Toxicological characterization and central nervous system effects of Calotropis procera Ait. aqueous extracts in mice
Prosper T. Kinda, Samson Guenné, Moussa Compaoré, Balé Bayala, Alin Ciobica,Raymond Belemtougri, Martin Kiendrebéogo
1Laboratory of Applied Biochemistry and Chemistry, University Joseph KI-ZERBO, 03 BP 7021 Ouagadougou 03, Burkina Faso
2Laboratory of Animal Physiology, Reproduction and endocrinology team, University Joseph KI-ZERBO, 03 BP 7021 Ouagadougou 03, Burkina Faso
3Department of Research, Faculty of Biology, Alexandru Ioan Cuza University, B dul Carol I, no 11, Iasi, Romania
4Academy of Romanian Scientists, Splaiul Independentei nr. 54, sector 5, 050094 Bucuresti, Romania
5Center of Biomedical Research, Romanian Academy, Iasi, B dul Carol I, no 8, Romania
6Laboratory of Forensic Sciences, General Direction of National Police, 01 BP 22 Ouagadougou 01, Burkina Faso
Keywords:Calotropis procera Toxicity Behavioral Burkina Faso
ABSTRACT Objective: To evaluate the toxicological and psychotropic properties of Calotropis (C.) procera.Methods: C. procera leaves and root-bark aqueous extracts were evaluated for their toxic and behavioral effects using adult mice. Toxicity studies were carried out using Organisation for Economic Cooperation and Development guidelines 423 and 407 for acute and subacute evaluation. Behavioral studies were performed using traction test, fireplace test, hole-board test and forced-swimming test to evaluate the sedative, anxiety and depressive-like activities of the extracts.Results: Very low acute toxicity was observed in mice that received both leaves and rootbark extracts. The subacute test showed some morphological, biochemical and hematological changes in the treated groups. Behavioral assessment demonstrated anxiety effects on mice for C. procera leaf extract (400 mg/kg of body weight).Conclusions: The acute use of C. procera (leaves and root-barks) aqueous extracts could be considered as low toxic. However, their repeated uses could have harmful effect on some organs. Likewise, a single dose up to 400 mg/kg body weight of these extracts produce no sedative or depressive-like effect, but they possess possible dose dependent anxiety effect. Yet,more studies are necessary to relate these results to the chemical profile of the plant extracts.
1. Introduction
Herbal drugs have received greater attention as an alternative to clinical therapy and the use for these herbal remedies has greatly increased recently. Their utilization is often based on long-term clinical experience. Despite their use in folk medicine, these plants began to receive toxicology attention from scientists[1].Some psychoactive plants are used more and more in the treatment of neuropsychiatric disorders. However, the continuous use of psychoactive therapies tends to have negative side effects including respiratory, cognitive function, physical dependence and tolerance[2].In our previous study, we reported many plants from Burkina Faso used in neuropsychiatric diseases treatment[3]. Calotropis (C.) procera belongs to Asclepiadaceae family, which is used in traditional medicine to treat various diseases such as epilepsy, madness, malaria,otitis, ulcer, tumors, knife bite, liver diseases[3,4]. The leaves and the latex are used in vertigo, hair fall, tooth aches, intermittent fevers and paralysis treatment[5]. The root, specially root-barks from C. procera,is used to cure leprosy, eczema, bronchitis, asthma, elephantiasis,hepatitis, drepanocytosis, fever, malaria and snake bite[5,6]. C.procera is widely studied for its numerous pharmacological properties[7,8], and reported to have rich phytochemical contents.It possesses cardenolides, triterpenes, flavonoids, sterols, saponins,diterpenes, resins, tannins, alkaloids and steroids[9-11]. Some of these chemical contents are well known to be toxic and act on the nervous system[10,12]. In the pharmacopeia of Burkina Faso, it appears that C.procera is one of plants specifically used by the older (experienced)traditional healers because of its potential toxicity[3]. In the aim to evaluate the possible noxious effect of C. procera, toxicological and behavioral studies were carried out on its leaves and root-barks aqueous extracts in mice models. Acute and subacute toxicities were evaluated. Behavioral, sedative, anxiety and depressive-like effects of this plant extracts were determined.
2. Materials and methods
2.1. Plant collection and extract preparations
C. procera (leaves and root-bark) samples were collected from natural habitats of Gampéla, in the Centre area of Burkina Faso.They were identified by botanists from the Plants Department of University Joseph KI-ZERBO (Burkina Faso). The voucher specimen was deposited at the herbarium of this University (ID:16971).
