CNB-001 reduces paraplegia in rabbits following spinal cord ischemia
Paul A. Lapchak , Paul D. Boitano, Rene Bombien, Daisy Chou, Margot Knight, Anja Muehle, Mihaela Te Winkel, Ali Khoynezhad
1 Neurocore LLC, Pomona, CA, USA
2 Department of Surgery, Memorial Care Health System, Long Beach, CA, USA
Abstract Spinal cord ischemia associated with trauma and surgical procedures including thoraco-abdominal aortic aneurysm repair and thoracic endovascular aortic repair results in devastating clinical deficits in patients. Because spinal cord ischemia is inadequately treated, we studied the effects of [4-((1E)-2-(5-(4-hydroxy-3-methoxystyryl-)-1-phenyl-1H-pyrazoyl-3-yl) vinyl)-2-methoxy-phenol)] (CNB-001), a novel curcumin-based compound, in a rabbit SCI model. CNB-001 is known to inhibit human 5-lipoxygenase and 15-lipoxygenase and reduce the ischemia-induced inflammatory response. Moreover, CNB-001 can reduce the level of oxidative stress markers and potentiate brain-derived neurotrophic factor and brain-derived neurotrophic factor receptor signaling. The Tarlov scale and quantal analysis technique results revealed that CNB-001 administered as an intravenous dose (bolus) 30 minutes prior to spinal cord ischemia improved the behaviors of female New Zealand White rabbits. The improvements were similar to those produced by the uncompetitive N-methyl-D-aspartate receptor antagonist memantine. At 48 hours after aortic occlusion, there was a 42.7% increase (P < 0.05) in tolerated ischemia duration (n = 14) for rabbits treated with CNB-001 (n = 16), and a 72.3% increase for rabbits treated with the positive control memantine (P < 0.05) (n = 23) compared to vehicle-treated ischemic rabbits (n = 22). CNB-001 is a potential important novel treatment for spinal cord ischemia induced by aortic occlusion. All experiments were approved by the CSMC Institutional Animal Care and Use Committee (IACUC #4311) on November 1,2012.
Key Words: curcumin analog; spinal cord injury; spinal cord ischemia; thoraco-abdominal aortic aneurysm;thoracic endovascular aortic repair; motor function; neuroprotection; neurorepair
Introduction
Spinal cord ischemia (SCI) is inadequately treated in the clinic due to the lack of efficacious therapeutic neuroprotective options (Lapchak et al., 2000a; Lapchak, 2004; Arbo et al., 2018)and high cost of drug research and development (Lapchak and Zhang, 2017). SCI and its sequelae are the most devastating and feared complications during the perioperative phase in patients undergoing open thoraco-abdominal aortic aneurysm (TAAA) repair and thoracic endovascular aortic repair(TEVAR) (Shaw, 1986; Skillman, 1986; Lintott et al., 1998;Rectenwald et al., 2002; Terada et al., 2004; Jacobs et al., 2007;Bicknell et al., 2009; Min et al., 2010; Uchida, 2014; Fleissner et al., 2015; Nardone et al., 2016; Miranda et al., 2018). Aortic surgery may lead to athero-embolic stroke (Nardone et al.,2016) and long-term disability and even result in high morbidity and mortality (Kornberger et al., 2018; Agostinelli et al.,2019; Stern et al., 2019).
Paraplegia resulting from TEVAR or TAAA has been reportedly to be associated with ischemia and a prolonged inflammatory response (Delamarter and Coyle, 1999; Fu and Tummala, 2005; Nardone et al., 2016). Evidence exists that the incidence rate of paraplegia after TEVAR or TAAA is highly variable, it is estimated that 3-22% of patients subjected to TEVAR or TAA will become paraplegic during or after surgical procedures, and the prognosis is highly poorer in female patients than in male patients (Shaw, 1986; Skillman, 1986;Lintott et al., 1998; Rectenwald et al., 2002; Terada et al., 2004;Jacobs et al., 2007; Bicknell et al., 2009; Min et al., 2010; Uchida, 2014; Fleissner et al., 2015; Nardone et al., 2016).
