Axonotmesis-evoked plantar vasodilatation as a novel assessment of C-fiber afferent function after sciatic nerve injury in rats
Xue-Song Wang , Xue Chen , Tian-Wen Gu , Ya-Xian Wang Da-Guo Mi , Wen Hu ,
1 Department of Orthopedics, The Affiliated Hospital of Jiangnan University (The Third People's Hospital of Wuxi City), Wuxi, Jiangsu Province,China
2 Wuxi School of Medicine, Jiangnan University, Wuxi, Jiangsu Province, China
3 Key Laboratory for Neuroregeneration of Ministry of Education and Co-innovation Center for Neuroregeneration of Jiangsu Province, Nantong University, Nantong, Jiangsu Province, China
4 Department of Orthopedics, Nantong Hospital of Traditional Chinese Medicine, Nantong, Jiangsu Province, China
Abstract Quantitative assessment of the recovery of nerve function, especially sensory and autonomic nerve function, remains a challenge in the field of nerve regeneration research. We previously found that neural control of vasomotor activity could be potentially harnessed to evaluate nerve function. In the present study, five different models of left sciatic nerve injury in rats were established: nerve crush injury, nerve transection/suturing, nerve defect/autografting, nerve defect/conduit repair, and nerve defect/non-regeneration. Laser Doppler perfusion imaging was used to analyze blood perfusion of the hind feet. The toe pinch test and walking track analysis were used to assess sensory and motor functions of the rat hind limb, respectively. Transmission electron microscopy was used to observe the density of unmyelinated axons in the injured sciatic nerve. Our results showed that axonotmesis-evoked vasodilatation in the foot 6 months after nerve injury/repair recovered to normal levels in the nerve crush injury group and partially in the other three repair groups; whereas the nerve defect/non-regeneration group exhibited no recovery in vasodilatation. Furthermore, the recovery index of axonotmesis-evoked vasodilatation was positively correlated with toe pinch reflex scores and the density of unmyelinated nerve fibers in the regenerated nerve. As C-fiber afferents are predominantly responsible for dilatation of the superficial vasculature in the glabrous skin in rats, the present findings indicate that axonotmesis-evoked vasodilatation can be used as a novel way to assess C-afferent function recovery after peripheral nerve injury. This study was approved by the Ethics Committee for Laboratory Animals of Nantong University of China (approval No. 20130410-006) on April 10, 2013.
Key Words: nerve regeneration; axonotmesis-evoked vasodilatation; laser Doppler perfusion imaging; nerve function; autonomic nerve; C-fiber afferent function; peripheral nerve injury; unmyelinated afferent fiber regeneration; neural regeneration
Introduction
Comprehensive assessment of peripheral nerve function is important for the diagnosis and monitoring of treatment efficacy for peripheral neuropathy, as seen in diabetes, nerve trauma, autoimmune diseases, and adverse effects of chemotherapy and radiation therapy. A variety of assessments to evaluate motor and sensory nerve functions have been developed in experimental studies on traumatic nerve injury and other neuropathies (Hare et al., 1992; Geuna and Varejão, 2008; Griffin et al., 2010; Wood et al., 2011; Wang et al., 2015). However, approaches that allow quantitative assessment of autonomic and/or C-fiber afferent functions,such as sweating (Hilz, 2002; Fan et al., 2008) and pain sensation (Beltramini et al., 2017; Horgas, 2017), are limited and remain to be standardized for clinical use. For experimental studies involving rodents, the evaluation of autonomic and/or C-afferent functions is sweating-based and rarely conducted (Sato and Sato, 1978; Michniak-Mikolajczak,1984); the evaluation is especially challenging in the case of neurotmesis or loss of continuity of the nerve trunk, where misdirection of regenerating axons is unavoidable and functional recovery is generally incomplete (Allodi et al., 2012;Liu et al., 2018), since the target is unevenly re-innervated.In cases of incomplete and misdirected re-innervation or uneven small-diameter nerve fiber degeneration (Lundborg and Rosen, 2007; Hamilton et al., 2011; Singh et al., 2014), it is preferable to assess the autonomous skin region of a given nerve as a whole rather than focally, as the results might not be representative due to uneven re-innervation.
Recent studies by our group and others have suggested that neural control of vasomotor function in the skin could be used to evaluate nerve function recovery (Hu et al., 2012;Deng et al., 2016; Kubasch et al., 2017). Neural regulation of cutaneous blood perfusion is orchestrated by postganglionic sympathetic efferents and C-fiber afferents, with the former likely to be mainly responsible for vasoconstriction and the latter for vasodilatation (Habler et al., 1998; Charkoudian,2003). One previous study (Hu et al., 2012) suggested that cutaneous vasculature in the plantar aspect of the hind foot was markedly dilated immediately after immersion of the hind foot in icy water, and this response was compromised when the sciatic nerve innervating that foot was injured;however, the foot with a permanently defected sciatic nerve,where no sciatic nerve function was expected, still exhibited an increase in blood perfusion after cold stress, despite a significantly lower level than observed in the foot with a repaired sciatic nerve injury. This limitation diminishes the value of post-cold vasodilatation in the foot for assessing nerve repair outcomes. Therefore, establishing a neurovascular response-based measurement that can facilitate better discrimination between different nerve repair groups remains a challenge. In the present study, we investigated whether axonotmesis (or nerve crush)-evoked vasodilatation (AEVD)quantitatively reflects the recovery of C-afferent nerve function after different types of nerve injury/repair in rats.
