Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve
Zhong-Yuan Zhang , Jin Yang , Zhen-Hai Fan , Da-Li Wang, Yu-Ying Wang , Tao Zhang , Li-Mei Yu , , Chang-Yin Yu
1 Key Laboratory of Cell Engineering in Guizhou Province, The Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, China 2 Department of Thyroid and Breast Surgery, Fifth People's Hospital of Chengdu, Chengdu, Sichuan Province, China
3 The Team of Scientific and Technological Innovation Talents on The Basic and Clinical Research of Amniotic Membrane and Bone Marrow Stem Cells in Guizhou Province, Zunyi, Guizhou Province, China
4 Department of Burn and Plastic Surgery, The Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, China
5 Department of Neurology, The Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, China
Abstract Suture and autologous nerve transplantation are the primary therapeutic measures for completely severed nerves. However, imbalances in the microenvironment and adhesion of surrounding tissues can affect the quality of nerve regeneration and repair. Previous studies have shown that human amniotic membrane can promote the healing of a variety of tissues. In this study, the right common peroneal nerve underwent a 5-mm transection in rats. Epineural nerve repair was performed using 10/0 non-absorbable surgical suture. The repair site was wrapped with a two-layer amniotic membrane with α-cyanoacrylate rapid medical adhesive after suture. Hindlimb motor function was assessed using footprint analysis. Conduction velocity of the common peroneal nerve was calculated by neural electrical stimulation. The retrograde axoplasmic transport of the common peroneal nerve was observed using fast blue BB salt retrograde fluorescent staining. Hematoxylin-eosin staining was used to detect the pathological changes of the common peroneal nerve sputum. The mRNA expression of axon regeneration-related neurotrophic factors and inhibitors was measured using real-time polymerase chain reaction. The results showed that the amniotic membrane significantly improved the function of the injured nerve; the toe spread function rapidly recovered, the nerve conduction velocity was restored, and the number of fast blue BB salt particles were increased in the spinal cord. The amniotic membrane also increased the recovery rate of the tibialis anterior muscle and improved the tissue structure of the muscle. Meanwhile, mRNA expression of nerve growth factor, growth associated protein-43, collapsin response mediator protein-2, and brain-derived neurotrophic factor recovered to near-normal levels, while Lingo-1 mRNA expression decreased significantly in spinal cord tissues. mRNA expression of glial-derived neurotrophic factor did not change significantly. Changes in mRNA levels were more significant in amniotic-membrane-wrapping-treated rats compared with model and nerve sutured rats. These results demonstrate that fresh amniotic membrane wrapping can promote the functional recovery of sutured common peroneal nerve via regulation of expression levels of neurotrophic factors and inhibitors associated with axonal regeneration. The study was approved by the Committee on Animal Research and Ethics at the Affiliate Hospital of Zunyi Medical University, China (approval No. 112) on December 1, 2017.
Key Words: nerve regeneration; human amniotic membrane; axonal; Schwann cells; α-cyanoacrylate rapid medical adhesive; neural suture;tibial anterior muscle; neuronal growth factor; common peroneal nerve injury; neural regeneration
Introduction
Common clinical peripheral nerve injury is primarily caused by trauma, but can also be a result of oppression, infection,ischemia, tumors, or nutritional disturbance. Peripheral nerve injury can lead to neurological motor and sensory dysfunction. Peripheral nerve injury can cause clinical muscle paralysis or atrophy, hypoesthesia or lack of sensation, rough skin without perspiration, ulceration, and blood circulation disorders (Smania et al., 2012; Castillo-Galván et al., 2014).A timely recovery of nerve continuity and integrity is important; this requires creating a suitable microenvi-ronment for nerve regeneration by suture, nerve transplantation, and neurotrophic drugs, then repairing the damaged nerve and restoring its regional function. When axonotmesis with an endoneurial tube is performed, the regenerating axons may still breed in the Schwann tube for self-repair. For completely severed nerves, suture and autologous nerve transplantation are the primary therapeutic measures. However, the microenvironment imbalance and the surrounding tissue adhesion affect the quality of regeneration and repair (Kim,2011; Lykissas, 2011). The neurolemma of Schwann cells protects and regenerates nerves, and also has some secretory functions (Rodrigues et al., 2012). After nerve rupture, axoplasmic transport is interrupted, and Wallerian degeneration also occurs within 1 or 2 days. Then, macrophages engulf the fragments of axons and myelin, and Schwann cells begin to proliferate and form nerve regeneration channels. While axonal hyperplasia and Schwann cell proliferation form pseudoneuroma on the proximal nerve, Wallerian degeneration of the distal nerve appears within around 4 weeks after damage(Liang et al., 2012; Pace et al., 2013; Yao et al., 2013). The repair of neural structures and recovery of neurological function are influenced by the surrounding conditions (inflammatory response, adhesions, and scar formation) and status of the damaged neuron (nutritional condition, Schwann tube formation, local edema, blood supply, and epineurium thickening), although the regeneration rate is generally about 1 mm/day (Yamada et al., 2009).
In recent years, a variety of neural tubes and bridging nerve terminals have been used in animal experiments and clinical trials with or without neurotrophic factors, including amnion muscle-combined graft conduits (Riccio et al.,2014; Marchesini et al., 2018). These tissue engineering technologies can significantly promote axonal regeneration,Schwann cell proliferation, and the establishment of nerve regeneration channels in injured nerves. Biological materials wrapped around damaged nerves can also reduce degeneration and prevent adhesion. Transplanted mesenchymal stem cells have anti-inflammatory, secretory, and angiogenesis functions, which means that they can improve nerve regeneration by optimizing the microenvironment and increasing blood supply (Jiao et al., 2009; Frattini et al., 2012; Quigley et al., 2013). Commonly used biological materials can also provide a relatively good microenvironment for nerve regeneration and prevent further nerve damage. For example,chitosan, polylactic acid, polyglycolic acid copolymer composite film, chitosan-collagen-dipropionic acid, and fibrin gel have good biocompatibility, biodegradability, plasticity,and biological functions in peripheral nerve injury therapy(Ding et al., 2010; Zheng et al., 2010; Yaniv et al., 2012; Pan et al., 2013).