For aortic surgery-caused SCI, the currently available therapeutic options include standard corticosteroid (methylprednisolone) administration to reduce chronic inflammation (Raiten et al., 2015a, b; Nardone et al., 2016) and the potential use of stem cell therapy to attempt to repair and regenerate affected neuronal populations (Curtis et al., 2018;Xie et al., 2018; Zhang, 2018; Zhu et al., 2018; Tsuji et al.,2019). Both of these two options are still in their infancy and have limited efficaciousness and clinical utility. It is important to note that neuroprotective strategies for SCI have not been abandoned by the research community, and are currently being studied in detail in relevant models because of the potential clinical use of such agents (Hilton et al., 2017;Sámano and Nistri, 2019; Cai et al., 2018; Chen et al., 2018;Cheng et al., 2018; Ishii et al., 2018; Jacobs and Lovejoy,2018; Ortmann and Hellenbrand, 2018; Ruzicka et al., 2018;Springer et al., 2018a, b; Zhang et al., 2018).
We have synthesized and extensively characterized (in vitro and in vivo) curcumin-based pleiotropic compound CNB-001 [4-((1E)-2-(5-(4-hydroxy-3-methoxystyryl-)-1-phenyl-1H-pyrazoyl-3-yl)vinyl)-2-methoxy-phenol)] (Lapchak, 2011; Lapchak and Boitano, 2014; Panzhinskiy et al.,2014; Lapchak et al., 2019). CNB-001 is a very promising drug candidate, a neuroprotective and cytoprotective molecule (Liu et al., 2008; Lapchak, 2011; Lapchak et al., 2011,2019; Jayaraj et al., 2013, 2014a, b; Lapchak and Boitano,2014; Panzhinskiy et al., 2014), in part due to its anti-inflammatory, anti-oxidant, anti-excitotoxic and neurotrophic-like activities, which are related to brain-derived neurotrophic factor and tyrosine receptor kinase (Trk).
In this study, we described the novel application of CNB-001 in the rabbit SCI model (RSCIM) that was developed by our group to test compounds for their efficacy to reduce the behavioral consequence of occlusion of the infrarenal aorta (Waterford et al., 2015). This study was conducted in consistence with currently applicable standardized research guidelines (Landis et al., 2012; Lapchak, 2013; Lapchak and Zhang, 2018). We studied the potential benefit of CNB-001,compared to a standardized positive control, the noncompetitive N-methyl-D-aspartate receptor (NMDA) antagonist, memantine (Chen et al., 1992; Chen and Lipton, 2005;Lipton, 2005; Lapchak, 2006). The large animal model that we used in this translational study has the advantage of reproducing paraplegia resulting from SCI induced by aortic occlusion, similar to the clinical situation (Lapchak et al.,2000a; Min et al., 2010; Ullery et al., 2011b, c), making it a suitable translationally relevant research and drug development tool.
Materials and Methods
Animals and surgery
Female New Zealand white rabbits (3.5-4 kg, 6 months old)acquired from IFPS, Norco, CA, USA were included in this study. All animals were quarantined for a minimum of 3 days, and were supplied food and water ad libitum while animals were maintained in an enriched environment prior to experimental use. All experiments were approved by the CSMC Institutional Animal Care and Use Committee (IACUC #4311) on November 1, 2012.
The rabbits were randomized to the following four groups:
Control group 1 (for memantine SCI group): normal animals were intravenously injected with 70% saline (n = 22).
Control group 2 (for CNB-001 SCI group): SCI animals were intravenously injected with solutol HS15 in 70% saline 30 minutes prior to aortic occlusion (n = 14).
CNB-001 SCI group: SCI animals were intravenously injected with CNB-001 (10 mg/kg) 30 minutes prior to aortic occlusion (n = 16).
Memantine SCI group: SCI animals were intravenously injected with memantine (20 mg/kg) 30 minutes prior to aortic occlusion (n = 23).
Drug preparation and administration
CNB-001 was synthesized by AQ BioPharma Co., Ltd.(Shanghai, China) as described previously (Lapchak et al.,2019). For intravenous administration, the test drug, CNB-001 (10 mg/mL) was dissolved in 30% solutol HS15 in 70%saline, whereas the positive control, memantine (20 mg/kg(10 mg/mL); Sigma Inc., St. Louis, MO, USA) was dissolved in saline. Both drugs were maintained at 37-39°C prior to intravenous injection (slow bolus) (see Figure 1 for experimental design).