Materials and Methods
Animals
Sixty-two adult female Sprague-Dawley rats aged 3-4 months were used. The rats were bred in a specific-pathogen-free facility in the Laboratory Animal Research Center of Nantong University, China (breeding license No. SCXK(Su) 2008-0010 and SCXK (Su) 2014-0001; animal use license No. SCXK (Su) 2012-0031). The animals were housed under a 12-hour light/dark schedule with free access to food and water. All animal procedures were carried out under the approval of the Ethics Committee for Laboratory Animals of Nantong University, China (approval No. 20130410-006) on April 10, 2013 and in accordance with US National Institutes of Health Guide for the Care and Use of Laboratory Animals published by the US National Academy of Sciences.
Nerve injury and immediate repair
A first cohort of naive rats was subjected to different injuries to the left sciatic nerve and immediate repair, and was sacrificed after a 6-month recovery period. Fifty rats, randomized into 5 groups (10 rats each), were anesthetized with intraperitoneal injection of 3% sodium pentobarbital (Sigma-Aldrich,St. Louis, MO, USA; 30 mg/kg body weight). The left sciatic nerve was exposed under aseptic condition and subjected to one of the five categories of injury/repair described below. These injury models have been well established and employed in our previous studies (Hu et al., 2012; Wang et al., 2015; Liu et al., 2018), and were as follows: (1) For nerve crush injury, the left sciatic nerve was completely crushed with a pair of 14-cm hemostatic forceps for 30 seconds at the mid-thigh level, and the site of crush was labeled with an 11/0 nylon suture placed in the epineurium. (2) For nerve transection/suturing, the left sciatic nerve was transversely cut with a pair of surgical scissors at the mid-thigh level, and the two nerve stumps were immediately anastomosed by placing three stitches of 11/0 nylon sutures in the epineurium. (3) For nerve defect/autografting repair, a 10 mm-long segment of the sciatic nerve was excised from 4 mm caudal to the piriformis muscle, and the excised nerve segment was immediately interpositioned to repair the nerve defect by the epineurial suturing described above. (4) For nerve defect/conduit repair, the sciatic nerve was subjected to the same injury as in nerve defect/autografting except that the 10 mmlong nerve defect was repaired with a 12 mm-long sterile silicone conduit, 1 mm in inner diameter and 0.2 mm in wall thickness, with 1 mm each of nerve stumps inserted into the conduit and the epineuria secured to the conduit wall with 9/0 nylon sutures. (5) For nerve defect/no regeneration, a 10 mm-long segment of the sciatic nerve was excised and the defect was left unrepaired, with the proximal nerve stump inserted in and secured to the adjacent intermuscular space so as to prevent axonal regeneration into the distal nerve stump. The incision was closed in layers with 4/0 silk sutures.Rats were allowed to completely recover on a soft heating pad before being returned to their home cages.
The rats were subjected to the toe pinch test, walking track analysis, and laser Doppler perfusion imaging (LDPI) analysis, and were sacrificed 6 months after nerve injury/repair.
The toe pinch test
The toe pinch test was performed at both 2 days and 6 months after nerve injury/repair using a modified version of previously described protocol (Kovacic et al., 2004; Ma et al., 2011). Briefly, the awake rat was gently restrained and the volar aspect of the fifth toe was gently pinched with a pair of eye dressing forceps. The pain perception response to pinch was scored based on the extent of hind limb withdrawal using a three-tier scoring paradigm, as follows: 0: no response;1: decreased response compared with normal; 2: strong and prompt withdrawal of the hind limb that is indistinguishable from the response of the counterpart on the contralateral normal side. The assessment was repeated three times and the highest score was selected to represent the response level. The naive fifth toe on the contralateral side was also assessed as a reference. Three of ten rats with nerve regeneration-preventing defect lost their fifth toe on the injured side 6 months after injury, and the fourth toe was therefore assessed instead.
Walking track analysis
Rats were subjected to walking track analysis to evaluate sensorimotor function at 2 months and 6 months after nerve injury/repair using well-established protocol (Hare et al.,1992). Briefly, the plantar aspects of both hind feet were painted with non-toxic red ink, and rats were allowed to walk and pass a 42 cm × 8.2 cm track, leaving foot prints on the paper. Three parameters, namely print length (PL), toe spread (TS), and intermediate toe spread (IT), were measured from both experimental (E) and contralateral normal(N) sides. The sciatic function index (SFI) was calculated using the following formula:
SFI = -38.3 × [(EPL-NPL)/NPL] + 109.5 × [(ETS-NTS)/NTS] + 13.3 × [(EIT-NIT)/NIT] - 8.8.
An SFI value of -100 represents complete loss of sciatic nerve function, and 0 represents normal nerve function. In the case of chronic flexion contracture or loss of toes that made measurement of any of the parameters impossible, the rat was excluded from analysis.
Bilateral nerve crush for blood perfusion analysis
To study whether both hind feet are equally responsive to AEVD and to determine the optimal time frame for LDPI analysis after bilateral nerve crush, a second cohort of naive rats (n = 6) was subjected to surgery. The bilateral sciatic nerves were crushed using the same surgical protocol described above; the right sciatic nerve was crushed immediately (~5 minutes delay) after crushing of the left one (Figure 1). LDPI analysis of the hind feet was performed prior to surgery (baseline perfusion) and 10, 20, and 40 minutes after completion of the surgery, and the rats were sacrificed thereafter.
