Pouteria campechiana leaf extract and its bioactive compound myricitrin are mosquitocidal against Aedes aegypti and Culex quinquefasciatus
Raju Sangeetha, Tamilselvan Pratheeba, Chinnasamy Ragavendran, Devarajan Natarajan
Natural Drug Research Laboratory, Department of Biotechnology, Periyar University, Salem-636 011, Tamil Nadu, India
Keywords:Pouteria campechiana Myricitrin Aedes aegypti Culex quinquefasciatus Mosquitocidal
ABSTRACT Objective: To test the mosquitocidal potential of leaf extracts of Pouteria campechiana prepared with different solvents and elucidate the structure of an isolated mosquitocidal compound.Methods: The leaf extracts of Pouteria campechiana prepared with three solvents (petroleum benzene, ethyl acetate and acetone) and potential bioactive fractions were tested against various stages of Aedes aegypti and Culex quinquefasciatus by using the WHO protocols, and the chemical profile and its functional groups were identified by GC-MS and Fourier transmissioninfrared spectroscopy (FT-IR). The structure of bioactive compound was characterized by nuclear magnetic resonance (NMR) spectral technique.Results: The preliminary phytochemical results revealed the presence of alkaloids, amino acids, flavonoids, quinones, saponins, steroids, tannins, and terpenoids in the acetone extract.A significant toxic potential was observed in the acetone extract against both Aedes aegypti and Culex quinquefasciatus mosquitoes. The acetone extract exhibits remarkable larvicidal(LC50: 12.232 μg/mL and LC90: 63.970 μg/mL), pupicidal (LC50: 18.949 μg/mL and LC90: 167.669 μg/mL) and adulticidal (LC50: 20.689 μg/mL and LC90: 72.881 μg/mL) effects against Aedes aegypti.Furthermore, the same extract was subjected to isolation of bioactive compound by GCMS and FT-IR analysis. GC-MS results showed the presence of 5 major compounds, and octacosane (18.440%) was detected as the predominant compound. The FT-IR result of acetone extract demonstrated the presence of various functional groups like alkanes/alkynes, ester,aromatic and amides. The NMR spectrum results of isolated compound were well matched to glycoside linked flavonoids. Based on the chromatography and spectral techniques the isolate molecule was identified as myricitrin by FT-IR and nuclear magnetic resonance spectral data.Conclusion: The isolated compound myricitrin possesses a significant toxic effect in all stages of Aedes aegypti and Culex quinquefasciatus mosquito’s with lowest LC50 and LC90 values.
1. Introduction
Mosquitoes are a major group of insect that creates a number of health problems around the globe. A total of 3 492 species of mosquitoes were recorded worldwide, and more than hundred species are able to transmit various diseases in humans and other vertebrates[1] e.g. malaria, dengue, yellow fever, filariasis and Japanese encephalitis[2]. Mosquito bites cause several allergic responses, including skin problems, urticaria, and angioedema[3].
Mosquito-borne diseases contribute to disease burden, death and social debility in the entire world, especially in tropical and subtropical countries[4].
Aedes (Ae.) aegypti is an important vector for dengue and yellow fever and adapted to the human environment where it mainly breeds in the domestic or peridomestic regions. Over the last decades the number of dengue infections has been increased in the worldwide,with the occurrence of serious outbreaks with dengue haemorrhagic fever[5,6]. Culex (C.) quinquefasciatus is responsible for lymphatic filariasis, which is commonly found in tropical regions and it affects around 120 million people every year[7].
The major problems in the mosquito control are associated with the intensive use of synthetic chemicals that lead to the mosquito resistance, environmental hazards and undesirable effects on nontargeted organisms[8,9]. Approximately 355 000 deaths per year are associated with pesticide poisoning globally[10]. However,the compounds isolated and/or derived from plants possess high mosquitocidal potential without any impact on the environment.Plant extracts and their essential oils are the best sources of many bioactive compounds, including insecticides for control of mosquitoes[11].
Plant products have been utilized traditionally by human communities in worldwide against most of the insect vectors and parasites. Over the past 50 years more than 2 000 plant species have been reported to contain toxic principles against several insects. Thus, pharmaceutical and pesticide companies have spared huge money and time for the isolation of natural compounds for developing new eco-friendly, cost-effective products from plants[12].
In India, several plants are well known for their insecticidal property and also used as natural pesticides/insecticidal agents. Many plant extracts have been tested for its toxic effect on various stages (larvae,pupa and adult) of mosquitoes[13]. More than 1 000 plant species in India are found to possess good insecticidal properties among them 384 contain antifeedants, 297 repellents, 27 attractants and growth inhibitors[14-16]. For example, previously many scientists/researchers who have done the mosquitocidal potential of several plants against target mosquitoes viz. Clausena anisata[17], Curcuma and other species[18], Carum copticum[19], Eclipta alba[20], Clausena dentata[21], Gliricidia sepium[22] and Azardirachta indica[23].