Rabbit spinal cord ischemia models (RSCIM)
The SCI procedure achieved by aortic occlusion using balloon inflation was described in detail elsewhere (Waterford et al., 2015).
Motor function assessment
In the treatment group, rabbit behavior was rated by the author MTW at 4, 24, and 48 hours using the standard Tarlov scoring scale (Sirlak et al., 2008). If the animal was considered paraplegic with a Tarlov score of 0 or 1 at 24 hours, it was euthanized by intravenous injection of a lethal amount of pentobarbital (Virbac Animal Health, Fort Worth, TX,USA) on day 1 post-surgery as per the recommendation of Institutional Animal Care and Use Committee (IACUC). All other animals were euthanized after the 48 hour rating with a lethal intravenous injection of pentobarbital.
Briefly, Tarlov scale (Tarlov et al., 1953; Tarlov, 1954, 1955,1972; Tarlov and Herz, 1954; Tarlov and Klinger, 1954; Gelfan and Tarlov, 1955, 1956) was defined as follows: a score of 0, paraplegia with no lower extremity function; 1, poor lower extremity function and weak antigravity movement; 2, some lower extremity function with good antigravity strength, but unable to draw legs under body or hop; 3, the ability to draw legs under body and hop, but abnormal function; 4, normal motor function.
The quantal analysis technique was used for statistical analysis. We utilized a wide range of ischemia durations in each treatment group as described in detail previously (Lapchak et al., 2000a, 2001; Lapchak, 2004).
To encompass population response, we designated Tarlov scores of 3-4 as normal behavior, while Tarlov scores of 1-2 were designated as abnormal. We plotted quantal analysis curves of ischemia duration versus behavior (normal or abnormal as defined above), which allowed us to calculate the P50value in minute. A curve was produced for each treatment condition. A statistically significant increase in P50value of the drug-treated group compared to a control group indicated beneficial neuroprotection, as measured by behavioral improvement in the rabbit SCI study population.
RIGOR, power and statistical analysis
This study was conducted in a randomized and blinded manner as per stroke therapy academic industry roundtable(STAIR) (STAIR, 1999; Fisher, 2003) and RIGOR guidelines and criteria (Lapchak and Zhang, 2018). Rabbits were randomized to treatment groups before the surgical procedure.The allocation was concealed by an independent party technical associate, and was not revealed until all analyses were completed.
Power analysis of the quantal dose-response curves for rabbits indicates that, assuming α = 0.05, a standard deviation of 25%, and a sample size of 14 animals per treatment group, we had 90% power to detect a 50% change in mean P50values. Quantal analysis curve P50values were calculated using an iterative curve-fitting algorithm as described previously (Lapchak et al., 2000a, b, 2001, 2003; Lapchak, 2004).Quantal analysis data are presented as P50(mean ± SEM) in minutes for the number of rabbits in each group (n) for the RSCIM. Data were analyzed for significance using the unpaired t-test (MedCalc Version 9.4.1.0; MedCalc Software,Mariakerke, Belgium).
Results
CNB-001 reduces paraplegia incidence in female rabbits following SCI
In this study, six rabbits were excluded due to the absence of paraplegia verification after balloon inflation, and additional five rabbits were excluded because of reperfusion-injury pain when rabbits vocalized.
To determine if CNB-001 would be efficacious in the RSCIM, we studied the effects of treatment with either intravenous CNB-001 (10 mg/kg) or memantine (20 mg/kg) (Chen et al., 1992; Chen and Lipton, 2005; Lapchak, 2006; Lipton,2006), a noncompetitive low affinity NMDA open-channel antagonist, on behavioral deficits of female rabbits following infrarenal aortic occlusion. In this study, memantine was first used as a positive control to demonstrate that behavioral improvement could be measured using Tarlov scores and quantal analysis.