Twenty five grams (25 g) of dried powder were used for decoction in 500 mL of distilled water at 100 ℃ for 30 min. Extract was filtered with muslin cloth and centrifuged at 4 000 rpm for 10 min.The supernatant was collected and lyophilized to dryness. The residue was weighed to obtain the extracted yield and kept at 4 ℃ in waterproof plastic flasks until use.
2.2. Chemicals
The diazepam and the tramadol were obtained from local pharmacy.All solvents used were analytical grade and purchased from Sigma-Aldrich (Germany).
2.3. Experimental animals
The mice were from the Naval Medical and Research Institute(NMRI), obtained from the animal house of University Joseph KIZERBO. The animals were housed for a week under controlled conditions for acclimatization before the experiments. All mice were aged from 11 to 12 weeks at the start of experiments. Female mice that weighed 28-37 g were used for acute and subacute toxicity study. Male mice weighed 27-34 g were used for behavioral studies.Animals were kept in plastic cages under identical animal house condition and were provided with standard pellet and water ad libitum. Each cage contained a group of mice (n=6 or n=5), with a bedding of shavings regularly renewed. Twelve-hour light and dark alternate cycles (started at 6: 00 AM) were provided, temperature was maintained at (22±3) ℃ and relative humidity was at (50±10)%.Mice were treated in accordance with the guidelines of animal bioethics from the Act on Animal Experimentation and Animal Health and Welfare Act from Burkina Faso (ethic community acceptance No. CE-UOI-2018-03) and all procedures were in compliance with the European Council Directive of 24 November 1986 (No. 86/609/EEC). All behavioral evaluations were performed between 9 am and 4 pm.
2.4. Toxicity effect assessment
2.4.1. Acute toxicity study
The acute toxicity study was conducted under Organisation for Economic Cooperation and Development 423 guidelines[13] with slight modifications. The mice were randomized into 3 groups and each group contained 6 animals. The 1st group (Control) received saline (0.9% NaCl), the 2nd group received C. procera leaves extract at 3 000 mg/kg body weight (b.w.) and the 3rd group received C. procera root-barks extract (3 000 mg/kg b.w.). After extract administration, the animals were observed continuously for the first 4 h to detect eventual behavioral changes. Then, they were observed periodically for 72 h for any mortality. Animals were maintained during two weeks and they were weighed each week. At the end,mice were sacrificed by cerebral dislocation and organs such as liver,spleen, kidney, lung, heart and brain were observed for possible morphological change and were weighed.
2.4.2. Subacute toxicity study
Assessment of subacute toxicity was performed in accordance with Organisation for Economic Cooperation and Development 407 guidelines[14]. Animals were randomized into 7 groups (n=6).Each treated group was administrated by oral route with an extract dose of 100, 200 or 400 mg/kg of body weight (b.w.) daily for 28 consecutive days. Group 1-3 received C. procera leaves extract,group 4-6 received C. procera root-barks extract and the control group received saline (NaCl 0.9%). All animals were observed twice daily for mortality or behavioral change during the 28-days period.The weight of each mouse was recorded the first day and at weekly intervals during the study, then the weight gain was calculated. At the end of treatment, after to be fasted 12 h, the blood samples of each animal were collected by cardiac puncture for biochemical and hematological parameters evaluation. Mice were sacrificed and organs were removed, observed for possible morphological change and were weighed.
Biochemical analyses were performed in serum obtained after centrifugation of total blood in tubes without anticoagulants.Creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, total triglyceride and total cholesterol were estimated in serum. Hematological analyses were performed in total blood collected in tubes with ethylenediaminetetraacetic acid. The relative blood indices as white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular hemoglobin concentration, platelets, mean platelet volume were estimated.
2.5. Behavioral assessment
2.5.1. Drug administration
Six groups of mice (n=5) were randomized for each test of this study. A single dose of 100 or 400 mg/kg b.w. from each extract was administrated to treated groups. Group 1, 2 received C. procera leaves extract and Group 3, 4 received C. procera root-barks extract.The normal control group received saline (0.9% NaCl) and the positive control group received Diazepam 3 mg/kg of body weight or Tramadol 20 mg/kg (b.w.). All treatment were administrated using oral route 30 min before behavioral test. The extracts doses were fixed based on the toxicity studies.