The human amniotic membrane (AM) has a long history as a medical biomaterial. It has been widely used in ocular surface injury, corneal reconstruction, skin burns, tissue filling, scaffold material, and neural tube production (O'Neill et al., 2009; Fotopoulou et al., 2010; Kim et al., 2010; Fairbairn et al., 2016). AM has been found to be valuable in clinical applications because it has useful functional characteristics that can facilitate biological activities without ethical controversies concerning the use of human tissue (Feng and Yu,2014; Zhao, 2015). AM has non-tumorigenic and low immunogenicity, antibacterial, antiviral, and anti-inflammatory effects, and secretes a variety of active factors, such as transforming growth factor-β, epidermal growth factor, stromal cell-derived factor, vascular endothelial growth factor, and polymorphic collagen (Fotopoulou et al., 2010; Kang et al.,2012; Mamede et al., 2012). Some studies have reported that easily manufactured and biodegradable amnion bridging conduits can significantly promote nerve regeneration and functional recovery (Meng et al., 2011; Fairbairn et al., 2014;Fesli et al., 2014). AM tissues are also rich in mesenchymal stem cells and epithelial cells, which have multiple biological functions to promote nerve, axon, and myelin regeneration(Zhao, 2015). The specific action and mechanism of the reparation effect of AM has yet to be clarified.
The balance of neurotrophins and neuron growth inhibitors plays a key role in the process of axon regeneration and the formation of myelin structure. The lack of neurotrophic factors and higher nerve regeneration inhibitory factors also significantly delay the process of nerve repair. Multiple neurotrophic factors promote axonal regeneration by activating the cyclic adenosine monophosphate/protein kinase A signaling pathway, which involves nerve growth factor (NGF),brain-derived neurotrophic factor (BDNF), glial cell line derived neurotrophic factor (GDNF), and growth associated protein 43 (GAP-43) (Yaniv et al., 2012). On the contrary,axon growth is inhibited by the formation of a NgR1/p75 or Troy/Lingo-1 receptor complex when a myelin-associated inhibitor factor activates the Lingo-1/RhoA/RhoA kinase pathway. This affects the cytostatic kinetics of neurons while also causing neuronal growth cone collapse (Zhao et al.,2016).
The purpose of this study was to investigate the therapeutic effects and neuroregenerative molecular changes elicited by application of a new biological dressings. Specifically, we examined the effect of an AM wrap on the restoration of a common peroneal nerve that was completely severed in rats.The experimental and theoretical basis for the application of the bioactive AM in the clinical treatment of peripheral nerve injury was established.
Materials and Methods
Animals
Forty-eight young adult male Sprague-Dawley rats (clean grade, 6-8 weeks old, weight 200 ± 20 g) were housed in standard environmental conditions at room temperature with a 12-hour light/dark cycle, and were had ad libitum access to commercially available balanced rodent food. They were provided by the Experimental Animal Center of Daping Hospital, Third Military Medical University of Chinese PLA (license No. SCXK (Yu) 2007-0003). The rats were divided randomly into the following four groups: control,model (completely severed common peroneal nerve injury only), suture (completely severed common peroneal nerve injury + end-to-end suture), and AM wrap (completely severed common peroneal nerve injury only + end-to-end suture + two-layer AM wrapping around the sutured nerve)groups (n = 12 per group). The study was approved by the Committee on Animal Research and Ethics at the Affiliate Hospital of Zunyi Medical University, China (approval No.112) on December 1, 2017.
AM wrap preparation
AM was mechanically removed from the human placenta after informed consent had been obtained from volunteers at the obstetrical department in the Affiliated Hospital of Zunyi Medical University, China. All residual blood clots and mucus were lightly scraped from the AM by washing the tissue several times with sterile phosphate-buffered saline.The amnion was cut into 2.0 mm × 5.0 mm amniotic patches. AM segments were folded into two layers. Then, AM was wrapped around the transected and end-sutured common peroneal nerve in a tube as shown in Figure 1D and E. The edge of the AM wrap was closed with medical α-cyanoacrylate rapid medical adhesive glue (medical anastomosis, OB glue, license No. 2006-3650647, Baiyun Medical Adhesive Co., Ltd., Guangzhou, China). The study was approved by the Committee on Animal Research and Ethics at the Affiliate Hospital of Zunyi Medical University, China (approval No. 112) on December 1, 2017.
Model establishment of completely severed common peroneal nerve and AM wrapping
The rats received intraperitoneal injection of anesthesia with 7% chloral hydrate. The right common peroneal nerve trunk was transected at a 5 mm segment above their branch point, except for control rats that did not receive any further treatment after separation and exposure of the nerve (Valli et al., 1968). The epineurium of the severed common peroneal nerve was end-to-end sutured in the suture group using a 10/0 non-absorbable surgical suture (Huawei Healthcare Ltd., Hangzhou, China). Rats in the AM group received a two-layer AM wrapping around the sutured nerve. The severed nerve of model group was neither sutured nor wrapped(Figure 1B).
Footprint analysis
At 3, 7, 14, and 28 days after nerve dissection surgery, the rats were encouraged to walk through a 10 cm × 70 cm, partially enclosed paper walking channel with black ink footprints of the bilateral hind paws. The gait and footprint were observed and measured via analysis of footprints generated with black ink. Toe spread (TS) was measured from the surgical-side and normal-side footprints between the first to fifth toe width. If the toes appear clawed or there was a trailing footprint that could not be detected, the width between the unexpanded five toes was measured when the rat stood on white paper (the upper body of the rat was supported by an experimenter during this measurement). The TS function(TSF) was calculated (Bozkurt et al., 2011) as TSF (%) =ETS/NTS × 100, where E represents the operative side and N represents the normal side.
Nerve conduction velocity analysis
BL-420E system (Chengdu Taimeng Technology Co. Ltd.,Chengdu, China) was connected after the rats had been anesthetized. The stimulated electrode was punctured into the right sciatic notch (above the site of nerve transection).Recording electrode 1 was placed at the surface projection area of the sciatic nerve about 1.5 cm from the stimulating electrode. Recording electrode 2 was inserted into the tibial anterior muscle of the fibular head area (below the site of trisection). The distance between recording electrode 1 and electrode 2 was 2 cm. The test item was the action potential of the selected neural stem in the muscle nerve experiments.Using a stimulus duration of 0.1 ms, stimulus intensity was increased gradually until a compound muscle action potential was obtained at recording electrode 1 and electrode 2.The latency of the action potential between the two recording electrodes was also recorded (Sekiguchi et al., 2013). Nerve conduction velocity was automatically calculated by BL-New Century software (Chengdu Taimeng Technology Co. Ltd.).
