Seroprevalence of Cryptosporidium and risks of cryptosporidiosis in residents of Sothern Egypt: A cross-sectional study
Ismail Elshahawy, Fatma AbouElenien
1Department of Parasitology, Faculty of Veterinary Medicine, South Valley University, Egypt
2Department of Hygiene and Preventive Medicine, Kafrelsheikh University, Egypt
Keywords:Cryptosporidium Epidemiology ELISA test Risk factors Serum Egypt
ABSTRACT Objective: To identify the serological epidemiology of Cryptosporidium infections and to follow up on the changes in the infection profile in Southern Egypt in order to establish a suitable scheme for control and prevention of cryptosporidiosis.Methods: A total of 1 912 (960 from human and 952 from animals) stool specimens and sera were screened for Cryptosporidium species using modified Ziehl Neelsen technique and a newly-developed enzyme-linked immunosorbent assay (ELISA). Environmental risk factors and socioeconomic data were surveyed by questionnaire between September 2016 and December 2017.Results: Totally, 20.83% of the human subjects were positive for Cryptosporidium infection tested by ELISA. The seropositivity was positively correlated with age. The prevalence of Cryptosporidium infections in females was significantly higher than in males (P<0.05).The sensitivity and specificity of ELISA for Cryptosporidium were 99.06% and 88.88%,respectively. Furthermore, a high prevalence of Cryptosporidium in domestic animals (42.20%).Conclusions: The study observed that Cryptosporidium infections are common in the study area, with water sanitation, socioeconomic level; eating habits and hygienic status are considered the main risk factors for cryptosporidiosis. Therefore, environmental sanitation and health education will be useful in reducing the prevalence of infection.
1. Introduction
Cryptosporidium spp. are anthropozoonotic parasites which are directly transmitted via person-person (anthroponotic) and animalperson (zoonotic) interactions, and indirectly transmitted via the faecal-oral route by ingesting water or food contaminated with oocysts[1]. Cryptosporidium parvum and Cryptosporidium hominis are considered as the most common species in human and accounted for more than 90% of Cryptosporidium infections[2].
There are several techniques used in diagnosis and identification of Cryptosporidium species in the stool, which are all timeconsuming, cost-effective and require significant experience. The most commonly used methods employ concentration steps, usually,formalin-ethyl acetate, followed by staining procedures that include modified acid-fast stain[3].
Several conventional immunodiagnostic techniques incorporating antibodies against Cryptosporidium infection have been developed.ELISA is considered as the simplest, rapid and least labour intensive one, which can be done by relatively unskilled operators with little training.
Recently, several molecular methods targeting various genes,proteins and glycoproteins have been developed for identifying Cryptosporidium genotypes using polymerase chain reaction (PCR)[1].Although PCR has been shown to be a more accurate and highly sensitive diagnostic tool. Unfortunately, they are not yet in routine diagnostic use in low-income countries because of the high cost and the shortage of reagents[3].
Several global studies have considered the prevalence of Cryptosporidium infection in both developed and developing countries with special reference to the role of animal and climate in the dynamics of infection[4,5].
In the Nile River Delta, Egypt, Cryptosporidium has been recognized as a widespread and contagious agent of childhood diarrhoea[6],with limited studies on Cryptosporidium as anthropozoonotic parasite[7]. Previous studies have demonstrated big variations in the prevalence of cryptosporidiosis between 0% and 47% among immunocompetent individuals with diarrhoea in Egypt[8]. However,to the best of our knowledge, no report is available on the prevalence of Cryptosporidium spp. infection in the Southern region of Egypt.
Therefore, the purpose of the present study was to identify the risk factors associated with Cryptosporidium infections and to evaluate the diagnostic performance of the techniques used.
2. Materials and methods
2.1. Study area and participants
This one-year cross-sectional study was carried out from September 2016 to December 2017 at Qena Governorate, Southern Egypt.Qena located between 26°10′12″ North and 32°43′38″ East in the Southern part of Egypt. It lies on 165 km stretch of the Nile Valley,between Luxor and Sohag governorates, with a population of 2.5 million people. It is home to 152 villages of which 59 are among Egypt’s 1 000 poorest villages identified by Egypt’s Poverty Map[9].Qena is distributed across nine districts with 41 central villages and 111 smaller villages.