2.5.2. Traction test
This test was performed according to wire stretched method[15].Mice were individually suspended by anterior limbs to a wire stretched horizontally. Abnormal mice that fail to make a reestablishment at least one of its posterior limbs to reach the wire are considered as subject under a sedative action. When the animals perform normal reestablishment immediately, the reaction is known as positive; otherwise, the reaction is called negative. Each animal was tested for 2 min and the reestablishment time were measured.
2.5.3. Fireplace test
Fireplace test was validated as a suitable tool for predicting sedative effect. The apparatus used for this test consist to a cylindrical transparent tube (length: 30 cm; diameter: 3 cm) vertically disposed.Mice were individually placed in the test tube. A normal mouse typically attempts to escape in 30 s, and the mice considered as subject to the sedative effect when performing the rise of cylinder greater than 30 s[16]. The escape time of animals was measured.
2.5.4. Hole-board test
The hole-board test was performed using a wood floor board according to the method described by Vieira et al.[17]. It is frequently used to screen the effects of drugs on anxiety, sedative or rodent behavioral exploration. When placed in a new environment, the natural tendency of an animal is to explore the holes by plunging its head in. This test enables an assessment of the effects of drugs on anxiety and exploration activity. It is based on the assumption that the number of head dips of animal is inversely proportional to anxiety.The perforated board test was made by using a wood floor board,60 cm×60 cm×20 cm, in which 16 evenly spaced holes (3 cm of diameter) were made. Mice were individually placed in the center of a perforated board, and the number of head dips was counted during 5 min. Also, locomotor activity (total active movements) of animals was recorded during the period of the experiment.
2.5.5. Forced-swimming test
The Forced-swimming test is the most widely used and recognized pharmacological model for assessing depressive-like response. The development of immobility when mice are placed in an inescapable cylinder filled with water reflects the cessation of persistent escapedirected behavior[18]. The possible depressant effects of the C.procera aqueous extracts were assessed on this test. In the pre-test session, every mouse was placed individually into the cylindrical recipient (diameter 30 cm, height 35 cm) containing 25 cm of water(26±1) ℃ for 15 min swimming. The test was performed 24 h after the pre-test session, each animal was left to swim for 6 min, 30 min after the extract administration. For this test, the following behavioral responses were recorded: the immobility time (time spent floating with the minimal movements to keep the head above the water) and the swimming time (time spent with active swimming movements).
2.6. Statistical analysis
Results were expressed as the mean±SD. Statistical analysis was performed using GraphPad Prism 5.03 for Windows (Graph Pad Software, Inc., California USA). One-way ANOVA with Dunnett’s post hoc test for parametric multiple comparisons between the control and the treatment groups was used. Differences were considered significant when P<0.05.
3. Results
3.1. Extracts toxicity effects
3.1.1. Acute toxicity
No mortality or external toxicity sign were observed after oral administration of both C. procera leaves extract and its root barks extracts at the dose of 3 000 mg/kg of body weight. Likewise, no change in gross appearance of internal organs as liver, spleen, spleen,kidney, lung, heart and brain was observed after animals’ autopsy.Compared to the 1st day weight, no significant difference was observed on body weight after two weeks. Relative organ weights were not changed in treated groups as compared to control group(Table 1).

Table 1. Weekly body weight and relative organ weight after two weeks.
3.1.2. Subacute toxicity
3.1.2.1. Effect of plant extracts on behavioral, morphological and weight
No toxicity behavioral manifestation on animals was observed during the 28-days period. Moreover, there was no mortality both in groups treated with leaves and root-barks extracts. Body weight of animals was lightly decreased in all treated groups, but no significant difference (P>0.05) was observed when compared 7th, 14th or 28th day weights to the 1st day weight. Likewise, there were no difference of weekly body weight gain in treated groups as compared to control group. For relative organs weight, no significant change was noticed between treated and control animals (Table 2). However,there were organs morphological changes such as abnormal size of spleen. Indeed, a hypertrophy of spleen of some animals was noticed in groups treated with leaves extract (100 and 400 mg/kg) and rootbarks extract (100 and 200 mg/kg) as compared to control animal organs (Figures not shown).