Phytochemical screening, isolation and identification of bioactive secondary metabolites from plant source for the discovery of new,potent and lead insecticidal compounds for mosquito control[12].Pouteria belongs to the family of Sapotaceae, which is commonly found in the globe. Several species of Pouteria have been used in folk medicine for treating inflammation, skin eruptions, ulcers and back pain[24]. Pouteria (P.) campechiana seeds are used in folk medicine to treat ulcers[25] and possess many biological activities like anti-inflammatory[26], anti-mitotic activity[27], antioxidant and hepatoprotective effects[28]. To the best of our knowledge, there are no reports on the mosquitocidal potential of P. campechiana.Therefore, the present study was aimed to investigate the larvicidal,pupicidal and adulticidal efficacy of P. campechiana extracts and isolated its bioactive principle against Ae. aegypti and Cx.quinquefasciatus.
2. Materials and methods
2.1. Solvents and reagents used
All the analytical reagents and solvents were used in this experiment was procured from Merck, Germany. Pre-coated TLC silica gel plates and HPLC grade solvents were obtained from Hi-Media (Mumbai, India).
2.1.1. Collection and extraction of plant material
Healthy, fresh leaves of P. campechiana (PU/BT/NDRL/03/2018)were collected from Munnar, Kerala, India. The plant specimens were deposited in the Natural Drug Research Laboratory,Department of Biotechnology, Periyar University, Salem,India,as voucher no-01 (2018). The collected plant was identified by a botanist, Dr. Pushpa Kumara, ICRAF-Country Liaison Scientist,Department of Crop Science, University of Peradeniya, Sri Lanka.The collected plant material was washed thoroughly in running tap water and dried at room temperature. Dried plant material was powdered using an electrical blender. The powdered plant material was extracted successively using three different organic solvents namely, petroleum benzene, ethyl acetate and acetone in a Soxhlet apparatus (for 9 h). All extracts were filtered through Whatman no.1 filter paper and the residual solvents were evaporated at room temperature. The dried extracts were weighed and stored at 4 ℃ for further study.
2.2. Collection of the test organisms
The larvae of Ae. aegypti and Cx. quinquefasciatus were collected from the Botanical garden of Periyar University, Salem, Tamil Nadu and India. The collected larvae were identified by a zoologist Dr.M.S. Shivakumar, Assistant Professor, Department of Biotechnology,Periyar University, Salem, Tamil Nadu, India and cross-referred with available books[29]. The larvae were reared in a plastic tray (24 cm×35 cm×5 cm) containing dechlorinated tap water and maintained at(25±2) ℃, with 70%-80% of relative humidity and 16:8h of light and dark photoperiods. The larvae were fed with a powdered feed contains dog biscuits and baker’s yeast (3:1 ratio). The pupae of each mosquito was cultured in a separate container covered with mosqui to net cages [(26±2) ℃, (85±3)% RH, 16:8 h (L: D) photoperiod] for the purpose of emerging adults.
2.2.1. Larvicidal and pupicidal bioassay
The larvicidal and pupicidal activity of plant crude extracts and isolated fractions were assessed by the standard WHO protocol[30].The desired concentrations of plant extracts and isolated fractions were dissolved in 1 mL of 10% DMSO for its solubility in water.From the stock solutions (1 mg/mL) different test concentrations(viz., 200, 400, 600, 800 and 1 000 μg/mL for extracts and 200, 400,600, 800 and 1 000 μg/mL for isolated fraction) were prepared and tested against target mosquitoes. The larvae and pupae of selected mosquito species (n=25) were introduced in 500 mL enamel tray containing 250 mL of distilled water and 1 mL of plant extracts/isolated fractions. About 1 mL of 10% DMSO was served as a negative control. The mortality rate was recorded (after 24 h and 48 h of post-treatment) and corrected with Abbott’s formula. All the experiments were conducted at room temperature [(28±2) ℃] and five replicates were tested at a time.
2.2.2. Adulticidal activity
The adulticidal potential of plant extracts and isolated fractions were performed as per the modified procedure of WHO[30]. The adulticidal bioassays of samples (crude and fraction) were performed in the test tubes (at horizontal position). The test tubes contains desired concentrations of extracts and isolated fractions (200, 400,600, 800 and 1 000 μg/mL), as well as the carrier solvent alone used as a control. The adult mosquitoes (n=15) were gently introduced into a glass holding tube for 30 min, rearing and transferred into the coated test tubes. The number of dead and/or alive mosquitoes was recorded after 24 h of treatment and the mortality rate was corrected using Abbott’s formula. All the bioassays were carried out in triplicates along with control providing ambient environmental [at room temperature with suitable relative humidity i.e. (80±2)%].