Fast blue BB salt retrograde fluorescent tracer
At 28 days after surgery, 5 μL 2.5% Fast blue BB salt (FB;excitation wavelength wave 340-380 nm, Fluka BioChemika, Buchs, Switzerland) was injected with a microinjector into the distal side 5 mm of the severed site in the common peroneal nerve for observation of retrograde axoplasmic transport. All rats were sacrificed 24 hours after injection.L4-5 and the lumbosacral enlargement of the spinal cord were frozen, then sliced 8-μm thick sections were observed and pictures were obtained under a DMIRB inverted fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
Histomorphometry
On the 28thday after surgery, the AM wrap absorption, surrounding tissue inflammation, and local adhesion were visually inspected in rats. Healing, swelling, and skin ulcers of the hind limb were also manually observed by experimenters. The myophagism was evaluated in the surgical side and normal lateral tibial anterior muscle. The complete surgical side and normal lateral tibial anterior muscle were obtained and accurately weighed. The tibial anterior muscle recovery rate (%) = the surgical side muscle wet weight/normal side muscle wet weight × 100. Histopathological changes in the muscle were examined through a microscope (Leica Microsystems, Wetzlar, Germany). After fixing the tissue with formaldehyde, we embedded, sliced, dewaxed, and hydrated the tissue, and then stained the bilateral tibial anterior muscle slices with hematoxylin-eosin.
Real-time polymerase chain reaction
The total RNA was isolated from the lumbosacral portion of the spinal cord by Trizol (Applied Biosystems Inc., Foster City, CA, USA) according to the manufacturer's instructions 28 days after the nerve operation. The RNA was subsequently reverse-transcribed using Taqman Reverse Transcription Reagents (Applied Biosystems Inc.). The synthesized cDNA was subjected to quantitative real-time polymerase chain reaction(PCR). The primers of neuroregenerative genes were synthesized for real-time PCR by Bio-Engineering Dalian Co. Ltd.(Table 1). The levels of mRNA expression were quantitatively assessed using an ABI PRISM 7500 Sequence Detection System (Applied Biosystems Inc.). The relative mRNA expression of target gene was normalized to β-actin expression and calculated as described preciously (Xu et al., 2012).
Statistical analysis
Statistical analyses were performed using the statistical package SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Data were presented as the mean ± standard deviation (SD). One-way analysis of variance and least significant difference tests were used to analyze all experimental data. A P-value < 0.05 was considered statistically significant.
Results
AM wrap improves walking and TSF in rats with completely severed common peroneal nerve injury
On the 3rdday after the operation, the control rats limped and footprints were clearer and wider, indicating that the rats had normal TSF. However, rats in the model group visibly limped, with footprints that were illegible or with an injured right hind paw that did not touch the ground (Figure 2A). The TS and TSF of the damaged paw were significantly lower in the model, suture, and AM groups than in the control group and the preoperative paw (P < 0.01, n = 8).The TS and TSF were not significantly different between the model, suture, and AM groups (P > 0.05). At 7, 14, and 28 days after the operation, pronation, foot arching, and claudication worsened in the model rats, and the footprints were inde-cipherable, with a trailing footprint. A model rat had toe swelling and skin ulcers of the right hind paw. The TSF of operative paws in the suture and AM groups was significantly wider compared with that of model rats at 14 and 28 days (P < 0.01; Figure 2), although the TSF of the AM group was lower than that of the control rats. In particular, the TSF was higher in the AM group than the suture group (P < 0.01,n = 8). The TSF of control rats gradually increased from the 3rdday. TSF recovery was faster in the AM group than in the suture group from the 7thday to the 14thday.
AM wrap increases nerve conduction velocity in rats with completely severed common peroneal nerve
The nerve conduction velocity and the amplitude from the right common peroneal nerve of rats are shown in Figure 3.On the 28thday after surgery, action potentials appeared in the AM, suture, and control rats, but not in model rats. The nerve conduction velocity and the amplitudes of the AM and suture groups were markedly increased compared with those of the model group (P < 0.01, n = 6), but were not significantly different to those of the control group (P > 0.05).The amplitudes of the control, AM, and suture groups were 1.18 ± 0.15, 1.17 ± 0.41, and 1.37 ± 0.55 mV, and no action potentials were generated in the model group.
AM wrap increases fluorescent tracer of FB in spinal cord in rats with completely severed common peroneal nerve
In the frozen spinal cord sections, FB fluorescence was present in the suture, AM, and control groups, but not in the model group (Figure 4). This indicated that there was reverse axoplasmic transport of the nerve and better nerve continuity in the suture and AM groups compared with the model group. From the dark field of view, the number of blue fluorescence particles was more abundant in control rats than in the AM wrap or suture-treated rats.
AM wrap improves local gross appearance and AM absorption
The re-explored right common peroneal nerve and adhesion data are shown in Figure 5. The perineural fibrosis and degree of adhesion was remarkably diminished in the AM group compared with the suture group. The model rats had the most severe adhesions. A common peroneal nerve defect was obvious in the model rats at 28 days after the operation,whereas better nerve continuity was seen in the AM andsuture groups, which were similar to the control group, and exhibited no neuroma formation. Most of the wrapped AM had been absorbed around the nerve in the AM group so that the adhesion was lighter than that seen in sutured rats,and the remanent AM was easily isolated from the nerve.