To validate complete disruption of axons in the sciatic nerve by the crush procedure described above, we included a third cohort of rats (n = 6). In these animals, the right sciatic nerve was transected using surgical scissors as an internal control to nerve crush injury to the left sciatic nerve (Figure 1E). LDPI analysis was performed 10 minutes after the crush/transection injury and rats were sacrificed immediately after the analysis.
Six months after nerve injury/repair and one day after the toe pinch test, rats of the first cohort were first subjected to LDPI analysis of the hind feet and then bilateral sciatic nerve crush (assessment crush) followed by LDPI analysis.Rats were anesthetized as described above. After the baseline perfusion of the hind feet had been measured, the leftsciatic nerve was re-exposed and completely crushed at 2 mm proximal to the previous injury/repair site with a pair of 14-cm hemostatic forceps for 30 seconds. The right sciatic nerve was also completely crushed in the same way immediately after crushing of the left one. The skin incisions were closed and both hind feet were subjected to LDPI analysis as described below at 10 minutes after bilateral nerve crush.
LDPI analysis
Blood perfusion of the hind feet was measured by LDPI using the PeriScan PIM 3 system (Perimed AB, Järfälla, Sweden) as previously described (Hu et al., 2012, 2013). Briefly,rats acclimatized to the test room, 23°C and 60% humidity,for at least 30 minutes before LDPI analysis. Rats were anesthetized as described above and placed in a prone position on a soft green pad, leaving the plantar aspect of both hind feet exposed to the laser beam overhead. The plantar aspect of the hind feet was then scanned twice with the repeated scan mode at a distance of ~18 cm. The arbitrary perfusion units measured from the two scanned images for each foot were averaged. In some cases, the relative perfusion level was calculated as the ratio of the injured side over the contralateral normal side. For the first cohort of rats in which LDPI analysis was performed to assess functional recovery 6 months after nerve injury/repair, a relative perfusion increase (or recovery ratio) was calculated using the following formula: Recovery index = (IUa- IUb) / (CUa- CUb) × 100%,where I and C stand for ipsilateral and contralateral sides of the previous injury/repair, respectively, U stands for arbitrary perfusion units, and a and b for measures after and before the bilateral nerve crush, respectively.
Electron microscopy
Three randomly selected rats from each group in the first cohort, which had received nerve injury/repair 6 months before, were transcardially perfused with saline followed by 1% paraformaldehyde plus 1.25% glutaraldehyde in 0.1 M phosphate buffer. A 2 mm-long segment of the sciatic nerve 2 mm distal to the previous injury/repair was excised and immediately soaked in 4% glutaraldehyde overnight at 4°C.The nerve specimens were post-fixed with 1% osmium tetroxide, dehydrated in ethanol, and embedded in Epon 812 epoxy resin. Ultrathin transverse sections of the nerve cut on a microtome were stained with lead citrate and uranyl acetate. Photomicrographs of 5-6 randomly selected visual fields at 3000× magnification were taken on a JEM-1230 transmission electron microscope (JEOL Ltd., Tokyo, Japan).Unmyelinated axons were counted and their density for each group was estimated.
Statistical analysis
All quantitative data were analyzed with GraphPad Prism 5.0 software package (GraphPad Software, San Diego, CA,USA). Between-group differences were compared with paired t-tests, repeated measures two-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test, oneway ANOVA followed by Tukey's multiple comparisons or non-linear regression or one-sample t test, as detailed in legends to figures. Non-parametric Spearman correlation was employed. For non-linear regression, the Michaelis-Menten model was chosen for best fit of the data. P < 0.05 was considered statistically significant.
Results
There are similar AEVD levels in both hind feet
Bilateral sciatic nerve crush and LDPI analysis of the hind feet in naive rats were performed to test whether the two feet show equal levels of AEVD (Figure 1A and B). As expected,no significant difference (F = 0.3271, P = 0.5800, repeated measures two-way ANOVA) in the increase of blood perfusion in the two feet was detected 10-45 minutes after bilateral nerve crush, despite a 5-minute delay for the injury to the right sciatic nerve (Figure 1C and D). To validate that the crush procedure we employed resulted in complete axonal disruption in the sciatic nerve, the left sciatic nerve was crushed and the right one was transected in an additional cohort of rats (Figure 1E). We detected no statistically significant difference (t = 0.51861, P = 0.6262, paired t-test) in axonal damage-evoked perfusion increase between foot with nerve crush and that with nerve transection (Figure 1F and G), which was indicative of complete disruption of axons in the sciatic nerve by the crush procedure. Collectively, these data indicate that AEVD in one hind foot can be used as an internal control for the other.
Varying levels of AEVD in rats with different types of nerve injury/repair
To test the ability of AEVD to reflect nerve function recovery after injury, we performed five different types of nerve injury/repair that are known to exhibit varying levels of regeneration and functional recovery. These injury/repair methods were applied to the left sciatic nerve in adult rats. At 2 days and 6 months after nerve injury/repair, the recovery of sensory function and sensorimotor function was evaluated using the toe pinch reflex and walking track analysis (Figure 2A). The baseline blood perfusion was detected in the hind feet (Figure 2A and B). LDPI analysis showed a similar level of baseline perfusion in the foot with a previous nerve injury/repair and the contralateral normal foot (F = 0.3271, P =0.9494, one-way ANOVA) regardless of the type of injury/repair (Figure 2D and E).