2.3. GC-MS analysis
The acetone extract was chosen for the isolation of the bioactive compound(s) and further analysis like GC-MS and Fourier transmission-infrared spectroscopy (FT-IR). GC-MS analysis of acetone extract was carried out in a GC Clarus 500 Perkin Elmer system comprised with an AOC-20i auto-sampler and gas chromatograph interfaced to a mass spectrometer with the following GC instrument conditions. GC column - Elite-1 fused with silica capillary (30 m×0.25 mm ID×1 μ Mdf, composed of 100%Dimethyl polysiloxane); mass spectrometer operated in electron impact mode at 70 eV; Helium gas (99.99%) was used as carrier gas at a constant flow of 1 mL /min and 0.5 μL of sample injected (split ratio of 10:1) with suitable injector (250 ℃) and ion-source temperatures (280 ℃). The oven temperature was programmed from 110 ℃ (isothermal for 2 min), with an increase of 10 ℃/min,to 200 ℃, then 5 ℃/min to 280 ℃, ending with a 9 min isothermal at 280 ℃. Mass spectra were taken at 70eV with a scan interval of 0.5 s and fragments from 40 to 450 Da and total running time of GC is 36 min. The relative percentage amount of each component was calculated by comparing its average peak area to the total areas. The comparison mass spectra of identified compounds in the chromatogram was done by adopting Turbomass Ver. 5.2.0 software.
2.4. FT-IR analysis
FT-IR is a valuable tool for the identification of functional groups of the plant molecules. Acetone extract of P. campechiana and the isolated fraction was subjected to FT-IR analysis. About 10 mg of the sample was encapsulated in 100 mg of KBr pellet, in order to prepare translucent sample discs. The sample disc was loaded in FTIR spectroscopy and scanned (range between 400 to 4 000 cm-1) with a resolution of 4 cm-1.
2.5. TLC analysis
TLC profile of plant samples was carried out as per the standard protocol. The acetone extract and isolated fractions were applied as a spot about (1 cm) above the edge of TLC (pre-coated silica gel F254, Merck, Germany) plate of the capillary tube. The TLC plate was placed in a closed container saturated with vapors of desired developing solvents (i.e. 7.1: 2.9-Chloroform: Methanol for extract and 7.1: 2.9-Chloroform: Methanol for isolated fraction) as a mobile phase. The well developed spots of TLC plates were examined under ultraviolet chamber (254 nm) and the Rf values were calculated.
2.6. Column chromatography of isolated fraction
The bioactive compounds from the acetone extract were isolated by column chromatography technique. Acetone extract of P.campechiana (5 g) was eluted through a 50 g of silica gel (60-120 mesh) column (60 × 4 cm) with mixtures of chloroform/methanol(7.1:2.9) to yield fractions (F). The individual fraction was monitored by TLC for its purity. The residual solvents of isolated fractions were evaporated at room temperature and the concentrated fractions were stored at 4 ℃ for further spectral analysis.
2.7. Nuclear magnetic resonance (NMR) spectroscopy
The structure of isolated fraction containing bioactive compound was determined using NMR spectroscopy. The proton (1H) and carbon (13C) NMR spectral study investigation was performed to elucidate the structure of the isolated compound. The pure fraction(10 mg) was dissolved in 1 mL of deuterated solvent. The NMR spectra of the isolated compound containing fracton were recorded in Bruker AV-600 NMR instrument. The spectral data were analyzed and compared with published literature in order to derive the structure of the isolated compound.
2.8. Statistical analysis
The larval mortality data were subjected to Probit analysis[31] to calculate the LC50and LC90, fiducial limits of upper confidence limit(UCL) and lower confidence limit (LCL) and chi-square and degree of freedom values by SPSS software version-16.0.
3. Results
3.1. Preliminary phytochemical screening
Preliminary phytochemical analysis of the sample showed the presence of alkaloids, flavanoid, phenols, quinones, saponins,steroids, tannins and aminoacid, terpenoids which were absent in acetone extract. The alkaloids were present in the ethyl acetate extract, but the flavanoids, phenols, quinones, saponins, steroids,tannins, aminoacids and terpenoids were absent. The presence of alkaloids and steroids was noticed in the petroleum benzene extract and the remaining chemicals like aminoacids, flavonoids, phenols,quinones, saponins, tannins and terpenoids were absent (Table 1).
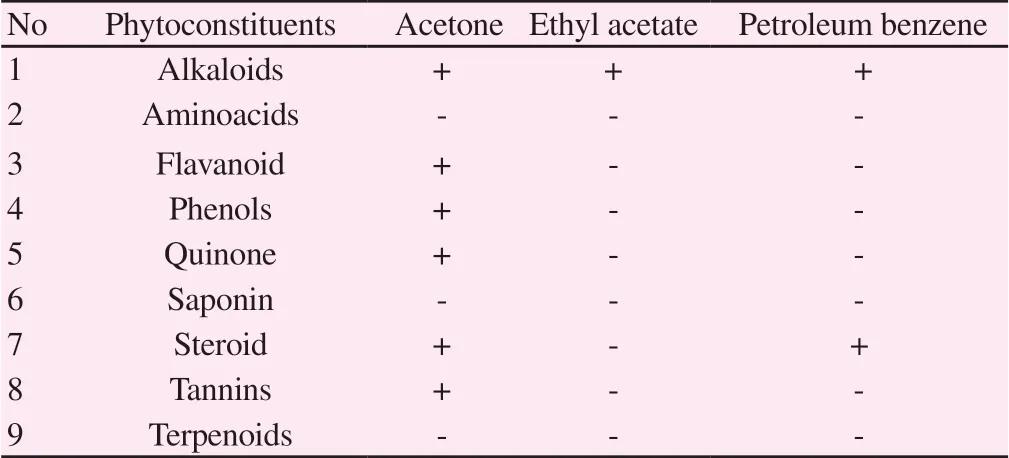
Table 1. Phytoconstituents of Pouteria campechiana extract using different solvent.