3.1.2.2. Effect of plant extracts on biochemical and hematological parameters
The results of biochemical tests are presented in Table 3. All extract treated groups showed significant decrease (P<0.01 to P<0.001) of creatinine concentration compared to control group.However, AST were increased (P<0.001) in dose of 100 mg/kg group and decreased (P<0.05) in dose of 400 mg/kg group of C. procera root-barks extract as compared to control group.No significant changes were observed on ALT, urea and total triglyceride content.
For hematological parameters, the treated groups were compared to the control group and the results are recorded in Table 3. With the root-barks extract, we observed significant increase (P<0.01) of white blood cell and mean corpuscular hemoglobin at the dose of 400 mg/kg. However, in all the rootbarks extract groups (100-400 mg/kg) significant decrease(P<0.01 to P<0.001) of the platelets count were noticed, and mean platelet volume was decreased (P<0.05) in dose of 100 mg/kg root extract treated group. For leaves extract, the platelets count lowering (P<0.05) were observed in the 100 mg/kg leave extract treated group.
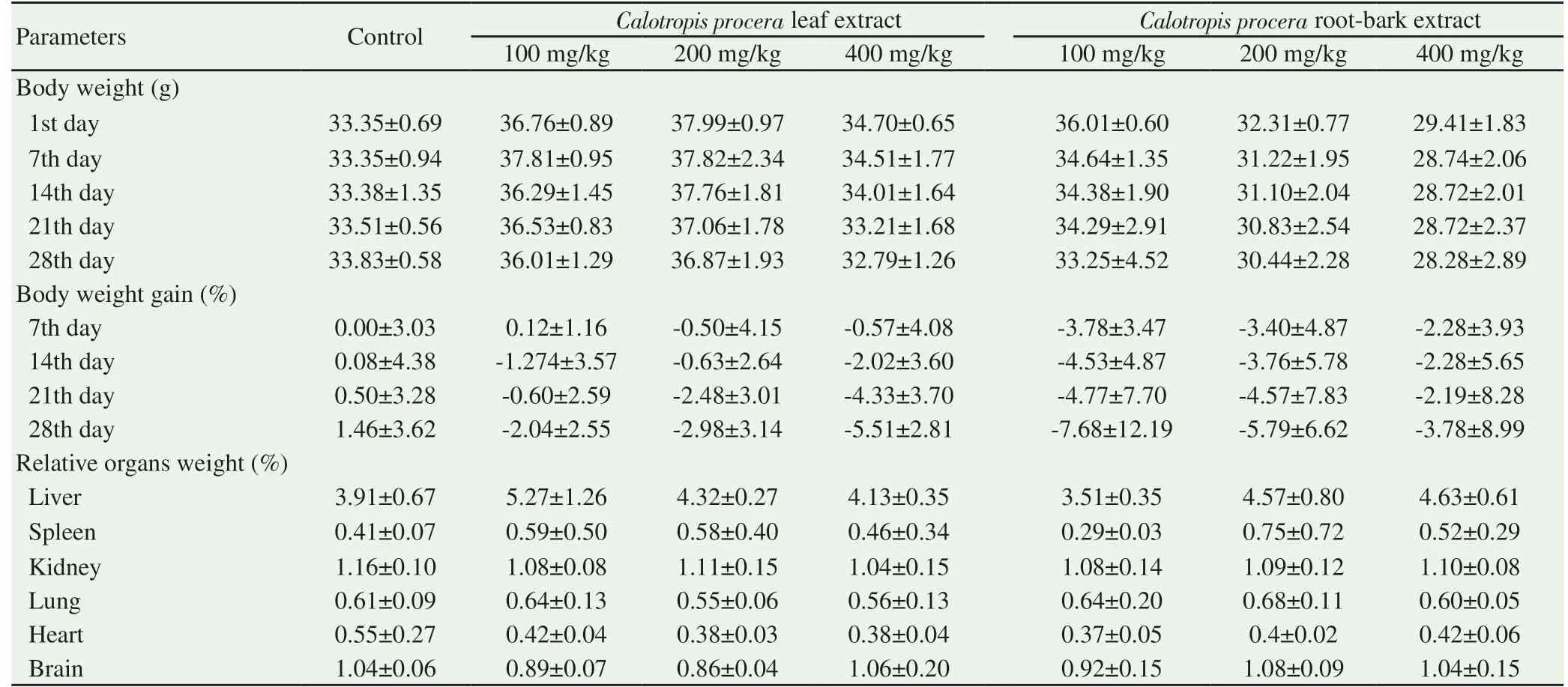
Table 2. Weekly body weight and relative organ weight after four weeks.