As shown in Figure 2, there was a 46% increase in the P50value (19.38 ± 2.03 minutes, n = 16) for CNB-001 pre-treated rabbits compared to the memantine SCI group (P50=13.58 ± 0.90 minutes, n = 14; P < 0.05). As shown in Figure 3, memantine produced a 72% increase in ischemia duration(P50value = 22.52 ± 2.98 minutes, n = 23) in rabbits with a paraplegic Tarlov score of 1-2 (See Table 1 for raw data)compared to the memantine SCI group (13.07 ± 2.12 minute, n = 22; P < 0.05). The P50values for CNB-001 and memantine were not statistically different (P > 0.05).
Discussion
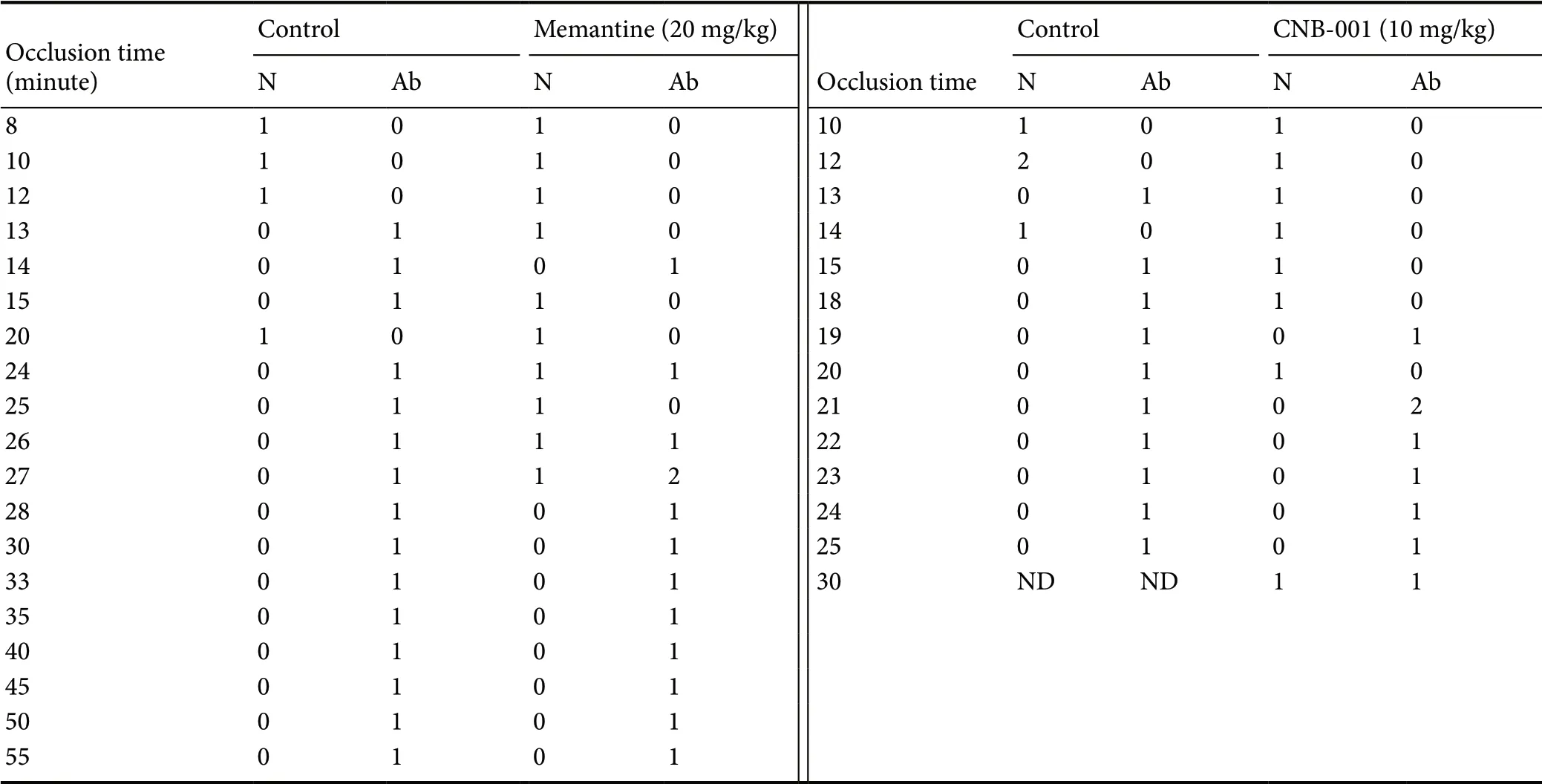
Table 1 Raw quantal curve data for 30 minute pretreatment regimen
In this proof-of-concept translational study, we used a previously established method (Waterford et al., 2015) to produce ischemia by inflating a Fogarty balloon in the infrarenal aorta of rabbits. This procedure resulted in paraplegia as measured by the loss of left lower extremity blood pressure and the disappearance of lower extremity motor potential. There were no discernable changes in the extent of blood pressure decrease or paraplegia between the four groups studied. No significant difference in morbidity or mortality was observed among the four groups.