We next performed complete crush injury (assessment crush/axonotmesis) of both the left and the right sciatic nerves in anesthetized rats and detected AEVD in the hind feet (Figure 2A, and C). We found that blood perfusion in the left foot with a previous nerve injury/repair increased in response to acute axonotmesis, and the levels of response in varying nerve injury/repair groups were different (F = 142.1,P < 0.0001, one-way ANOVA; Figure 2D and F). For instance, the axonotmesis-evoked increase of blood perfusion in the left foot that had received a crush injury 6 months before was not significantly different to that of the contralateral side (t = 0.09817, P = 0.9240 compared with a theoretical mean of 100, one-sample t test), which served as an internal control; the foot with a previous sciatic nerve defect (no regeneration) did not show significant AEVD (t = 0.7473, P =0.4739 compared with a theoretical mean of 0, one-sample t-test) when the proximal sciatic nerve was crushed (Figure 2D and F). However, the left feet with previous nerve transection/suturing, nerve defect/autografting, or nerve defect/conduit repair showed mean axonotmesis-evoked increases of blood perfusion as normalized with that on the contralateral normal side (recovery index) of 63.3-75.9%; the nerve transection/suturing group showed a significantly greater recovery index of AEVD compared with the nerve defect/conduit repair group (q = 4.130, P < 0.05, Tukey's multiple comparison test; Figure 2D and F).
The AEVD recovery index is positively correlated with sensory function
We detected complete loss of the pinch reflex in the fifth toe and complete loss of sciatic nerve function in rats of all groups 2 days after nerve injury/repair, as evidenced by the toe pinch test and walking track analysis, respectively (Figure 3A and 3B). These results further confirmed that there had been a complete disruption of axons in the sciatic nerve by the crush procedure.
When rats were subjected to toe pinch test 6 months after nerve injury/repair, those in the nerve crush and nerve defect/no regeneration groups showed normal sensibility and no sensation in the toe, respectively (Figure 3A). No significant difference was observed in toe pinch scores between nerve suturing, nerve autografting, and conduit repair groups (P = 0.4706; Figure 3A). However, the AEVD recovery index showed strong positive correlation with toe pinch scores when all five groups were included (P < 0.0001,Spearman correlation; R2= 0.9330, Michaelis-Menten's curve fit; Figure 3C). We also performed walking track analysis 6 months after nerve injury/repair, but calculation of SFI was impossible due to inevitable chronic flexion contracture of the paw in all groups other than crush and defect/no regeneration; complete recovery of SFI (t = 1.143, P = 0.2827 compared a theoretical mean of 0, one-sample t-test) and loss of function (t = 0.1573, P = 0.8785 compared with a theoretical mean of -100, one-sample t-test) were observed in the crush and defect/no regeneration groups, respectively (Figure 3B).
The AEVD recovery index is positively correlated with the density of unmyelinated nerve fibers
Transmission electron microscopy of the nerve distal to injury was performed to identify the association between AEVD and regenerated unmyelinated nerve fibers. Transmission electron microscopy showed numerous unmyelinated nerve fibers, in addition to myelinated axons with a thinner myelin sheath than the normal control nerve, in all groups except for the nerve defect/no regeneration group (Figure 4A).Although the difference in the estimated density of unmyelinated nerve fibers between repair groups did not reach significance (F = 0.4583, P = 0.7649, one-way ANOVA;Figure 4B), there was a strong positive correlation between the AEVD recovery index and the density of unmyelinated nerve fibers (P = 0.0006, Spearman correlation; R2= 0.8714,Michaelis-Menten's curve fit; Figure 4C). These data indicated that the AEVD level was well correlated to nerve function recovery and nerve regeneration.
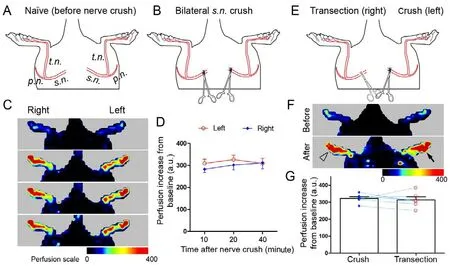
Figure 1 Crush injury to bilateral sciatic nerves results in a similar extent of vasodilatation in the hind feet.(A, B) The site of sciatic nerve axonotmesis (crush). The left sciatic nerve was completely crushed, followed by the same injury to the right sciatic nerve 5 minutes later. The laser Doppler perfusion imaging analysis of both hind feet was performed at 10, 20, and 40 minutes after the crush of the right sciatic nerve. (C) Laser Doppler perfusion imaging images and (D) line chart of perfusion increase in both feet after axonotmesis. (E) The sites of axonotmesis (left side) and transection (right side) of the sciatic nerves. (F) Laser Doppler perfusion imaging images and (G) bar chart with paired scattered dots showing the vasodilatation response to axonotmesis and nerve transection. Data are expressed as the mean ± SEM [n = 6 rats for each surgical paradigm, repeated measures two-way analysis of variance followed by Bonferroni's post hoc tests (D) or paired t-tests (G)]. No significant difference in nerve injury-induced perfusion increase was found between the two feet. The filled arrow and hollow arrowhead indicate the side of nerve crush and nerve transection, respectively. s.n.: Sciatic nerve; t.n.: tibial nerve; p.n.: peroneal nerve.