Qena region has a hot desert climate, and the hottest months in July and August see an average temperature of 39.9 ℃ and the lowest temperature recorded in January of 21 ℃ with a big difference in temperature between day and night (temperature difference may reach 16 ℃).
Four regions, two urban (Dishna and Nag Hammadi) and two rural cases (Abu Tesht and Qift), were selected for this study based on the level of the community, as the poverty rate of the regions chosen is the highest among Southern Egypt, specifically in rural areas (51.5%) followed by urban areas (29.4%). The population density per km2is 2 276 in rural areas. The residents are engaged in husbandry, and most of the livestock are housed within human houses.
2.2. Questionnaire
Before specimen collection, a questionnaire was developed and filled with direct interviews through household visiting. The personal information form provided personal details of the patient such as name, sex, age, place of residence, and education level. The questionnaire, on the other hand, sought information relating to personal hygiene habits that prompted a hospital visit, risk factors such as the source of water, washing hands before meals, washing before eating raw fruits and raw vegetables and after defecation.Information about livestock was collected in the participant house and their clinical signs. Each person was informed of the purpose of the study and the need for repeated stool collections, and then informed consent was obtained.
2.3. Sampling
Single blood (10 mL) and fecal samples were taken from each interviewed participant (n=960) under aseptic conditions. Sera were separated after sedimentation of blood cells and were kept at 20 ℃ and transported to the parasitology laboratory, South Valley University. Besides, fecal smears were concentrated from each sample using Sheather’s sugar floatation technique[10] and stained by a modified Ziehl-Neelsen procedure[11].
2.4. Enzyme-linked immunosorbent assay (ELISA)
A commercially available ELISA Kit (My-BioSource, Inc., San Diego, USA, MBS047372) is an enzyme immunoassay based on the detection of anti-Cryptosporidium antibodies (IgG) in the serum specimen. The test, which was carried out following the kit manufacturer’s protocol [intra-assay CV (%) and Inter-assay CV (%)are less than 15%].
2.5. Animal samples
Fecal samples of livestock nurtured were collected from 4 districts namely, Abu Tesht, Dishna, Nag Hammadi and Qift to examine the presence/absence of cryptosporidiosis. Among the 952 samples collected, 352 were sheep, 352 goats, and 248 were camels. Thin fecal smears were made, air dried, fixed and, stained by modified Ziehl-Neelsen procedure[11].
2.6. Statistical analysis
Chi-square test (SPSS statistical package version 20.0; IBM SPSS Institute, Inc., USA) was used to evaluate significant differences in the seroprevalence positive rates by different epidemiologic risk factors and P<0.05 was considered statistically significant.Furthermore, the sensitivity and specificity of microscopic examination and the detection of anti-Cryptosporidium antibodies in the serum samples from subjects were compared due to the absence of a standard method[12].
2.7. Ethical statement
The study suggestion was accepted by the Research Ethics Committee of the University (No. SVU100) and informed written consent was obtained from the subjects for blood and stool sampling.
3. Results
3.1. Prevalence of infection among participants
Of all 960 stool specimens screened by modified Ziehl-Neelsen(ZN) microscopy, Cryptosporidium oocysts were detected in 105(10.93%) samples.
Anti-Cryptosporidium antibodies using ELISA test were detected in the sera of 200 (20.83%) out of 960 participants. There was a significant difference (P<0.01) between positive and negative results detected by microscopy and ELISA methods.