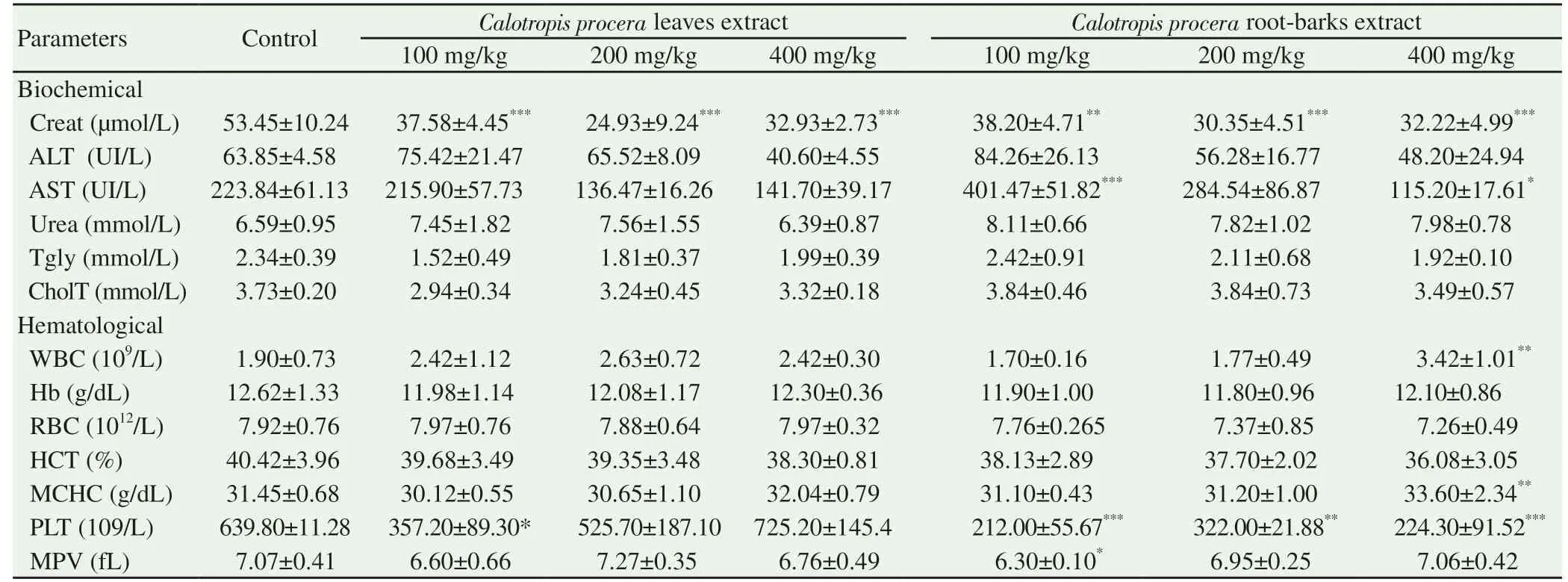
Table 3. Extract effect on biochemical and hematological parameters.
3.2. Psychotropic effect of extracts
3.2.1. Sedative effect
The sedative effects were determined by using two specific tests,the Traction test and the Fireplace test. In the Traction test, none of the extracts administrated groups showed significantly alteration(P>0.05) in reestablishment time as compared to the control group.All animals were performed reestablishment immediately after to be suspended. Diazepam group revealed significant sedative effect as indicated by the relative increase (P<0.001) of the reestablishment time (Figure 1A). For the Fireplace test, animals were treated with leaves and root-barks extracts and showed no significant difference (P>0.05) of the escape time when compared to control group. All animals were performed normal escape immediately. Diazepam groups presented relatively longer escape time (P<0.001) as compared to control group (Figure 1B). Indeed,the animals that received diazepam had difficulties to escape from apparatus or delayed to take escape initiative.
3.2.2. Anxiety effect
Assessed in the Hole-board test, a significant decrease (P<0.05) of head dipping was recorded in animals which received leaves extract 400 mg/kg as compared to control group. Likewise, diazepam group showed a light decrease of head dips number, but Dunnett’s post hoc analyses revealed non-significant statistical differences (P>0.05)when compared to control group (Figure 1C). During the test trial,no noticeable change of locomotors activity (total active movements)was observed in extracts treated animals. However, very low active movements were noticed in diazepam group.