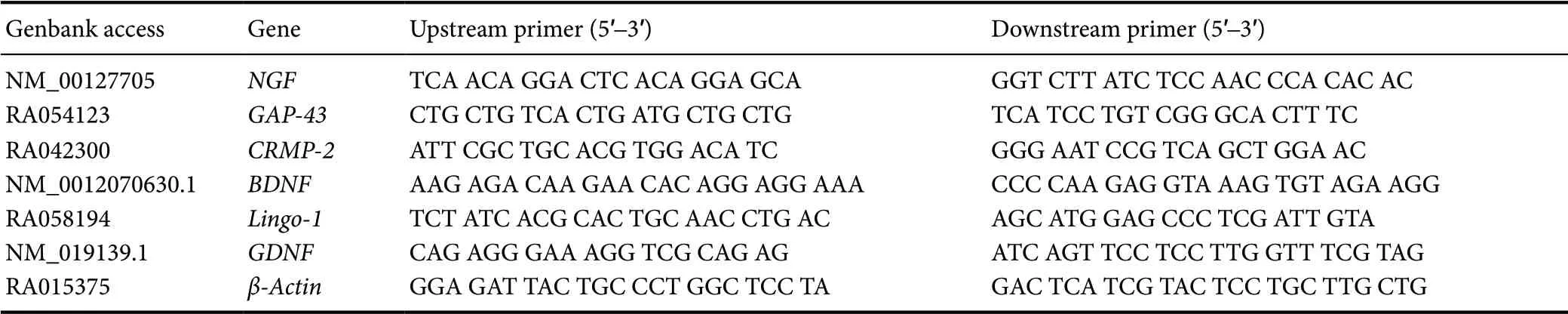
Table 1 Prime sequences of real-time polymerase chain reaction
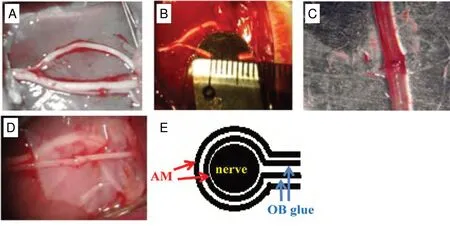
Figure 1 Different treatment for injured common peroneal nerve.(A) Control group: the nerve was separated, but not transected; (B) model group: the nerve was transected at 5 mm; (C) suture group: the 5-mm transected nerve was sutured to the end-toend transected nerve; (D) AM group:the sutured nerve was wrapped by a two-layer AM. (E) Schematic diagram of the double AM wrapping of the common peroneal nerve with an end-toend suture. AM: Amniotic membrane;OB glue: medical α-cyanoacrylate rapid medical adhesive glue.
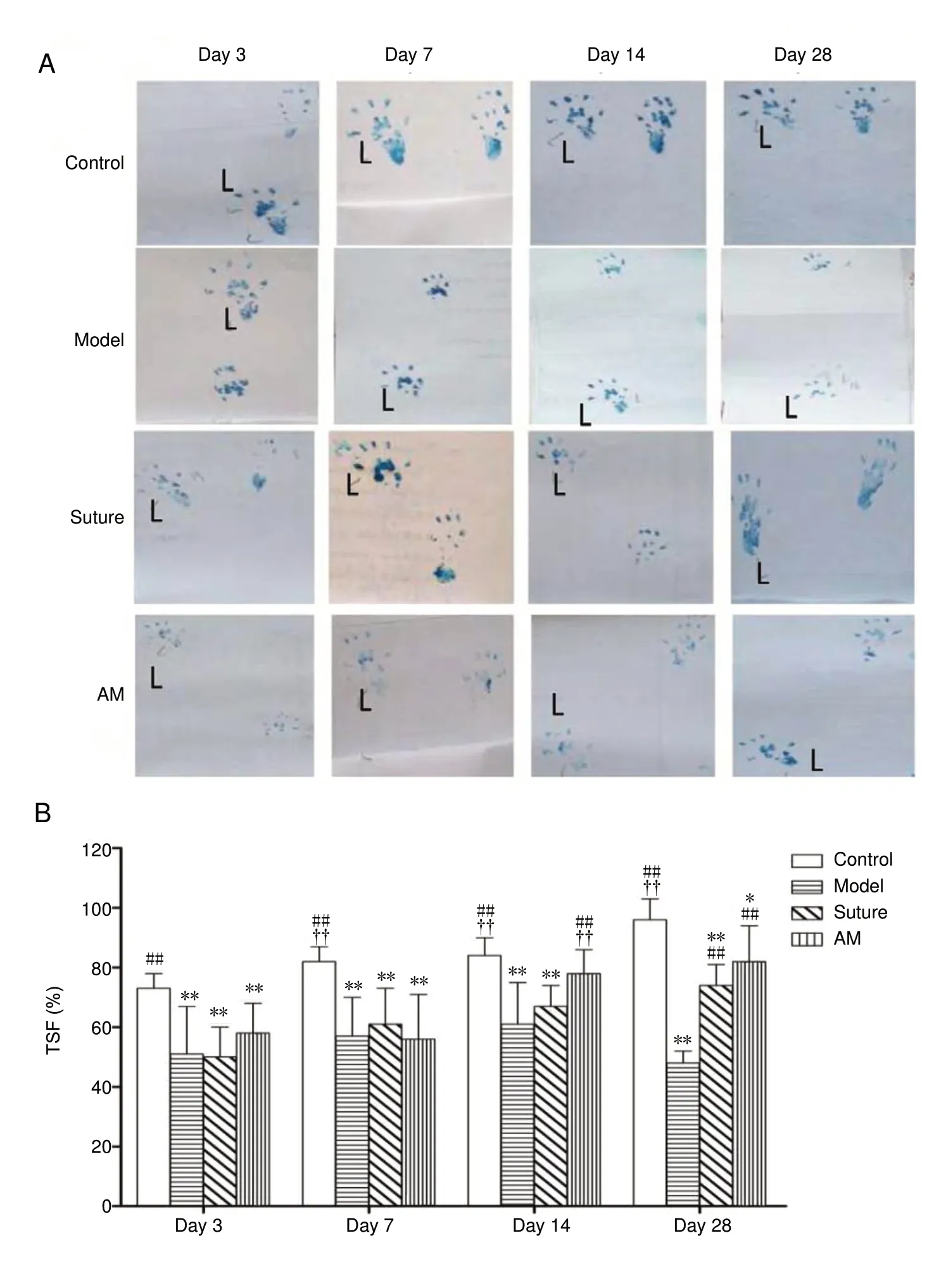
Figure 2 Effect of amniotic membrane on toe spread function changes in rats at 3,7, 14, and 28 days after common peroneal nerve injury.(A) Rat double hind limb marks; left (L) was normal side. At 7, 14, and 28 days after the operation, the right toe spread of the suture and AM groups were wider than those of the model group. The toe spread of the AM group had recovered better than that of the suture group at 14 days. (B) Quantitative evaluation of TSF. Data were expressed as the mean ± SD (n = 8) and analyzed using one-way analysis of variance followed by the least significant difference test. *P < 0.05,**P < 0.01, vs. control group; ##P < 0.01, vs.model group; ††P < 0.01, vs. suture group.AM: Amniotic membrane; TSF: toe spread function.