Discussion
Quantitative assessment of nerve function recovery, especially that of sensory and autonomic nerve function, remains a challenge in the field of nerve regeneration (Varejao et al.,2001; Hilz, 2002; Griffin et al., 2010; Wood et al., 2011). The perception of nociceptive stimulation in the plantar skin has been widely employed in animal studies of pain (Chen et al., 2017; Zhao et al., 2017; Jiang et al., 2018). However, the assessment of pain sensibility is seldom included in nerve regeneration research (Kovacic et al., 2004; Ma et al., 2011),and nor is autonomic nerve function (McLean et al., 2002;Wang et al., 2005; Yang et al., 2007), possibly due to large variation from animal to animal as a result of incomplete and uneven axonal regeneration after nerve transection or defect. In the present study, the extent of AEVD recovery was strongly correlated with the toe pinch score and estimated density of unmyelinated nerve fibers. In addition, the AEVD recover index appeared more sensitive than the toe pinch test in differentiating functional recovery between different nerve injury/repair groups, as seen in the between-group comparisons of the AEVD recovery index. These results suggest that the AEVD recover index could represent a novel and reliable quantitative assessment of C-afferent function recovery in nerve regeneration research in rodents.
Cutaneous vasculature is dilated or constricted in response to local warming/cooling and during challenges to thermal homeostasis so that the core body temperature can be maintained; the modulation of dermal vasomotor activity involves neural and non-neural mechanisms (Charkoudian, 2003).Both C-fiber afferents and sympathetic nerve fibers are involved in neural vasomotor control in the skin (Hamilton et al., 2011; Singh et al., 2014). In the glabrous skin in the rat,C-fiber afferents are predominantly responsible for dilatation of the superficial dermal vasculature whereas sympathetic nerve fibers contribute to constriction of both deep and superficial blood vessels (Habler et al., 1998). Resection of L2-4 sympathetic paravertebral ganglia, which project with their gray rami to L3-5 spinal nerves, results in pronounced vasodilatation and thus an increase in local temperature by~5°C in the rat plantar skin (Ringkamp et al., 1999); this surgical sympathetomy-induced vasodilatation could be the consequence of a loss of sympathetic tone in the dermal vasculature (Charkoudian, 2003). However, irritation of C-fiber afferents by tropical application of capsaicin, a vanilloid receptor subtype 1 (TRPV1) agonist, results in vasodilatation, and repeated application of capsaicin abolishes the antidromic vasodilatation induced by electrical stimulation of the severed peripheral nerve (Izumi and Karita, 1990). These results suggest that AEVD observed in the present study could be the joint effect of a loss of sympathetic tone and the antidromic release of vasoactive neuropeptides from C-afferents, but might be mainly attributed to the latter, which deserves further investigation. Whatever the underlying mechanisms might be, the present results indicate that the AEVD recover index is strongly correlated with the recovery of pain sensation and regeneration of unmyelinated nerve fibers. In addition, unlike the toe pinch reflex and von Frey test, which are semi-quantitative, or the Hargreaves test for thermal sensibility, which might cause foot burn, the AEVD recovery index represents a quantitative assessment of C-fiber nerve function after nerve injury. As the whole plantar surface is involved in LDPI analysis, the AEVD recovery index may reflect nerve function of the entire autonomic territory,regardless of whether the skin area is evenly re-innervated or not.