3.2. Larvicidal activity
The larval toxicity of different solvents crude extracts of P.campechiana was tested against 4th instar larvae of Ae. aegypti and Cx. quinquefasciatus. The obtained LC50, LC90values of different solvents extract tested against targeted mosquitoes after 24 and 48 h post treatment were shown in Table 2. The acetone extract expressed the good larval mortality against Ae. aegypti [LC50=15.161 and LC90=144.175 μg/mL, (after 24 h) and LC50=12.232 and LC90=63.970 μg/mL, (after 48 h), followed by Cx. quinquefasciatus LC50=33.778 and LC90=242.333 μg/mL, (after 24 h) and LC50=14.212 and LC90=187.648 μg/mL, (after 48 h)] respectively. The isolated fraction of acetone extract showed moderate larvicidal activity against tested mosquitoes, while compared to crude extracts. The lethal concentration of LC50=43.023 and LC90=243.537 μg/mL in Ae. aegypti and LC50=76.129 and LC90=499.822 μg/mL in Cx.quinquefasciatus (after 24 h). Similarly, after 48 h treatment showed LC50=20.555 and LC90=127.468 μg/mL in Ae. aegypti and LC50=51.068 and LC90=287.265 μg/mL (Table 3).
3.3. Pupicidal bioassay
The acetone, ethyl acetate and petroleum benzene extracts from P. campechiana leaves were tested against Ae. aegypti and Cx.quinquefasciatus pupae at 24h post treatment. The good mortality rate was observed in the acetone extract when compare with other extracts. Acetone crude extracts have low LC50and LC90values (LC50=51.735 and LC90=400.122 μg/mL in Ae. aegypti and LC50=65.004 and LC90=530.811 μg/mL in Cx. quinquefasciatus after 24 h; LC50=18.949 and LC90=167.669 μg/mL in Ae. aegypti and LC50=38.125 and LC90=337.437 μg μg/mL in