3.2.3. Depressive effect
Evaluated in the Forced swimming test, no significant change(P>0.05) of immobility time was observed in groups treated with plant extracts compared to the control group. Besides, there was not significant change (P>0.05) of immobility time between tramadol treated group and control group (Figure 1D).
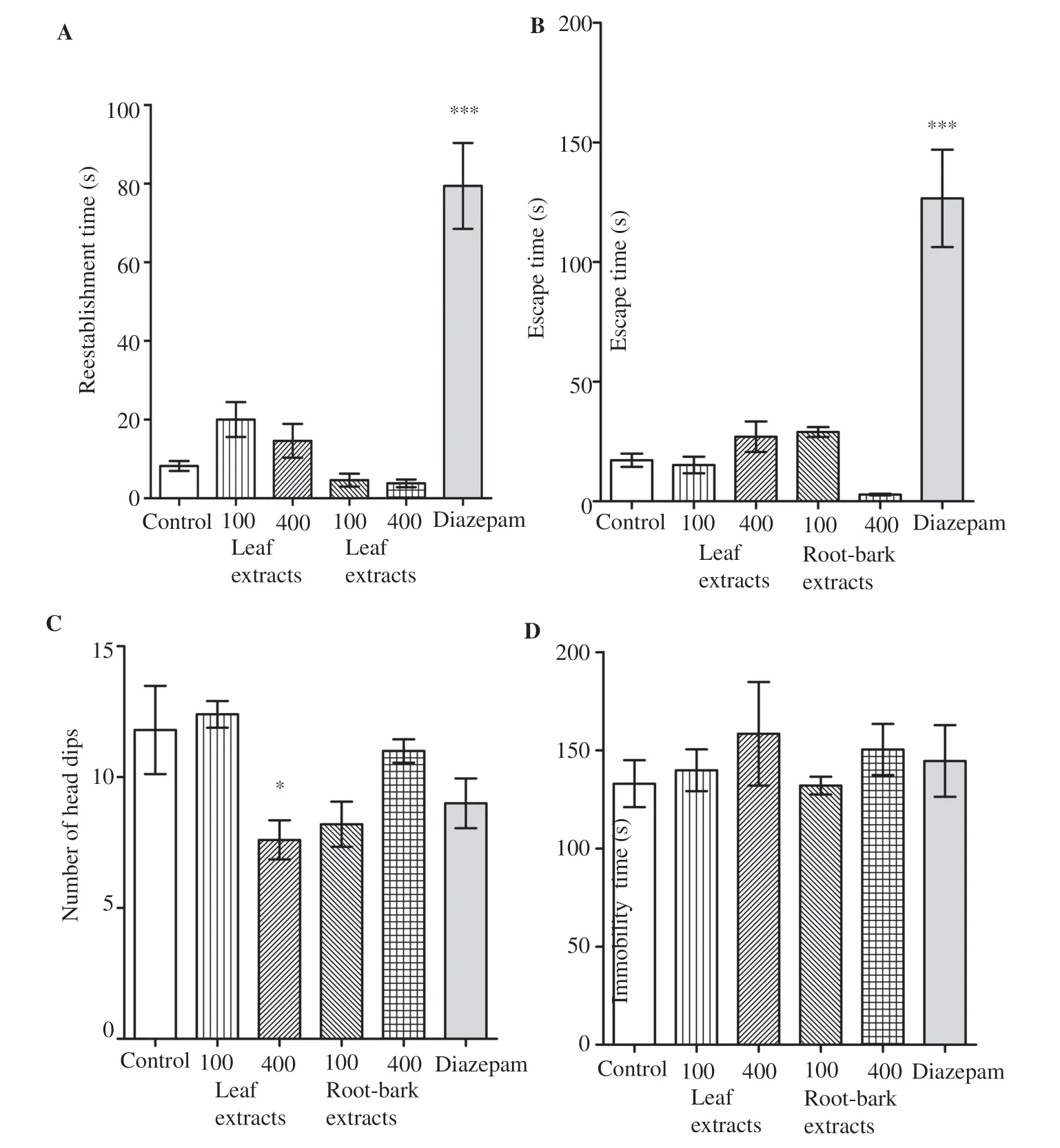
Figure 1. Psychotropic effect of Calotropis procera leave and root-bark extracts. A, B: Sedative effect; C: anxiety effect and D: depressive effect of C. procera leaves and C. procera root-bark aqueous extracts. *P<0.05 and ***P<0.001, significant difference when compared to the control group.