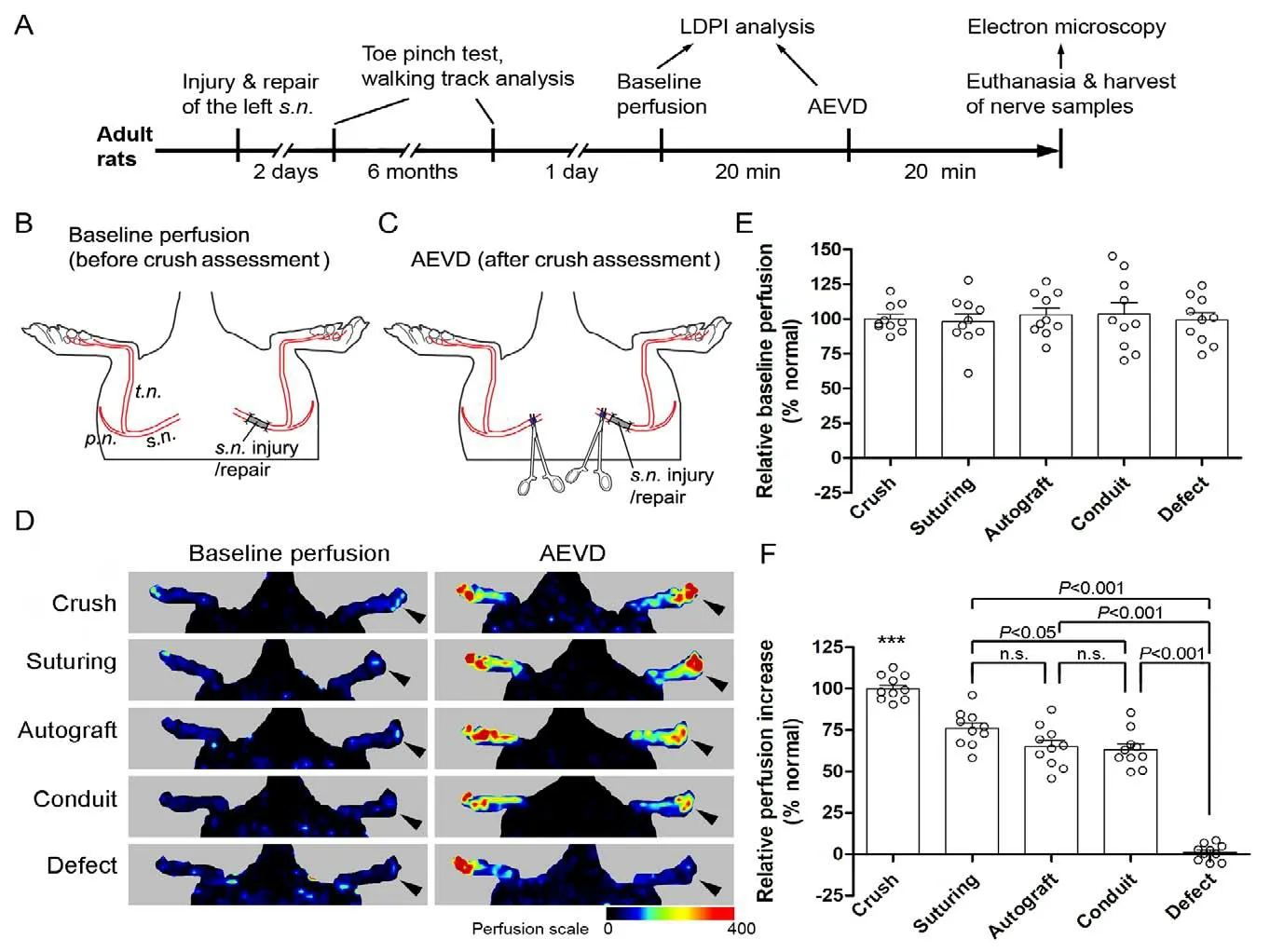
Figure 2 Recovery of AEVD differed between the different types of nerve injury/repair 6 months after injury.(A) Timeline (not proportional) of the experimental schedule. The left sciatic nerve was subjected to one of the following injury/repair protocols:(1) crush, (2) transection and direct epineurial suturing (suturing), (3) 10-mm defect repaired with autologous nerve grafting (autograft), (4) 10-mm defect repaired with a silicone conduit (conduit), and (5) 10-mm defect with a regeneration-preventing procedure (defect). Six months after nerve injury/repair, both hind feet were subjected to LDPI analysis to detect baseline blood perfusion, then the sciatic nerves of both sides were completely crushed proximal to the previous injury/repair site and LDPI analysis of both hind feet was performed within 20 minutes (min) after the bilateral nerve crush. (B, C) The site of the original nerve injury/repair and the bilateral nerve crush. (D) LDPI images of the hind feet before and after the bilateral nerve crush. Arrowheads indicate the left side, which was subjected to a previous nerve injury/repair. (E) Quantification of baseline perfusion. (F) Quantification of relative response (recovery index) of AEVD. Data are expressed as scattered dots plus the mean ± SEM (n = 10 rats/group; one-way analysis of variance followed by Tukey's post hoc test for multiple comparisons). ***P < 0.001, vs. any of the other groups. Each dot represents the data from one rat. AEVD: Axonotmesis-evoked vasodilatation; LDPI: laser Doppler perfusion imaging.
We recently found that transection of the rat sciatic nerve results in a robust increase in blood perfusion in the skin territory, namely the glabrous aspect of the foot, and that this neurogenic vasodilatation disappears within a few days(Hu et al., 2012; Shen et al., 2013). Both neural and humoral mechanisms are involved in vasomotion control and skin perfusion is sensitive to ambient temperature (Charkoudian,2003). The equal level of perfusion increase in both hind feet observed in the present study indicates that the contralateral side could serve as an internal control for assessing AEVD response in the foot after nerve injury/repair, as both hind feet are exposed to the same humoral influence and ambient temperature.
Sciatic nerve crush is known to be completely reversible in terms of nerve function (Wood et al., 2011), which was also evident from the walking track analysis in the present study.Six months after sciatic nerve crush injury in rats, the hind foot on the injured side showed an AEVD that was 90.5%to 112.8% (99.8 ± 7.0%) of that on the contralateral normal side. In contrast, rats in the nerve defect group (no regeneration) did not show a significant increase in blood perfusion in the foot following assessment nerve crush (1.1 ± 4.9% of the internal normal control). The AEVD response observed in the present study substantially differs from the post-cold vasodilatation employed previously (Hu et al., 2012) in that the nerve defect/no regeneration group showed a detectable response in post-cold vasodilatation. Furthermore, the AEVD recovery index could differentiate between the level of function recovery after nerve transection/suturing and that after repair of a 10-mm nerve defect with a silicone conduit; 10 mm is considered a critical defect length in the rat sciatic nerve, beyond which no suitable regeneration or functional recovery can be ensured by repairing with a non-degradable tubular implant (Fields et al., 1989; Chen et al., 2000; Gu et al., 2011). In this regard, AEVD appears to be a better assessment of nerve function than vasodilatation after cold stress (Hu et al., 2012).