AM wrap enhance tibialis anterior muscle recovery in rats with a completely severed common peroneal nerve
Visual inspection revealed a more obvious myophagism in the surgical tibialis anterior of the model rats compared with control, AM, and suture rats, including myophagism in the normal lateral tibial anterior muscle. The tibialis anterior muscle recovery rate was slower in the model group than that in the control, suture, and AM groups. There was no difference between the AM and control groups (P < 0.01;Figure 6).
Histological examination of the right tibialis anterior muscle showed serious atrophy with broken and dissolved muscle fibers and a lot of inflammatory cell infiltration in the model group, while this was not the case in the control and AM groups. The aligned muscle fiber was regular in size and shape in the AM group and control group, and the sarcomere was complete without degeneration or necrosis.In the suture group, a few fibers showed muscle atrophy and rupture, with little infiltration of inflammatory cells(Figure 6C).
AM wrap regulates expression of axonal regenerationrelated genes in the spinal cord of rats with a completely severed common peroneal nerve
The quantitative PCR results showed that the expression of NGF, GAP-43, collapsin response mediator protein-2(CRMP-2), BDNF, and Lingo-1 mRNA was significantly higher in the spinal cord tissue of the model group compared with the control group, but GDNF was lower. The mRNA expression levels of NGF, GAP-43, CRMP-2, BDNF,and Lingo-1 genes were significantly lower in the AM group compared with the model group, while only the mRNA expression of CRMP-2 and BDNF genes were lower in the sutured group compared with the model group (P < 0.05 and P < 0.01, respectively; Figure 7). The NGF, GAP-43, and Lingo-1 mRNA expression levels were not significantly different between the AM and suture groups. The mRNA expression levels of BDNF and CRMP-2 were lower in the AM group compared with the suture group, and returned to control levels on the 28thday after the operation (P < 0.01; Figure 7).GDNF mRNA expression levels were not different among the control, sutured, and AM groups.
Discussion
In this study, an AM wrap applied to an end-sutured common peroneal nerve led to obvious improvements in behavior, histopathology, and nerve function compared with the model and sutured rats. Suturing is important in the recognition of impaired neural structure and functional recovery(Klein, 2010; Yaniv et al., 2012; Godinho et al., 2013). These results indicate that fresh human AM wrapping can significantly accelerate axon and Schwann tube regeneration after timely suture of the epineurium. On the 14thday after AM treatment, the AM group showed a restored TSF. At the 28thday, the TSF and nerve conduction velocity were comparable to those of the control group. Limping was reduced and the TS increased in the AM wrapping group on the 14thday.The nerve conduction velocity in the AM and suture groups was significantly faster than that in the model group, and the muscle recovery rate of the right tibialis anterior muscle also improved with no obvious muscle atrophy. In the lumbosacral spinal cord, we observed significant recovery in retrograde axoplasm transport in the AM and suture groups.However, there was a little muscle atrophy and slight rupture of muscle fibers in the suture group at 28 days. End-sutured common peroneal nerve function recovered more quickly in the AM group compared with the suture group. Previous studies have reported that AM can secrete a variety of repair promoting cytokines and anti-inflammatory factors. Thus,we speculate that the effect of the fresh AM wrap treatment may be related to the action of the AM in reducing inflammation and secreting a variety of neurotrophic growth factors, such that the AM provides a suitable micro-environment for axonal regeneration. After rupture of the common peroneal nerve, the model group exhibited a right limb limp,narrow footprints, and small TS. TSF was reduced and nerve conduction velocity was slower on the injured side in the model group compared with that in the other three groups.Further, the right tibial anterior muscle showed significantly amyotrophy, indicating that the tibialis anterior muscle recovery rate was slower in the model group compared with the other three groups. The retrograde axoplasm transport disappeared from commen peroneal nerve to the spinal cord. The results for the model rat group are consistent with previous literature (Fairbairn et al., 2014; Deng et al., 2017).Given the above, our data indicate that the AM wrap could be used for clinical treatment of common peroneal nerve injury for restoring neurological function earlier, thus promoting nerve injury repair and alleviating adhesion. However,whether the AM wrap or AM wrap compounded with mesenchymal stem cells or nerve cells for forming neural tubes produce similar results for longer peripheral nerve trunk defects remains to be studied.
It is well known that the AM can reduce adhesion of the surrounding tissues and scar formation in the treatment of tissue lesions in the skin, cornea, or sclera (Kim et al., 2010).Consistent with the literature (Fairbairn et al., 2016), layers 1-6 of the AM had different rates of degradation and absorption in the preliminary experiment. Therefore, we chose to use two layers of fresh AM wrapped around the damaged common peroneal nerve. At 28 days, the AM had been degraded and absorbed. The surrounding tissue adhesion of the repaired common peroneal nerve was significantly lighter in the AM group. Our experiment supports previous findings that AM has a good biocompatibility and low immunogenicity (Fotopoulou et al., 2010; Mamede et al., 2012). This may be associated with the secretory functions of many of growth factors and the anti-inflammatory effects of the AM or AM-derived stem cells (Kang et al., 2012; Mamede et al., 2012).
TSF, nerve conduction velocity, and muscle recovery rate all are important indexes to estimate the transmission of excitation in recovered motor nerves from the center to the effector. The incoming functions of nerve impulses are related to nerve continuity, while FB retrograde fluorescent tracers of spinal cord primarily activity reflect reverse axoplaic transport (Gu et al., 2015). There were more fluorescent particles in the lumbosacral spinal cord of the control group than in that of the AM-treated and sutured rats. There were more fluorescent particles in the AM wrapping rats than in sutured rats. This result indicates that the non-motor functions of the nerve in the AM-treated group underwent a greater degree of recovery compared with that in the suture and model groups, such as sensory nerve function from peripheral nerve impulse into the central nervous system.