The primary strength of this work is the translational value of utilizing the rabbit paraplegia model with quantal analysis to demonstrate the significant efficacy of the curcumin compound CNB-001 that effectively crosses the blood-brain barrier (Lapchak et al., 2019). Briefly, we found that intravenous CNB-001 administered pre-treatment, produced statistically significant clinical improvement in female rabbits,adding to a wealth of knowledge of the effects of CNB-001 in stroke and ischemia models (Lapchak et al., 2011, 2019). The pre-treatment regimen adopted for CNB-001 study in the RSCIM is justified, because the RSCIM that was developed parallels in some aspects SCI that results from modern surgical procedures, including TAAA repair and TEVAR (Shaw,1986; Skillman, 1986; Lintott et al., 1998; Rectenwald et al.,2002; Terada et al., 2004; Jacobs et al., 2007; Bicknell et al.,2009; Min et al., 2010; Uchida, 2014; Fleissner et al., 2015;Nardone et al., 2016; Miranda et al., 2018).
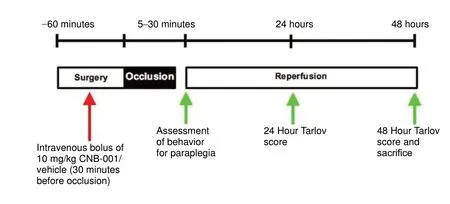
Figure 1 Experimental design temporal profile.Female rabbits underwent the aortic occlusion procedure for various durations as shown in the figure. Rabbits were pretreated by intravenous bolus of 10 mg/kg CNB-001/vehicle 30 minutes before aortic occlusion. Behavior was assessed at 24 and 48 hours. CNB-001: 4-((1E)-2-(5-(4-hydroxy-3-methoxystyryl-)-1-phenyl-1H-pyrazoyl-3-yl)vinyl)-2-methoxy-phenol).
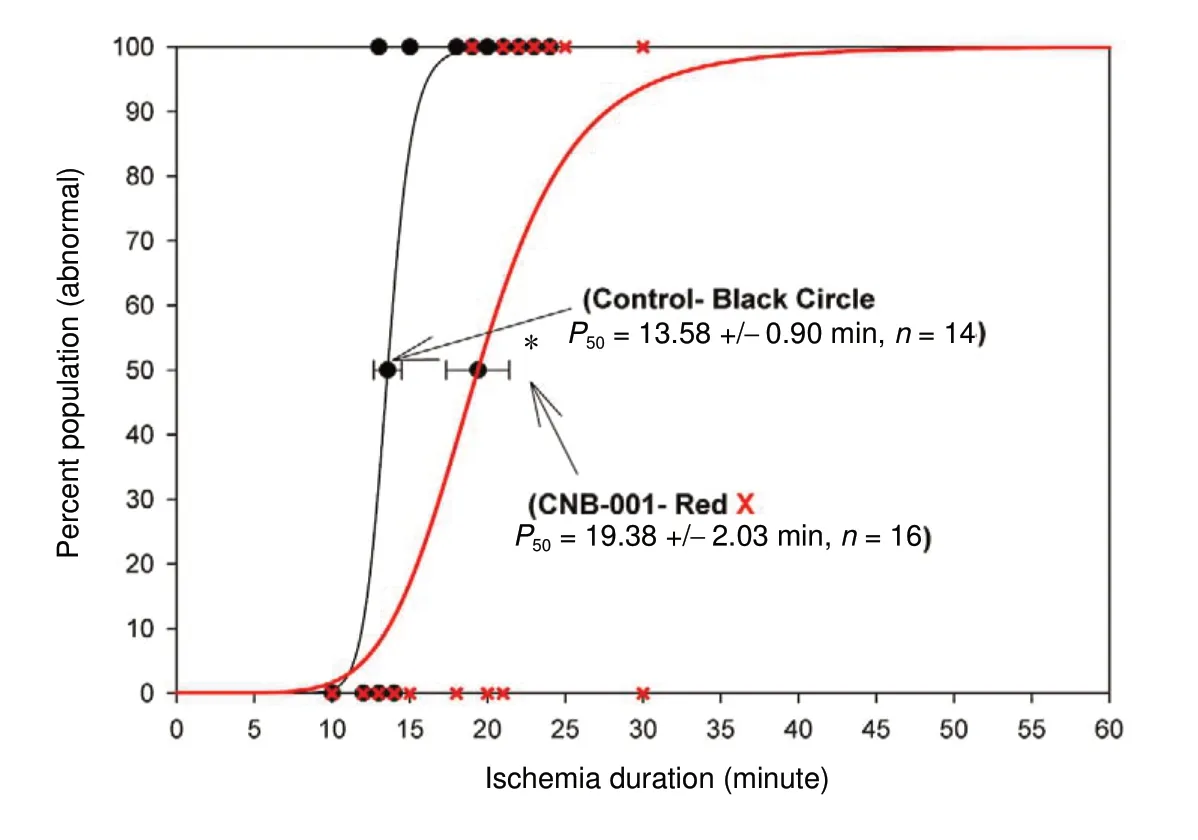