Considering the age of participants, the people less than 6 years old had the highest percentage of positive results using ELISA(37.60%), followed by those of 20-30 years (24.30%), 7-19 years(23.40%), and 31-40 years (11.30%), while the lowest value was recorded in participants over 40 years old (7.70%). On the basis of statistical analysis, a significant difference (OR=7.19, 95% CI: 3.88-13.3, OR=3.83, 95% CI: 2.02-7.27, OR=3.60, 95% CI: 1.90-6.97 and OR=1.52, 95% CI: 0.76-3.02, P<0.01) was recorded among different age groups. The infection percentage in female (27.10%)was significantly (χ2=22.736 8, P<0.01) higher than their male counterparts (14.58%) (Table 1).

Table 1. Univariable analyses for risk factors associated with Cryptosporidium seropositivity.
The rural areas had shown significantly higher (χ2=10.105 3,P<0.01) seropositivity (25.00% vs.16.70%) as compared with that in urban areas.
In respect to educational level, the obtained results demonstrated significant higher (χ2=68.72, P<0.01) seroprevalence among participants with no formal education level (35.20%) than those of higher education. Likewise, there were significant differences in epidemiologic parameters between Cryptosporidium- infected cases with clinical signs like watery diarrhea (26.70%), abdominal pain(15.80%), fever (14.30%) and bloody diarrhea (5.00%) (χ2=35.901 3,P<0.01, Table 1).
The prevalence of positive samples was higher in participants using raw surface water (32.96%) than groundwater (13.48%).
As a result of the seasonal dynamic of cryptosporidiosis in Qena governorate, the Cryptosporidium seropositive rate was lower during summer (11.80%) than in the winter (36.80%), spring (24.00%) and autumn seasons (15.70%), with obvious seasonal significant variance(χ2=28.078 8, P<0.01).
3.2. Risk factors of being anti-Cryptosporidium antibodies seropositive
The results of the univariable analysis for risk factors associated with Cryptosporidium infection are summarized in Table 1. These comparative analyses showed that the following factors were freely concomitant with the higher prevalence of cryptosporidiosis: keeping livestock, the source of water, washing fruits and raw vegetables and marital status. Furthermore, significant associations were also observed between Cryptosporidium infections and hygienic habits including washing hands before meals, level of hygiene, keeping livestock within human houses and contact with soil-contaminated with fecal matter. However, an insignificant relationship was detected between Cryptosporidium seropositivity and the habit of washing of hands after defecation (χ2=0.004, P=0.95).
3.3. Prevalence of Cryptosporidium infection among examined livestock
The prevalence of Cryptosporidium infection among the studied animal at different sampling regions was 53.4% in Abu Tesht, 37.4%in Dishna, 32.8% in Nag Hammadi and 45.4% in Qift districts.The current study proved that the infection rate was significantly(χ2=81.48, P<0.01) more prevalent among goats (57.1%) as compared to other animals (Table 2).
3.4. Diagnostic achievement of the tested procedures
Taking the modified ZN-stained microscopy test results as the gold standard, the newly-developed ELISA test showed sensitivity,specificity, positive predictive value, and negative predictive value of 99.06%, 88.88%, 52.50%, and 99.87%, respectively (Table 3).Likewise, a good level of similarity between the used techniques was found.
4. Discussion
The present investigation revealed that 10.93% of the diagnosed participants were positive for Cryptosporidium infection by using stool analysis. The literature on microscopy recorded that the prevalence of Cryptosporidium infection in Egyptian patients varied significantly from 0% to 47%[8]. The broad range in prevalence could be most likely to explain by many causes such as the immune state of the patients, environmental factors, personal habitats or seasonal variation[13]. Additionally, the present findings were higher than previous investigation carried out in Cairo (Egypt) (5.88%)indicating that Cryptosporidium infection is an important public health problem, especially in southern Egypt as it is inked to living standard and poverty[14]. In contrast, the present results were much lower than previous studies carried out in Ismailia province (33.3%)[15], and Alexandria province (48.8%)[16]. Furthermore, worldwide high prevalence rates were reported from developing countries such as Nicaragua displaying a prevalence rate of 35.7%, respectively[17]. The higher rates in these communities may be attributed to improper hygiene and agricultural backgrounds.