Neurokines, which include nutritional and inhibitory factors, are produced in the body of neurons and surrounding cells in the spinal cord during axonal regeneration. NGF is widely distributed in both nerve tissue and non-nerve tissue,and participates in the regulation of neuron development and differentiation in the central nervous system. As a messenger molecule involved in synaptic function regulation,NGF can protect spinal anterior horn motor neurons and support neuron survival. It may promote axonal regeneration and myelination in the peripheral nervous system. After drug treatment for peripheral nerve injury, the expression of NGF in the spinal cord was lower than that in the model group. Lower NGF expression decreases the risk of pseudoneuroma formation and alleviates pain (Schmelz et al.,2019). BDNF not only stimulates the reaction of neurons to environmental changes elicited by glial cells, but also has neuroprotective effects. In the peripheral nervous system,BDNF promotes axon regeneration for proximal shaft bud growth to the end distal to the injury. BDNF facilitates the migration of Schwann cells into the lesions and the secretion of BDNF. When ultrashort waves combine with bone marrow mesenchymal stem cells to treat peripheral nerve injury, BDNF mRNA expression in the spinal cord and muscle increases in early in the repair (metaphase) (Godinho et al., 2013). GAP-43 mainly exists in the axis bud terminals of regenerative peripheral nerves. The GAP-43 protein plays an important role in axonal regeneration, axon orientation, and neuronal growth and development (Carriel et al.,2017). The change of GAP-43 gene expression may indicate impairment and repair of neurological function at different times. CRMP-2 is strongly expressed during differentiation of axis bud terminals, dendrites, and neurons. CRMP-2 can promote microtubule protein assembly and participates in the reconstruction of skeletal proteins; thus, the microtubule protein (α-tubulin and β-tubulin) can be transported to the axon growth cone to promote neuronal polarity and axonal extension (Rozés Salvador et al., 2016). We found that the expressions of NGF, GAP-43, CRMP-2, and BDNF mRNA were significantly higher in the spinal cord of the model group compared with those in the control group. These results may be indicative of important molecular changes or triggers for axonal regeneration that are involved in the slow self-repair process that occurs following common peroneal nerve injury, including pseudoneuroma formation in the model rats. Compared with the model group, BDNF and CRMP-2 mRNA expression were significantly lower in the AM group at 28 days after nerve injury, and although the levels were restored to pre-treatment values, they were below those of the suture group. NGF and GAP-43 expression also were reduced by AM treatment, although the levels were higher than in the normal group. These results indicate that regulation of BDNF, CRMP-2, NGF, and GAP-43 had a high concordance with the improvement of function or the degree of decrease following common peroneal nerve injury. This further supports the idea that AM wrapping is advantageous in terms of the regulation of neurotrophic factors, as indicated by the differences in the AM group compared with the suture group during regeneration and reparation. These results further demonstrate that the therapeutic effects of AM treatment were better than those of the suture treatment.
Lingo-1 is a transmembrane signaling protein that expressed in neurons and astrocytes. It is not expressed in the non-nervous tissue. Lingo-1 is most abundant in the cerebral cortex, and is scarce in the spinal cord. The function of Lingo-1 is the negative regulation of myelination, and inhibition of Lingo-1 expression can promote neural functional recovery and nerve axon growth (Shao et al., 2017). BDNF can reduce the inhibition of axonal growth by decreasing Lingo-1 levels. Although BDNF mRNA had already been restored to normal levels at 28 days after treatment, Lingo-1 mRNA was higher in the AM and suture groups than in the control group, but was lower in the AM group compared with that in the model and suture groups. The downregulation of Lingo mRNA expression may be one of the important mechanisms for the recovery of nervous function in the AM group. GDNF offers neuronal protection and enhances damaged nerve reparation in brachial plexus nerve injury, and is synthesized in neurons, target tissues, and glial cells. When peripheral nerves are injured, GDNF expression gradually decreases due to GDNF mRNA transport loss from the target tissue to the spinal cord. The level of GDNF was only decreased in the model rats, with no visible changes in the other groups. This may be related to disrupted reverse transport from the target tissue to the spinal cord caused by indirectly affecting interactions between neurons and glial cells
To conclude, this study demonstrated that a fresh human AM wrap with end-suture treatment resulted in enhanced recovery following common peroneal nerve injury and that AM improved neurologic function as well as neurophysiological and histological outcomes. The efficacy of AM wrapping is superior to that of single suture, and recovery time is shorter. Although the precise mechanism of the action of AM in axon regeneration is still unclear, we speculate that amniotic secretory functions or regulation of nerve growth and inhibitory factors play an important role. Future research should focus on how amniotic secretion participates in axon regeneration. Although we did not investigate the effects of AM wrapping on axon and Schwann cell regeneration or the dynamic changes in common peroneal nerves during repair, further investigation including these aspects could have great clinical significance. AM wrapping or tissue engineering techniques to construct AM neural tubes could be used in the regeneration and repair of large segments of damaged peripheral nerve.