4. Discussion
From obtained results of this study, no mortality was observed in any animal group treated with C. procera (leaves and root-barks)aqueous extracts during acute toxicity assessment. Consequently,extracts LD50are considered to be higher than 3 000 mg/kg (b.w.).According to the OCDE guideline, any pharmaceutical drug or compound with the oral LD50higher than 2 000 mg/kg could be considered safe or low toxic[13]. This result suggests that aqueous extracts of C. procera leaves and C. procera root-barks are practically non-toxic at single dose up to 3 000 mg/kg (b.w.) by oral route. It corroborates to the previous studies reported that C.procera aqueous extract is non-lethal by oral administration up to the dose of 2 000 mg/kg (b.w.) for root-barks extract[19], and the dose of 5 000 mg/kg (b.w.) for the leaves extract[20]. However, it disagrees with Mbako’s group data which obtained a LD50of 940 mg/kg (b.w.) for aqueous extract of the fresh leave of C. procera by oral route[21].
The subacute toxicity study revealed no mortality or noticeable behavioral change in the treated groups during the 28-day period. However, a light decrease of animal’s body weight and morphological changes of some organs were observed in groups treated. Decreases or increases in the body weight can be used as an indicator of adverse effects of drugs or chemicals[22].Nevertheless, body weight change might be due to an accumulation of fat or physiological adaptation response as decrease of appetite rather than the toxic effects of drugs[23].
Spleen hypertrophy (splenomegaly) was observed in some extracts dose treated groups. Liver, kidney and spleen are organs of metabolism and excretion which are likely affected by potentially toxic agents[1]. The spleen represents an important clearance site for some chemicals[24]. An accumulation of these substances leads to alterations and changes in spleen histology[24].However, splenomegaly could be result from many reasons such as hypertrophy due to an increased immune response, erythrocyte destruction, splenic congestion due to difficulty in venous drainage, infiltration of elements produced by metabolic disorders or haematological cancer[25].
The biochemical and hematological parameters observed could support these results. Indeed, biochemical tests showed significant decrease (P<0.01 to P<0.001) of creatinine levels in animals that received all doses of C. procera extracts compared to control group.Creatinine is known as a good indicator of renal function. Any rise of creatinine levels is observed if there is a marked damage to indicator of renal function[1]. However, a decrease of creatinine levels could indicate myopathy or damage of liver function.Indeed, creatinine is a product of the metabolism of creatine,which is produced in the liver and stored in muscles in order to be used as a source of energy once phosphorylated. In chronic liver disease, the reduction in the serum creatinine pool is due for 50%to decrease in hepatic production of creatine[26]. The significant decrease in creatinine levels in treated groups probably indicates that extracts act on the metabolism of creatinine production.
Likewise, AST showed significant changs in groups treated with root-barks extract. AST and ALT are well known enzymes which serve as biomarkers, able to predict toxic effects. AST is present in a wide variety of tissues including heart, kidney, skeletal muscle and liver, whereas ALT is primarily localized in liver. Many studies have reported that changes in AST and ALT levels in serum could be linked for liver damage or some cellular injuries[1]. The change observed in creatinine and AST levels in treated groups might be caused by some phytochemical that have toxic potential on liver,spleen or other organs.
Similarly, the hematological evaluation showed some differences when compared to control group. With C. procera root-barks extract, all the doses treated groups showed significant decrease(P<0.001) of platelets levels. The dose of 400 mg/kg presented an increase (P<0.01) of white blood cell count and mean corpuscular hemoglobin concentration. These results revealing blood cells disturbances are agreed with splenomegaly observed.The hematopoietic system is one of the most sensitive target for toxic compounds and an important index of physiological and pathological status in human and animals[27].
Some phytochemical contents from C. procera extracts such as cardenolides and alkaloids were known to have toxic effect[10].Their accrual after repeated dose administration of extracts might affect the thrombopoiesis and produce harmful effects on spleen and liver function. Previous studies reported similar disterbance in biochemical and hematological markers after long term administration of C. procera extract and suggested harmfull effects[28]. In this first part of the study, the purpose was to evaluate the toxicity profile of this plant extracts. These results suggest that both C. procera leaves and C. procera root-barks aqueous extracts could be safe at the single dose level up to 3 000 mg/kg (b.w.), but in case of the repeated dose use, they could have some toxicity effects.