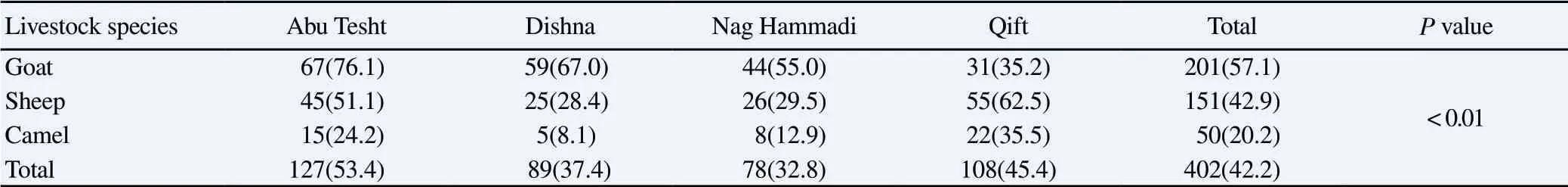
Table 2Infection rates of Cryptosporidium species in livestock raised in different districts in Qena region [n(%)].

Table 3Analytic concert of microscopy and ELISA of participants (n=960) with IgG antibodies against cryptosporidiosis.
Various past studies have focused on the response to Cryptosporidium spp. serum antibody in individuals residing in both developed and developing countries[18]. Similar results regarding the seropositivity were recorded in Peru (20%) and Venezuela(16%)[19]. On the other hand, a higher percentage (62%-83%) of Cryptosporidium seropositivity was found among southern Europe population[18].
The present records also revealed a significant positive correlation between the prevalence of infection among different age groups with peak values among children under 6 years age group. A high incidence of the disease in this age group has been reported in Canada, Unites States, New Zealand, England and Wales, and France [13]. Furthermore, the high seropositivity of Cryptosporidium infection in the young population (6-19 years) is similar to previous reports[20], while significantly lower than those recorded in rural China (70%) and Brazil (90%)[21]. The exact reason for this higher level is unknown, but it could be returned to immuno-physiological and ethological differences (more exposed due to water play games together with unawareness). However, low immunity response was the cause in those elder than 40 years.
The significant higher-level Cryptosporidium seropositive rate in female than male was matched with that obtained in Guatemala[22].However, in contrast to the current results, the higher level of infection was reported in males of Nigeria and Iran[23,24]. This difference suggested that male’s exposure to Cryptosporidium infection was affected by lifestyle risk factors that increased the exposure of the females to the untreated water in the study area. For instance, women in this area spend more time on farmlands cultivating vegetable than their male counterparts. They also do most of the house chores including cleaning of the toilets which in rural areas are often pit latrines. Hence women are often exposed to unhygienic conditions due to their living conditions.
When rates are compared between urban (Dishna and Nag Hammadi) and rural cases (Abu Tesht and Qift), a clear difference is apparent with rates within the rural population being significantly higher than the urban population. Globally, contaminated water bodies from livestock and agriculture wastes were considered as the major source of protozoan parasites[25-27]. Similarly, in England and
Wales, rates of cryptosporidiosis were higher in rural areas, areas with more agricultural manure application, and areas with poorly treated water supplies[28]. Currently, these results supported by the believing of some researchers who said that the sources of infection for humans are human feces and the feces of domestic animals,mostly the sheep in particular rural areas[29]. Differences in the prevalence of Cryptosporidium infection between rural and urban areas may be due to the presence of domestic animals and directly contacting with them[30]. Likewise, the present results indicate a negative correlation between the educational level and the prevalence of infection. As the literate patient is known or expected to be able to practice better hygiene as compared to illiterate ones, thereby preventing/reducing transmission of infection. The findings of this study complement those from a study in Brazil which reported the high presence of enteroparasites (80%) in children with illiterate mothers as compared to those with literate ones (26.3%)[31].
Several factors could account for seasonal variations in the occurrence of cryptosporidiosis, including factors affecting the number of oocysts present in the environment, such as rainfall or agricultural practices; factors affecting oocyst survival, such as humidity or temperature; and factors promoting exposure to oocysts,such as contact with animals or attendance at child care centers.However, in most studies, the highest numbers of cases have been detected during the rainy season[32,33]. Similarly, our results were in line with a former study in Kuwait which found the highest prevalence was observed during winter season[34].