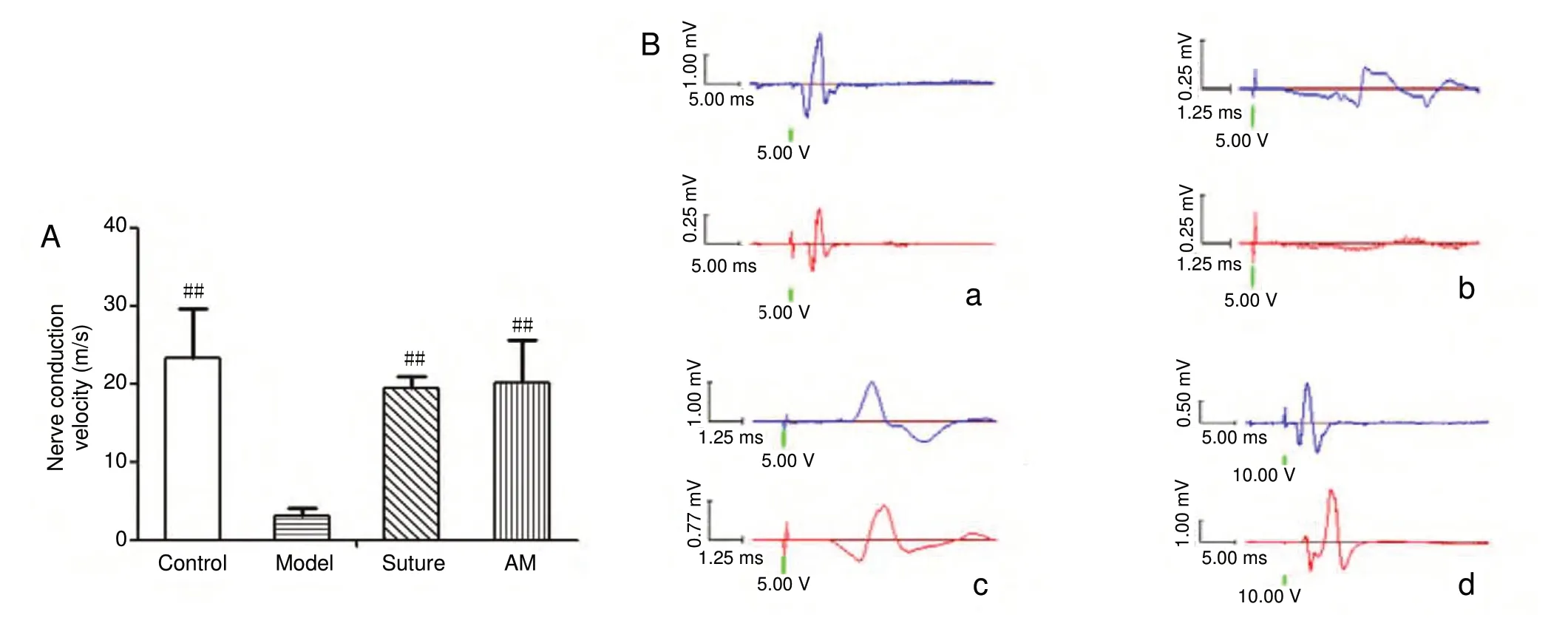
Figure 3 Effect of AM wrapping on the electrophysiology recovery in the damaged common peroneal nerve.(A) Nerve conduction velocity. Data were expressed as the mean ± SD (n = 6) and analyzed using one-way analysis of variance followed by the least significant difference test. ##P < 0.01, vs. model group. (B) Nerve voltage amplitude was examined for 4 weeks after nerve injury. (a-d) Control (a),model (b), suture (c), and AM (d) groups. Upward and downward peaks respectively indicate the two electrodes that received electrical signals at different times after triggering the action potential. The nerve conduction velocity in the suture and AM groups were lower than that of the control group, with no significant difference between the former groups. AM: Amniotic membrane.
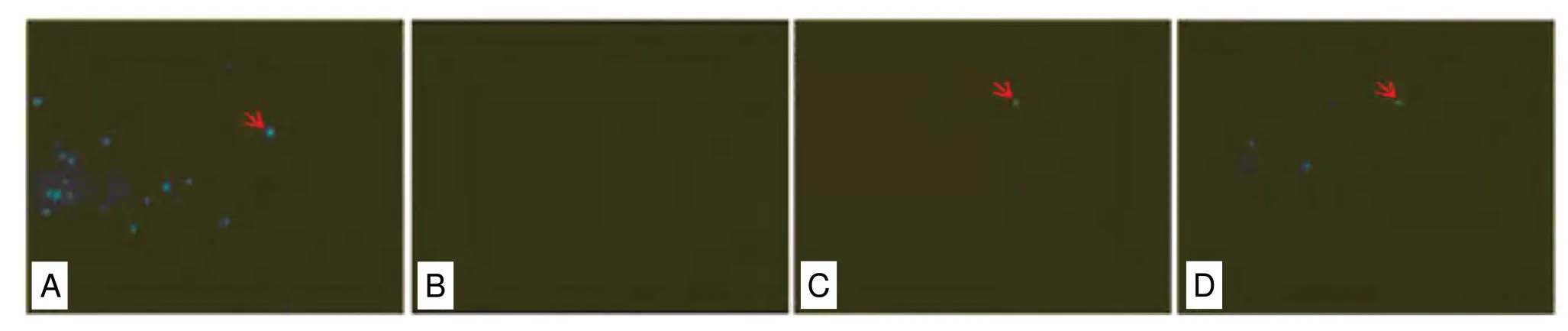
Figure 4 Effect of AM wrapping on the axoplasmic transportation in the sutured common peroneal nerve (fast blue BB salt retrograde fluorescent staining).Red arrows show blue fluorescence particles of fast blue BB salt. (A-D) Control (A), model (B), suture (C), and AM (D) groups. The number of blue fluorescence particles in the spinal cord was highest in control group, that of the AM group was higher than that of the suture group, and no blue fluorescence particles were observed in the model group. Original magnification, 200×. AM: Amniotic membrane.
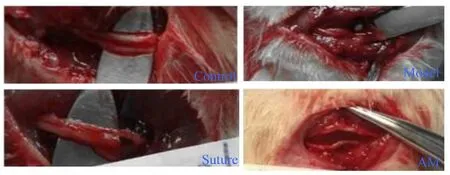
Figure 5 The perineural fibrosis and degree of adhesion in the damaged common peroneal nerve treated with AM.The perineural fibrosis and degree of adhesion were remarkably diminished in the repaired nerve with AM wrapping compared with the model group. The wrapped AM had been absorbed at 28 days after AM wrapping. AM: Amniotic membrane.