Figure 2 CNB-001 pre-treatment prevents paraplegia in female rabbits subjected to aortic occlusion.Rabbits were pretreated by intravenous vehicle (30% Solutol HS15 in saline, n = 14) or CNB-001 (10 mg/mL, n = 16) 30 minutes (min) before aortic occlusion. CNB-001 (red line,red X) significantly increased the P50 value (ischemia duration by 5.8 minutes) measured at 48 hours after aortic occlusion (unpaired t-test, *P < 0.05) compared to vehicle control(black line, black circle), and improved rabbit behaviors. For the superimposed graphs, behaviorally normal animals are plotted on the y-axis at 0 and abnormal animals are plotted at 100. CNB-001: 4-((1E)-2-(5-(4-hydroxy-3-methoxystyryl-)-1-phenyl-1H-pyrazoyl-3-yl) vinyl)-2-methoxy-phenol).
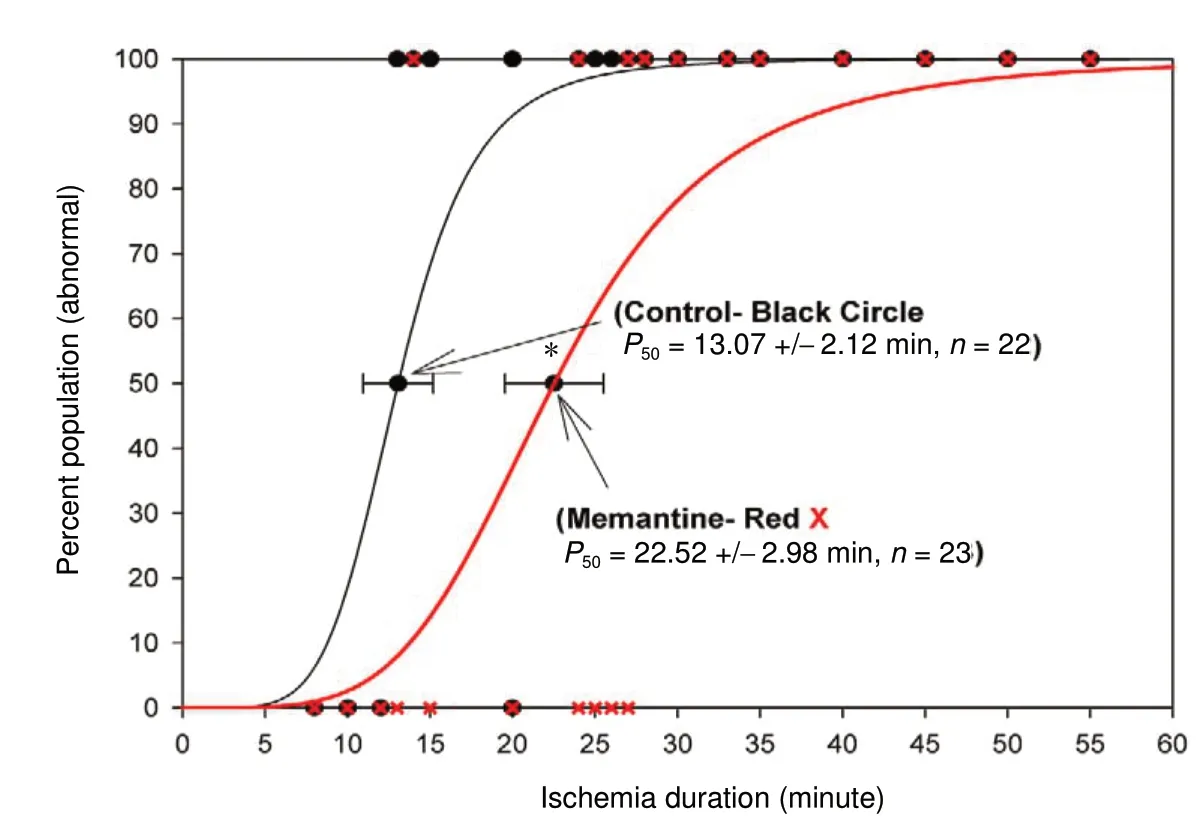
Figure 3 Memantine pre-treatment prevents paraplegia in female rabbits subjected to aortic occlusion.Female rabbits underwent the aortic occlusion procedure for various durations as shown in the figure. Rabbits were pretreated with intravenous either vehicle (saline, black line,black circle, n = 22) or memantine (20 mg/kg; red line, red X, n = 23) 30 minutes (min) before aortic occlusion. Memantine significantly increased the P50 value (ischemia duration by 9.45 minutes) measured at 48 hours after aortic occlusion(unpaired t-test, *P < 0.05), and improved rabbit behaviors.For graphical analysis, normal animal data are plotted on the y-axis at 0 and abnormal animal data are plotted at 100.