Cx. quinquefasciatus after 48 h(Table 2). The tested fraction of acetone fraction showed LC50and LC90values against Ae. aegypti (LC50=88.044 and LC90=536.880 μg/mL for 24 h treatment; LC50=47.190 and LC90=355.178 μg/mL for 48 h treatment) and Cx. quinquefasciatus ((LC50=100.407 and LC90=599.240 μg/mL), (LC50=65.190 and LC90=449.762 μg/mL)) in 24 h and 48 h (Table 3).
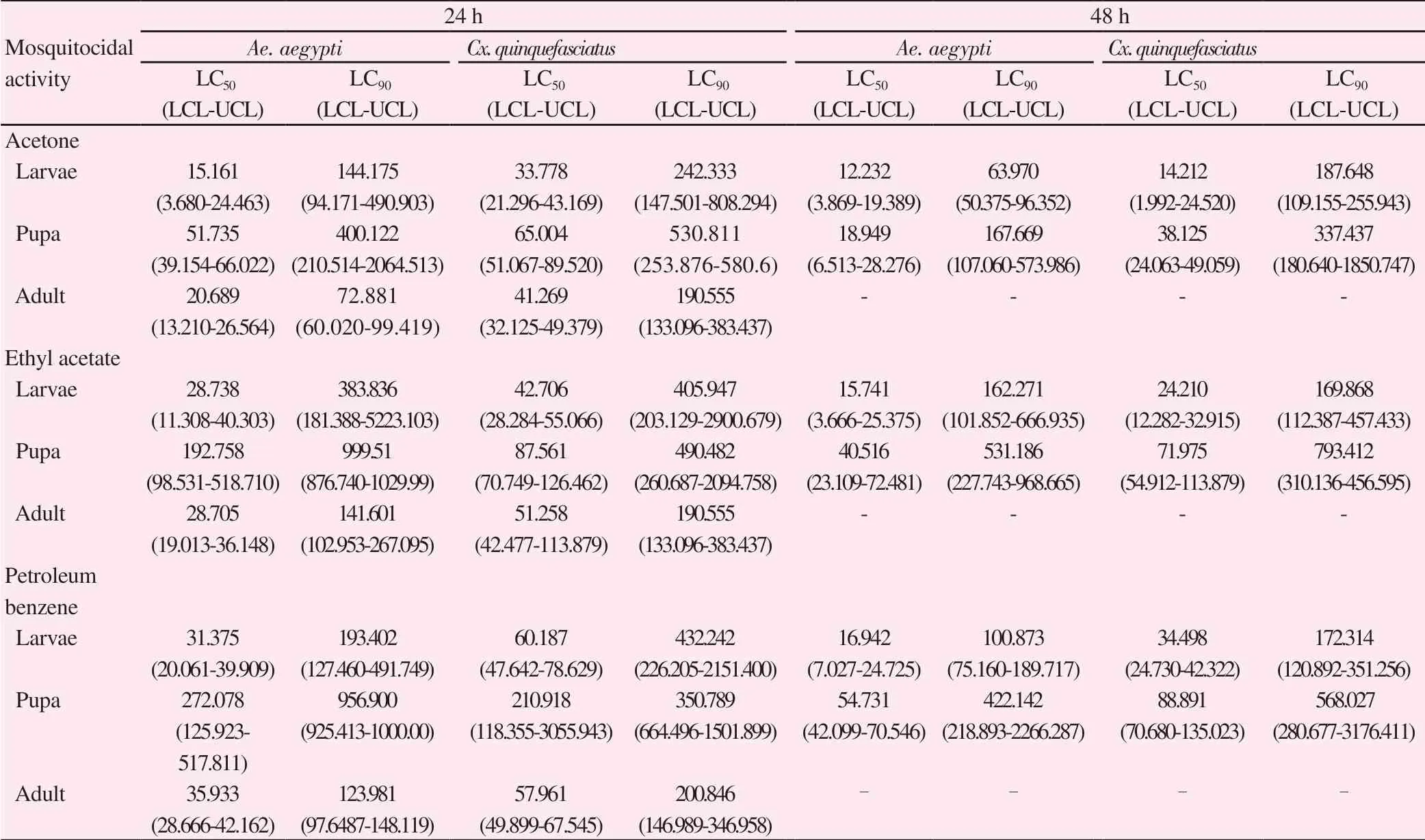
Table 2. Larvicidal, pupicidal and adulticidal activity of Pouteria campechiana against Aedes aegypti and Culex quinquefasciatus (after 24-48 h) μg/mL.
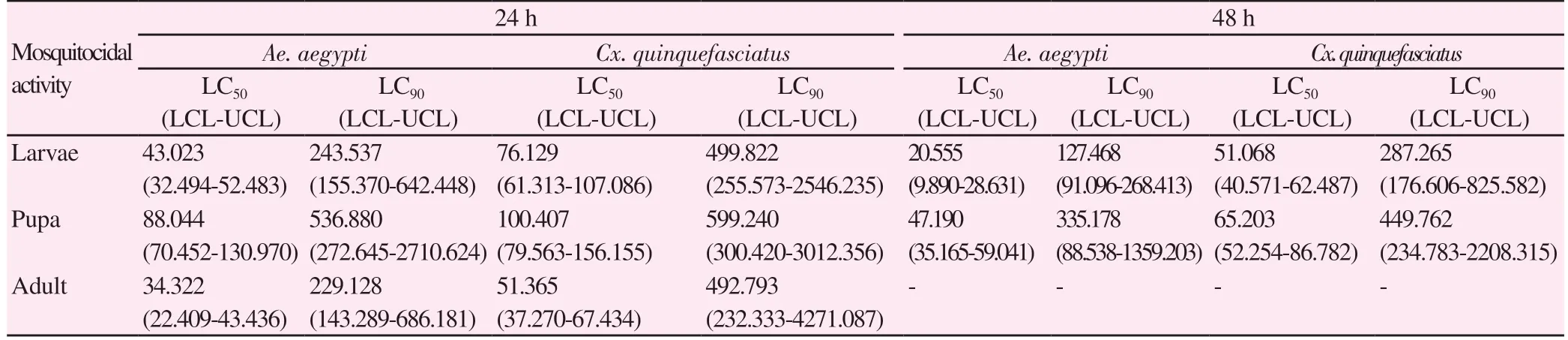
Table 3. Mosquitocidal potency of myricitrin from Pouteria campechiana acetone extracts against Aedes aegypti and Culex quinquefasciatus (μg/mL).
3.4. Adulticidal activity
All extract shows varied levels of adulticidal activity against target mosquitoes. After 24 h exposure, the petroleum benzene extract of P. campechina shows the lowest activity with LC50and LC90values of Ae. aegypti (35.933 and 123.981 μg/mL, after 24 h) and Cx.quinquefasciatus (57.961 and 200.846 μg/mL, after 24 h). Ethyl acetate extract reflects moderate activity with the LC50and LC90values of Ae.aegypti (28.705 and 141.601 μg/mL, after 24 h) and Cx. quinquefasciatus(51.258 and 190.555 μg/mL, after 24 h). While, the leaf acetone extract shows maximum adulticidal effect against Ae. aegypti with the best LC50and LC90values (20.689 and 72.881 μg/mL, after 24 h) (Table 2).The isolated fraction of acetone extract reported adulticidal activity of Ae. aegypti (LC50=34.322 and LC90=229.128 μg/mL) and Cx.quinquefasciatus (LC50=51.365 and LC90=492.793 μg/mL) after 24 h(Table 3).