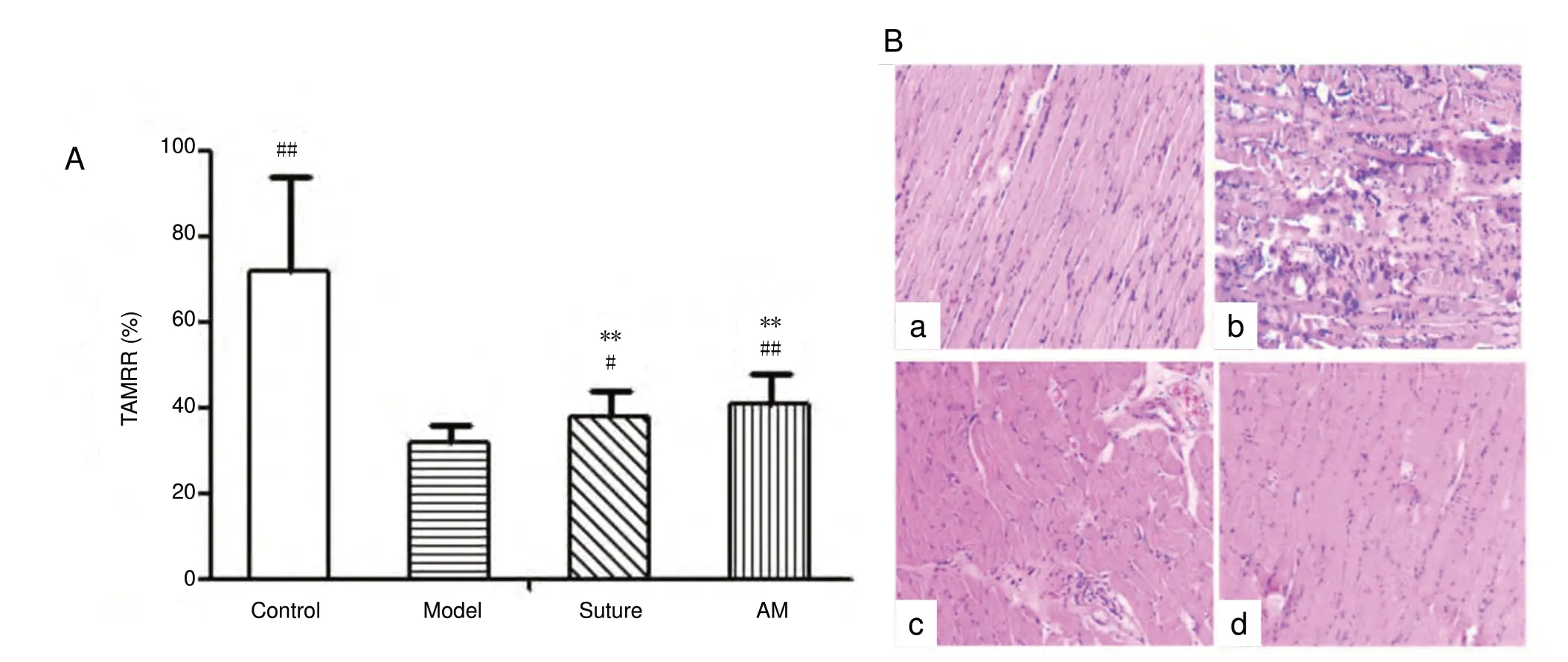
Figure 6 Effects of amniotic membrane wrapping on the muscle recovery rate (A) and the changes of histomorphology (B, hematoxylin-eosin staining) in the tibialis anterior.Data were expressed as the mean ± SD (n = 8) and analyzed using one-way analysis of variance followed by the least significant difference test. **P< 0.01, vs. control group; #P < 0.05, ##P< 0.01, vs. model group. (a-d) Control (a), model (b), suture (c), and AM (d) groups. Original magnification, 200×. TAMRR: Tibialis anterior muscle recovery rate; AM: amniotic membrane.
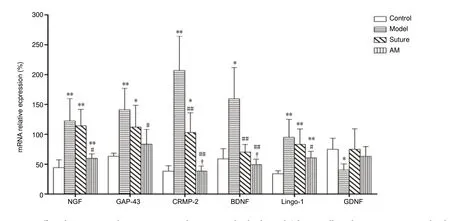
Figure 7 Effect of amniotic membrane wrapping on the expression levels of axonal, Schwann cells, and nerve regeneration-related genes detected in the spinal cord by real-time polymerase chain reaction.Data were expressed as the mean ± SD (n = 4) and analyzed using one-way analysis of variance followed by the least significant difference test. *P< 0.05, **P < 0.01, vs. control group; #P < 0.05, ##P < 0.01, vs. model group; †P < 0.05, vs. suture group. AM: Amniotic membrane; NGF: nerve growth factor; GAP-43: growth associated protein-43; CRMP-2: collapsin response mediator protein-2; BDNF: brain-derived neurotrophic factor;GDNF: glial cell line derived neurotrophic factor.
Acknowledgments:We would like to thank the personal staff in Department of Physiology, The Affiliated Hospital of Zunyi Medical University,China, who contributed electrophysiological detection for this study.
Author contributions:Study design: LMY, CYY; experimental implementation: ZYZ, JY, ZHF, YYW; manuscript writing: LMY, CYY, ZYZ,JY, ZHF, YYW; data acquisition and analysis: DLW, TZ. All authors approved the final version of the paper.
Conflicts of interest: The authors declare no conflicts of interest.
Financial support:This study was supported by Guizhou Province Major Special Projects in Science and Technology, China, No. Qin Ke He Zhong Da Zhuan Xiang Zi [2011]6002; and the Special Co-operation Funds of the Science and Technology Administration in Provinces and Cities,China, No. Sheng Shi He (2014) 59 (both to LMY). The funding bodies played no role in the study design, collection, analysis and in-terpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Institutional review board statement:The study was approved by the Committee on Animal Research and Ethics at the Affiliate Hospital of Zunyi Medical University, China (approval No. 112) on December 1, 2017.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-Share Alike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Michele R. Colonna, University of Messina, Italy.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- CNB-001 reduces paraplegia in rabbits following spinal cord ischemia
- Differential gene and protein expression between rat tibial nerve and common peroneal nerve during Wallerian degeneration
- Application of custom anatomy-based nerve conduits on rabbit sciatic nerve defects: in vitro and in vivo evaluations
- Axonotmesis-evoked plantar vasodilatation as a novel assessment of C-fiber afferent function after sciatic nerve injury in rats
- Relationship between high dietary fat intake and Parkinson's disease risk: a meta-analysis
- Optogenetics-induced activation of glutamate receptors improves memory function in mice with Alzheimer's disease