TAAA repair and TEVAR are fraught with challenges that can possibly be overcome if a safe and efficacious neuroprotective therapy can be used prior to and/or concomitantly with difficult surgical procedures. Moreover, since aortic surgery can occasionally involve athero-embolic events (Nardone et al., 2016), the use of CNB-001 as demonstrated in both rabbits and primates (Lapchak et al., 2019) could potentially reduce the extent of clinical deficits in stroke patients.
In this study, CNB-001 was directly compared to memantine in parallel studies that required the use of two different vehicle control groups because of the differential solubility characteristics of CNB-001 and memantine. The data showed that the baseline P50value for the vehicle control was not significantly different. Moreover, when compared to the appropriate vehicle control, CNB-001 increased P50value by 42.7% and memantine increased P50value by 72.3%.Clearly there were differences in the magnitude of the efficacy that may be dose-related. It is of importance to note that this proof-of-concept study utilized only single doses of both CNB-001 and memantine, and the doses were both selected based upon previous work done in our group (Lapchak, 2006; Lapchak et al., 2011, 2019). The selected dose of CNB-001 was previously shown to be efficacious in male rabbits, male non-human primates and male rats (data not shown). This study adds to the knowledge base for CNB-001 by demonstrating statistically significant efficacy in females.Based on this, both dose-response analysis for maximum efficacy following aortic occlusion and temporal analysis studies regarding pre- and post-treatment timing for maximum durability can be conducted once they are funded by NIH/NINDS or another funding agency. Moreover, complete gender analysis can be conducted.