We have recently shown that baseline cutaneous perfusion in the palm of humans largely depends on skin innervation;denervated skin is markedly hypoperfused, whereas the skin blood perfusion gradually recovers to normal levels during reinnervation (Deng et al., 2016). However, in the present study, we did not observe a markedly reduced baseline skin perfusion in the hind foot 6 months after denervation,which is consistent with our previous rat study (Hu et al.,2012). The discrepancy between findings from humans and rats could be attributed, at least in part, to higher baseline perfusion in the palm of the human as opposed to relatively low baseline perfusion in the plantar skin of hind feet in the rat. The relatively high baseline perfusion in human hands,especially in the finger pulps, seems to correspond to high sensitivity of the hand function as a sensory organ (Lundborg and Rosen, 2007). The distinct baseline perfusion and its dependence on innervation suggest that the cutaneous vasomotor control of different species can be remarkably different, which should be taken into account when considering the clinical implications and applications of data collected from animal studies.
One concern in the use of AEVD to evaluate nerve function is that the bilateral nerve crush might lead to pathophysiological alterations in the distal nerve, which may in turn compromise histological or immunohistochemical assessments of the nerve. However, the vasculature of the nerve remains intact after nerve crush, and the assessment crush and subsequent LDPI analysis can be completed within 30 minutes by a well-trained experimenter. Given that the peripheral nerve starts to show signs of Wallerian degeneration at the ultrastructural level a few hours after nerve transection injury in rodents (Cullen, 1988; Rotshenker, 2011), the AEVD assessment procedure proposed here will not significantly change the morphology of the nerve if the nerve sample is taken immediately after the procedure.In the present study, we tested the correlation between the AEVD recovery index and pain sensation and unmyelinated nerve fibers because C-afferents are predominantly involved in neurogenic vasodilatation in the superficial area of the glabrous skin (Habler et al., 1998).
A limitation of AEVD as an assessment of nerve function is that it is only suitable in animal studies, and cannot apply to clinical practice. However, in the clinic other vasomotion-based assessments, such as baseline cutaneous perfusion, cold-induced vasoconstriction and deep breathevoked vasoconstriction, could be utilized, as proposed in our previous study (Deng et al., 2016). Whether the AEVD recovery index is also correlated with other indicators of nerve regeneration and function recovery remains an unanswered question in the present study. In addition, further understanding of how AEVD response changes over time is another important issue to address in future studies using one or more injury/repair models.
In summary, the recovery index of AEVD was positively correlated with toe pinch reflex scores and estimated density of unmyelinated nerve fibers in the regenerated nerve. These findings indicate the potential value of AEVD recovery index in the evaluation of C-afferent function in nerve regeneration research.
Author contributions:Experimental implementation: XSW, XC, TWG,YXW; study concept, experimental implementation, data analysis: DGM;study concept, design, manuscript writing: WH. All authors approved the final version of the paper.
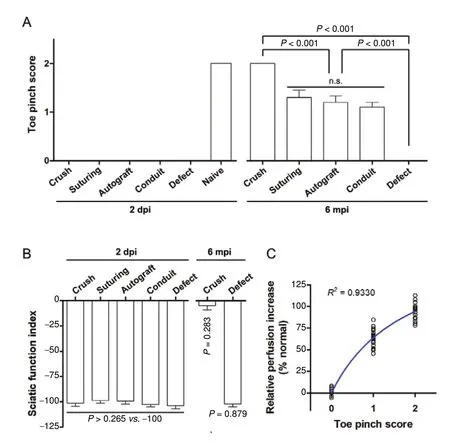
Figure 3 AEVD is correlated with sensory recovery 6 months after injury/repair.(A) The toe pinch test of sensory recovery in the plantar aspect of the fifth toe at 2 days and 6 months after injury/repair. (B) Walking track analysis of sciatic function index at 2 days and 6 months after injury/repair. The data for nerve suturing, nerve autograft, and conduit repair groups are not available due to chronic flexion contracture of the paw 6 months after injury/repair. (C) Non-linear regression between relative perfusion increase, or AEVD recovery index,and toe pinch scores. Data are expressed as the mean ± SEM(A and B, n = 7 for defect at 6 months after injury/repair in B, otherwise n = 10 rats/group) or scatter dots (C), and were analyzed using one-way analysis of variance followed by Tukey's multiple comparisons tests (A and left part of B),one sample t-tests for a theoretical mean of either 0 (crush)or -100 (defect) (right part of B), or Michaelis-Menten non-linear regression (C). Each dot in C represents the data from one rat. dpi: days post-injury; mpi: months post-injury. AEVD: Axonotmesis-evoked vasodilatation.