3.5. GC-MS analysis results
Totally 51 chemical constituents were identified from the acetone extract of P. campechiana by GC-MS. Among them, 5 peaks were noticed after comparison of the mass spectra with WILEY and NIST libraries and identified as follows, Octacosane (18.440%),Hexatriacontane (9.435%), Sulfurous acid pentadecyl2-propylester(6.837%), D1-N-Octyl phthalate (10.377%) and Tetracosane(4.394%), respectively (Table 4 and supplementary Figure 1).
3.6. FT-IR analysis results
In the FT-IR spectrum of acetone extract of P. campechaina reflected nine functional groups. The peak at 2 925.70 cm-1could be attributed to alkanes/alkynes group with C-H stretching vibration.The wavelength of 1 722.85 cm-1could be shown to esters, aromatic group with C=O stretching vibration, 1 601.03 cm-1could be reflected to amides group with N-H bens stretching, 1 501.65 cm-1could be attributed to aromatic compounds group with Ring C=C stretching vibration. The peak at 1 461.05 cm-1indicates alkanes/alkynes with C-H bend stretching and 1 332.05 cm-1interpreted to alkyl halides with C-F stretching vibration, 1 267.06 cm-1could be attributed to ethers with ethers stretching vibration (C-O-C), 1 056.14 cm-1could be interpreted to alcohol with C=O stretching vibration and peak at, 821.08 cm-1could be corresponds to aromatic compounds with C-H bend stretch (Supplementary Figure 2).
3.7. Column chromatography
Column chromatography was performed from the acetone extract of P. campechiana using the solvent system of chloroform/methanol in the ratio of 70.1:29.9 resulted two active fractions and it can be separated based on the extract ingredients resolution. The Rf value of each fraction was calculated and marked as Band-1: 0.83 and Band-2: 0.85 respectively.
3.8. 1H-NMR spectrum
The1H-NMR spectrum of isolated fraction from acetone extract has revealed the number of protons in the isolated compound(Supplementary Figure 3). TheδH values at 7.73, 7.70, 7.03 and 6.98 ppm of four singlet peaks revealed the aromatic protons. The peaks atδH 3.98, 3.96, 3.92, 3.91 and 3.87 ppm noticed, -CH and-CH2alkane protons. BesidesδH 1.34 singlet peak has revealed the-CH3proton and evident of this peak confirmed the glycoside group is attached with the isolated compound. The OH protons of isolated compound would be exchanged with DMSO-D6solvent therefore hydroxyl proton peaks are not appeared in their selected chemical shift. Based on the screening test, FT-IR and1H-NMR spectrum data of the isolated compound was assumed as glycoside linked flavonoid compound and the spectral data is well matched with ‘Myricitrin’compound.
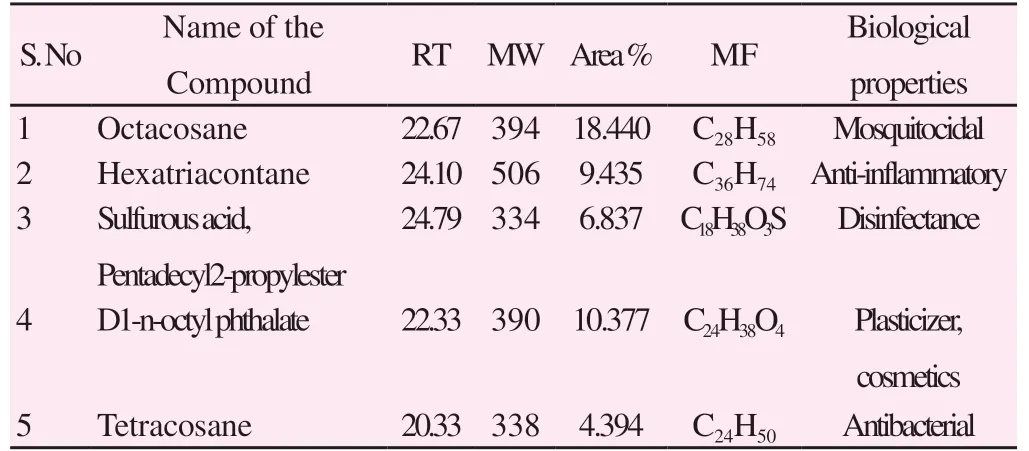
Table 4. GC-MS analysis of acetone extract from Pouteria campechiana.