Strong evidence exists that the pleiotropic properties of the molecule CNB-001 are extremely important (Liu et al.,2008; Lapchak, 2011; Lapchak et al., 2011, 2019; Jayaraj et al., 2013, 2014a, b; Lapchak and Boitano, 2014; Panzhinskiy et al., 2014) for neuroprotection. CNB-001 is reported to decrease the generation of reactive oxygen species, reduce the production of a variety of inflammatory mediators including tumor necrosis factor-α and interleukin-6, and attenuate deleterious membrane lipid peroxidation. CNB-001 also effectively preserves normal physiological mitochondrial membrane potential, which is important for cellular energy production and function. These characteristics of CNB-001 indicate that it would have utility in the treatment of ischemic diseases (Liu et al., 2008; Maher et al., 2010; Sharma et al., 2010; Lapchak, 2011; Lapchak et al., 2011, 2019; Wu et al., 2011; Jayaraj et al., 2013, 2014a, b; Valera et al., 2013;Lapchak and Boitano, 2014; Panzhinskiy et al., 2014). In addition, CNB-001 stimulates the production of brain-derived neurotrophic factor (i.e., pro-survival neurotrophic factors)and activates the pathways involved in cytoprotection (Liu et al., 2008; Sharma et al., 2010; Lapchak et al., 2011, 2019; Wu et al., 2011; Valera et al., 2013). Future studies will elucidate the mechanisms involved in reduced functional deficits using the RSCIM.
CNB-001 can reduce behavioral dysfunction in the RSCIM (as shown in the current study), narrow the spread of ischemia (i.e., infarct growth) in cynomolgus monkeys,and improve the clinical function of the rabbit small clot embolic stroke model (RSCEM) (Lapchak et al., 2011,2019) when administered either pre- or post-ischemia. It is promising to use a molecule like CNB-001 to halt the consequence of ischemic damage when provided to patients prior to TAAA repair and TEVAR where there is a high incidence of SCI, which can result in paraplegia (Svensson et al., 2003;Svensson, 2005; Dillavou and Makaroun, 2008; Ullery et al., 2011a, b, c). As hypothesized previously (Lapchak et al.,2019), it may be possible to “freeze” penumbra in patients in the field, immediately upon first contact of spinal cord injury (ischemia) patients or victims with emergency vehicle personnel, paramedics or medics (i.e., athletes, football or soccer players as an example, car accident victims, enlisted military personnel), and ischemic stroke patients prior to current conventional reperfusion therapies (Bratane et al.,2011; Lapchak et al., 2019).
The current study showed that pretreatment with CNB-001 significantly reduced ischemia-induced paraplegia in female rabbits with SCI. The early effects of CNB-001 to interfere with the ischemic cascade following ischemia in the SCI model may in part be linked to its ability to counteract the deleterious effects of glutamate on cells (Liu et al., 2008; Lapchak et al., 2011) and to reduce the propagation of ischemia.CNB-001 is a valid candidate to treat ischemic disease and should be further pursued in well-designed clinical trials.
Author contributions:PAL: Study design and implementation and manuscript writing; PDB, MK, AK: study implementation and technical support; RB: study design and implementation and manuscript editing;DC: study design and implementation; MTW: study implementation and primary Tarlov ratings; AK: study design and implementation. All authors approved the final version of this manuscript.
Conflicts of interest:Funding for this project was provided by anonymous donors to support translational research. The authors declare no conflict of interest relating to the material presented in this Article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
Financial support: None.
Institutional review board statement: The study was approved by the CSMC Institutional Animal Care and Use Committee (IACUC #4311) on November 1, 2012.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Syoichi Tashiro, Keio University School of Medicine, Japan; Adriana Vizuete, Universidade Federal do Rio Grande do Sul Instituto de Ciencias Basicas da Saude, Brazil.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Fresh human amniotic membrane effectively promotes the repair of injured common peroneal nerve
- Differential gene and protein expression between rat tibial nerve and common peroneal nerve during Wallerian degeneration
- Application of custom anatomy-based nerve conduits on rabbit sciatic nerve defects: in vitro and in vivo evaluations
- Axonotmesis-evoked plantar vasodilatation as a novel assessment of C-fiber afferent function after sciatic nerve injury in rats
- Relationship between high dietary fat intake and Parkinson's disease risk: a meta-analysis
- Optogenetics-induced activation of glutamate receptors improves memory function in mice with Alzheimer's disease