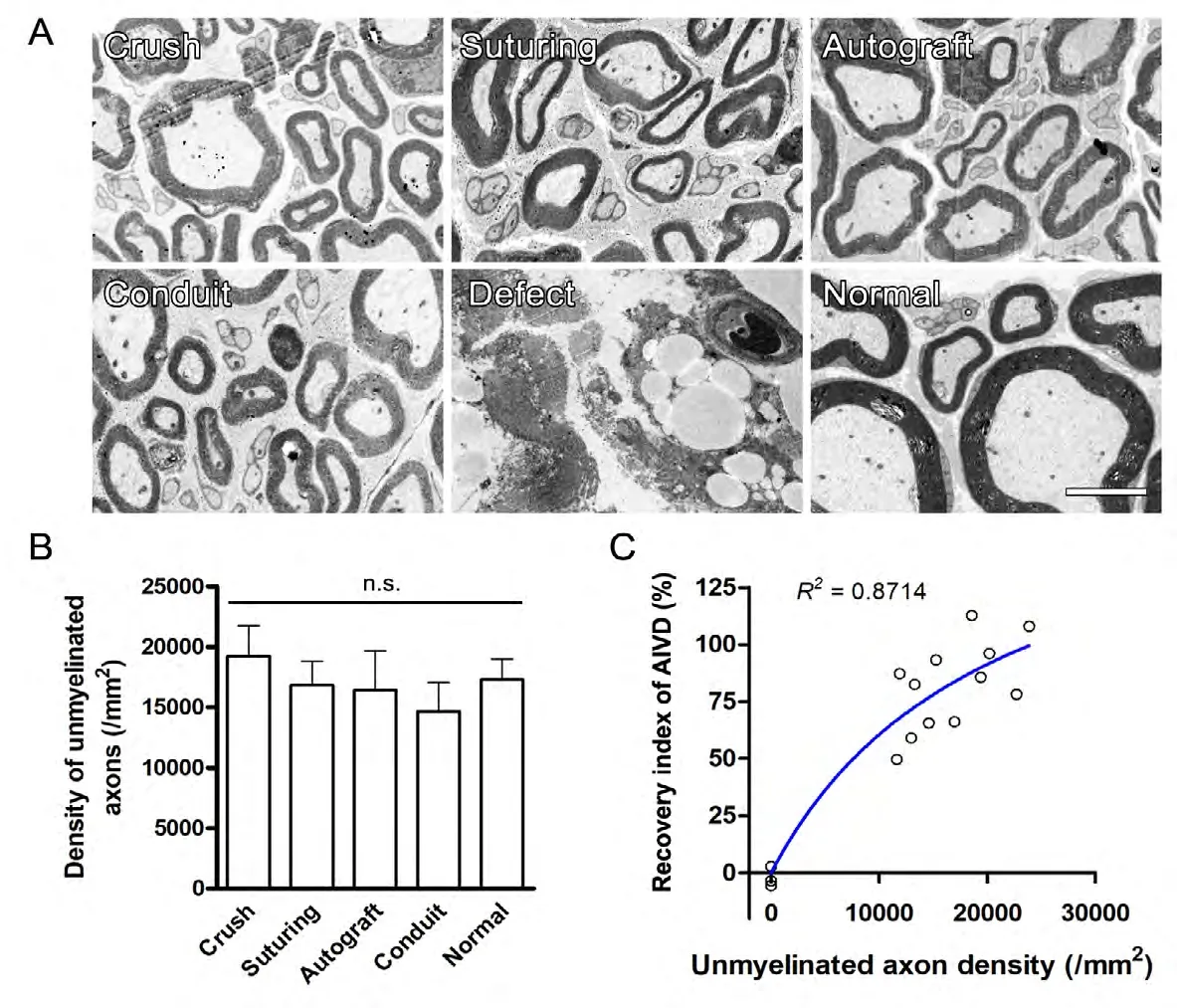
Figure 4 AEVD is correlated with the density of unmyelinated fibers 6 months after nerve injury/repair.(A) Representative transmission electron micrographs of the sciatic nerve distal to injury/repair show regenerated nerve fibers. (B) Estimated unmyelinated fiber density. No nerve fibers were observed in the nerve defect group. (C) Non-linear regression between relative perfusion increase and estimated density of unmyelinated nerve fibers. Data are expressed as the mean ± SEM (B, n = 10 rats/group) or scatter dots (C),and were analyzed using one-way analysis of variance followed by Tukey's multiple comparisons tests (B) or Michaelis-Menten non-linear regression (C). Each dot in (C) represents the data from one rat. AEVD: Axonotmesis-evoked vasodilatation.
Conflicts of interest: There were no conflicts of interest in this experiment.
Financial support:This work was supported in part by the National Natural Science Foundation of China, No. 81100939 and 81773713 (to WH),No. 81501610 (to XC); the Research Project funded by Jiangsu Provincial Government of China, No. BRA2018223 (to DGM); the Public Health Center at Jiangnan University of China, No. JUPH201808 (to XSW); the Wuxi Commission of Public Health and Family Planning of China, No.MS201717 (to XSW); the Project of Academic Development Program by Governments of Jiangsu Province and Nantong City of China (to DGM).The funding bodies played no role in the study design, in the collection,analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement:The study was approved by Ethics Committee for Laboratory Animals of Nantong University of China(approval No. 20130410-006) on April 10, 2013.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement: This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ruslan Masgutov, Kazanskij Federal'nyj Universitet Institut Fundamental'noj Mediciny i Biologii, Russian Federation.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve
- CNB-001 reduces paraplegia in rabbits following spinal cord ischemia
- Differential gene and protein expression between rat tibial nerve and common peroneal nerve during Wallerian degeneration
- Application of custom anatomy-based nerve conduits on rabbit sciatic nerve defects: in vitro and in vivo evaluations
- Relationship between high dietary fat intake and Parkinson's disease risk: a meta-analysis
- Optogenetics-induced activation of glutamate receptors improves memory function in mice with Alzheimer's disease