3.9. 13C-NMR spectrum
The isolated bioactive compound from P. campechiana was subjected to13C-NMR analysis revealed the number of carbon and helpful to predict the carbon skeleton structure of isolated compound(Supplementary Figure 4). The carbon chemical shift of isolated compound atδH values 29.57, 55.95, 59.81, 61.44, 62.09 ppm shows aliphatic methyl (-CH3) and methylene (-CH2) carbon which is the strong evidence for isolated compound as glycoside containing flavonoid compound. The chemical shift at 95.92 and 111.13 ppm,indicates the aromatic carbons. And notably, the chemical shift at 173.57 ppm represented the carbonyl carbon (C=O) 73, 7.70, 7.03 and 6.98 ppm of four singlet peaks shown the aromatic protons.The chemical shift at 112.94 ppm revealed the linkage carbon of glycoside and flavonoid ring and 148.57 and 153.43 ppm shows that carbon of A and C hydroxyl group containing carbon. Additionally,the chemical shift at 150.83 indicates the B and C linked carbon(C-O-C) of isolated carbon. Based on the above, the13C-NMR spectrum of isolated compound was strictly matched with the compound Myricitrin (Figure 1).
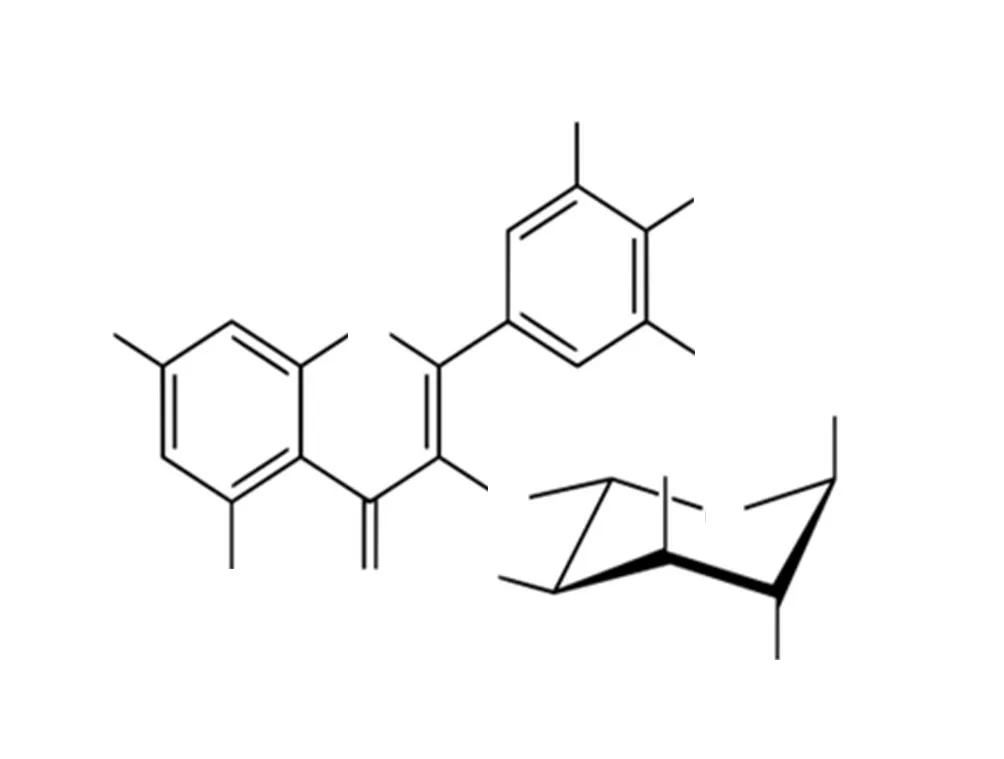
Figure 1. Structure of myricitrin isolated from acetone extract.
3.10. FT-IR spectrum of myricitrin
FT-IR spectrum analysis of the isolated compound confirmed the presence of various functional groups in the wave length ranged from 4 000-400 cm-1by Bruker instrument. The sharp peak at 3 776 cm-1indicates the -OH group of phenolic compound, which means the isolated compound may contain phenolic ring. The peaks in the range of 3 262-2 361 cm-1corresponding to C-H stretching vibration.Peaks at 1 954 and 1 632 cm-1expressed the presence C=O and C=C group. The short peak at 1 384 cm-1revealed the stretching of C-C alkane group presence. Moreover, the broad peak at 1 106 cm-1shows the C-O-C group, which means the isolated compound may contain glycoside moiety. From the result of FT-IR spectrum of the isolated compound may consists of glycoside linkage with flavonoid compound.
4. Discussion
Plants source of innumerable therapeutic agents are important to the health care of individual as well as communities. Secondary metabolites from plants can be acted as protective agents against microorganisms, predator insects and natural candidates for the discovery of new products to combat against mosquito borne diseases. Several studies have been focused on natural products for controlling mosquitoes as insecticides and larvicides[32,20].
In the present study, preliminary phytochemical analysis of different extracts from P. campachiana was found to occurrence of alkalioids, flavanoids, phenols, quinones, saponins, steroids, tannins in the acetone extracts. Previously, Mehraj et al.[33] reported the P. campechiana fruit extract showed the presence of all chemical constituents. Similarly, Ramanji et al.[34] identified the presence of alkaloids, carbohydrates, tannins, flavonoids, saponins, phytosterols,mucilages in P. campechiana plant extract. Previously, several plant species viz., Pouteria sapota[35]; Pouteria ramiflora[36]; Ocimum gratissimum[37] reported to contain various phytochemicals.