In the second part of this work, four tests were performed to assess behavioral (sedative, anxiety and depressive) effects of the same extracts on animal models. In the Traction test, all treated animals performed normal reestablishment time compared to the control animals. Fireplace test also enables to predict sedative effect. All animals treated with aqueous extracts escaped the apparatus normally. Diazepam is a conventional sedative control, it is known to possess anxiolytic effect[16]. It binds to the benzodiazepine receptors and increase GABA (gammaaminobuturic acid) affinity to GABAA receptors, accentuating the effect of the natural neurotransmitter present in the nervous system[29]. Diazepam administrate at single dose of 3 mg/kg,produced significant sedative effect on mice compared with control or treated groups. The result of these tests reveals that C. procera(leaves and root-barks) aqueous extracts at single doses of 100 or 400 mg/kg (b.w.) produce no potential sedative effect.
In the Hole-board test, significant decrease (P<0.05) of head dips was recorded in the 400 mg/kg (b.w.) leaves extract groups.This result suggests that extracts at these doses induce anxiety effect. Diazepam was used as positive control and produced a light decrease (non-significant) of head dips. This corroborated the low locomotor activity of this group. Indeed, diazepam produces sedative or anxiolitic effect according to where it binds to 1-GABAA orα2-GABAA receptors respectively[30]. It was reported that motor activity decreasing by diazepam in rodents is a valid behavioral manifestation of its sedative properties[31].
In preclinical studies of potential antidepressant properties of substances, the forced swimming test was used to assess emotional status of animals via behavioral despair[17]. When rodents were forced to swim in a confined space, after an initial period of struggling, they would become immobile, resembling a state of despair and mental depression[32]. In the present study, after being treated with C. procera aqueous extracts, the immobility time of animals was not significantly changed (P>0.05) when compared to control group. These results suggest no depressive-like effect of extracts in response. Tramadol is known to be an antagonist of the μ-opioid receptor, inhibiting the reuptake of serotonin and norepinephrine. It possesses the similar mechanism action as the tricyclic antidepressants and considered as an antidepressant[33,34].Used as positive control, tramadol administrates at single dose of 20 mg/kg by oral route produced no significant change in the immobility time. Ostadhadi’s group obtained the same result[35],but other authors observed significant antidepressant effect of tramadol at this dose when administrated intraperitoneally[33,34].The data of these behavioral tests lead to conclude that aqueous extract of C. procera leaves and root-barks at single doses of 100 and 400 mg/kg (b.w.) produce no sedative and depressive-like effect on mice treated, but the leaves extract at 400 mg/kg (b.w.)produced decreased head dipping records.
Despite these interesting results, we could also mention here the limitations of our study which were the lack of histopathological study. This could give more information from extracts effects on the organ’s histology.
This study provides very important data on toxicity and psychotropic profile of the aqueous extracts of C. procera on animal model. Both leaves and root-barks extracts were found to be safe at single dose up to 3 000 mg/kg of body weight by oral route, but their repeated dose uses in chronic treatment could have harmful effects on some organs. Besides, C. procera extracts revealed potential anxiety effect of leaves extract at the dose of 400 mg/kg body weight. This medicinal plant has effective pharmacological potentials in animal model and its use in traditional medicine should be careful. Results in this study demonstrated its real interest for these plants further use. However, specific studies are necessary to assess chemical profile and mechanisms related to the possible effects of these plants extracts.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgements
We thank Angel IROIDIS for language correction.
 Asian Pacific Journal of Tropical Medicine2019年7期
Asian Pacific Journal of Tropical Medicine2019年7期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Disseminated cysticercosis presenting with bilateral proptosis: A case report
- Pouteria campechiana leaf extract and its bioactive compound myricitrin are mosquitocidal against Aedes aegypti and Culex quinquefasciatus
- Impact of seasonality on the prevalence and risk factors of Giardia lamblia infections among the aborigines
- Visceral leishmaniasis among children in an endemic area of northwestern Iran between 2016 and 2017: An epidemiological study
- Leishmaniasis in the Argentine Republic: Temporal and geographical distribution from 2013 to 2017
- Potential of herbal constituents as new natural leads against helminthiasis:A neglected tropical disease