In the current study persistent diarrhea, bloody diarrhea, abdominal pain, and fever were the most observed signs significantly associated with Cryptosporidium infection. This result was in line with that obtained by Chalmers and Davies[35].
In the current study, the number of cases was highly correlated with the type of drinking water sources[36]. These findings were in line with previous studies that considered untreated water as the most important source for Cryptosporidium infection[37,38]. Possible reasons for constant contamination include inefficient coagulation,filtration and poor disinfection (e.g. no free-residual disinfectant and short contact times) during water treatment[39]. Moreover, posttreatment contamination may be happened and entered the piped distribution system[39].
The safety of water is threatened by the use of old metal water distribution pipes which are more prone to develop. In Egypt, the peak of infection was recorded in the winter season as a result of runoff from agriculture land after application of contaminated manure which results in contamination of surface water.
The current study illustrated that hand washing and vegetables and fruits washing before eating were significantly safeguarding against Cryptosporidium infection. It was observed that people who washed hands regularly before eating were less likely to be infected than those who only washed occasionally or do not wash at all, as well as,those who picked up contaminated dropped fruits from the ground and directly eating them without washing or eating raw vegetables,which resulted in high prevalence of Cryptosporidium infection.
The present investigation declared that contact with feces contaminated-soil, poverty and low standards of hygiene that resulted from substandard housing were significantly correlated with Cryptosporidium infection.
It was found that washing hands after defecation insignificantly affect the prevalence of Cryptosporidium infection among studied groups. This could be due to the use of bidet shower instead of toilet paper (Islamic rules) which decreases the probability of hand contamination after defecation.
People living in close contact with animal cases and carriers which continually disseminate Cryptosporidium oocysts that contaminate the environment, was considered as the serious source for human infection[40,41]. Similarly, the present results were supported by previous studies who demonstrated that 15% of young children with contact of animal had a Cryptosporidium infection[42].
Previous studies reported a remarkable lower prevalence (2.5%)of cryptosporidiosis among Egyptian sheep[43], but in contrast, the present results were in line with that recorded in camels with an infection rate of 20.3% in Iran[44], highlighting the effect of habitat on the prevalence of cryptosporidiosis.
Based on the current results, the sensitivity and specificity of newly-developed ELISA test in comparison with modified ZN microscopy was found to be 99.06% and 88.88% respectively.This is comparable to another study which showed sensitivity and specificity of another antigen rapid test (Crypto-Giardia kits) in comparison to microscopy as 86.7% and 100%, respectively[45]. This result was predictable and attributed to the fact that the detection of infection by MZN microscopy mainly depends on the experience and skills of microscopist[45].
The sensitivity and specificity of a screening test are characteristics of the test’s performance at a given cut-off point (criterion of positivity). However, the positive predictive value of a screening test will be influenced not only by the sensitivity and specificity of the test but also by the prevalence of the disease in the population that is being screened.
In conclusion, the most important environmental risk factors which associated with cryptosporidiosis in Southern Egypt are extreme poverty, poor environmental sanitation, contaminated water supplies and crowding which facilitate fecal-oral trans mission.Health education should be given to people to protect them from environmental sources of infection.
Conflict of interest statement
The authors declare that there is no conflict of interests.
 Asian Pacific Journal of Tropical Medicine2019年5期
Asian Pacific Journal of Tropical Medicine2019年5期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Parsonage-Turner syndrome following chikungunya virus infection:A case report
- Evaluation of phytochemical properties and larvicidal activities of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti
- Phylogeny of Culex theileri virus flavivirus in Spain, Myanmar, Portugal and Turkey
- Climate change and potential distribution of zoonotic cutaneous leishmaniasis in Central Iran: Horizon 2030 and 2050
- Antimalarial activity of a novel series of artemisinin-derived 1, 2,3-triazole dimers