The outcome of present study revealed the mosquitocidal property(larvicidal and pupicidal) of three different solvents extracts obtained from P. campechiana against Ae. aegypti and Cx. quinquefasciatus mosquito vectors. The highest mortality was observed in acetone extract. Similarly, Mukandiwa et al.[17] investigated the acetone,dichloromethane and hexane leaf extract of Clausena anisata acted as a potential larvicidal agent against Ae. aegypti. Rajasekaran and Duraikannan[38], observed the highest mortality in the petroleum ether extract of several medicinal plants against 4th instar larvae Ae.aegypti. Similarly, Govindarajan[39] reported the Sida acuta plant shows strongest repellent action against three mosquitoes. Kamaraj and Rahuman[40] studied the larvicidal and pupicidal activity of few plants against Cx. quinquefasciatus and Cx. gelidus. On the other hand, Bagavan and Rahuman[41] investigated the mosquitocidal effect on ethyl acetate extract of Ae. aspera, against target mosquito.
Botanical derived secondary metabolites/molecules have toxic larvae, pupae and adult mosquitoes. The plant derived secondary metabolites, essential oils, bioactive compounds and nanoparticles are highly toxic to insects[42]. Earlier, many researchers reported that the toxicity effect on larvae and pupae might be linked to the ability of plant derived molecules to penetrate into the outer layer of larvae and pupa. In the cell membrane space, molecules can bind to sulfur in proteins or phosphorus in the DNA, which might lead to denaturation of cells, organelles and metabolites are entering into cell membrane permeability and disturbed to proton motive force that may cause loss of cell organelle function and finally death of treated mosquitoes[43].
The gas chromatography and mass spectroscopic analysis of P.campechiana acetone extract reflected the presence of 5 major peaks. The mosquitocidal activity of Octocosane from Moschosma polystachyum, 7 bioactive compounds ((+)-gallocatechin,(+)-catechin, (-)-epicatechin, dihydromyricetin) from P.campechiana, P. sapota and P. viridis, 3 bioactive compounds(Friedeli, Epi-friedelanol and Taraxerol) from Pouteria ramiflora have been reported by Ma et al[44].
The functional groups of chemical compounds were identified by FT-IR analysis, and the results revealed the presence of major peaks corresponding to alkanes/alkynes (C-Hs), esters and aromatic(C=Os), amides (N-Hb), aromatic compounds (Ring C=Cs), alkanes/alkynes (C-Hb), alkylhalides (C-Fs), ethers (C-O-C), alcohol (C=Os),aromatic compounds (C-Hb). Previously, Likewise, Rodrigues et al[45] studied the FT-IR analysis of chloroform extract of Ocimum gratissimum revealed the presence of major amines, imines (N - Hstr), alkanes (-CH3), disubstituted alkenes (R1 CH=CHR2), Nitrates(O-NO2 v) stretching, wagging, bending of oxygenated bonding(O-H), C-N stretching alcohols, carboxylic acids, ethers and esters.
The acetone extract from the leaves of P. campechiana was subjected to TLC and column chromatography yielded 7 fractions and further fraction 2 from CC used to the isolation of compound.The structure of isolated bioactive compound was derived by1HNMR and13C-NMR spectral analysis. Based on the spectral data,the isolated compound was identified as ‘myricitrin’. Likewise,Elsayed et al.[26]; Hernandez et al.[27] and Ma et al.[44] identified several bioactive compounds, including myricetin (3-O-α rhamnopyranoside) from the same plant. The overall results from study suggest that bioactive compound from the acetone extracts of P. campechiana contains insecticidal property and may be used as potential candidature for control of Cx. quinquefasciatus and Ae.aegypti mosquito vectors in near future.
Conflict of interest statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
We would like to thank the Department of Biotechnology, Periyar University, Salem, for providing the infrastructural facility for carrying out this research work and we also thank the St. Joseph’s College, Trichy for FT-IR and Vellore Institute of Technology,Vellore for GC-MS analyses.
 Asian Pacific Journal of Tropical Medicine2019年7期
Asian Pacific Journal of Tropical Medicine2019年7期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Potential of herbal constituents as new natural leads against helminthiasis:A neglected tropical disease
- Leishmaniasis in the Argentine Republic: Temporal and geographical distribution from 2013 to 2017
- Visceral leishmaniasis among children in an endemic area of northwestern Iran between 2016 and 2017: An epidemiological study
- Impact of seasonality on the prevalence and risk factors of Giardia lamblia infections among the aborigines
- Toxicological characterization and central nervous system effects of Calotropis procera Ait. aqueous extracts in mice
- Disseminated cysticercosis presenting with bilateral proptosis: A case report
