stress caused more serious damage to millet seedlings than flooding.
Millet; Water stress; Growth; Lipid peroxidation; Osmotic adjustment substances
10.3969/j.issn.1000-6362.2019.08.003
韩志平,张海霞,张巽,等.水分胁迫对黍子幼苗生长和生理特性的影响[J].中国农业气象,2019,40(8):502-511
2019−01−03
山西省农业科技攻关项目(20150311010-1);大同市农业科技攻关项目(201468-2)
韩志平(1976−),博士,副教授,主要从事植物逆境生理研究。E-mail:13620629501@163.com

