Evaluation of phytochemical properties and larvicidal activities of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti
Ananta Swargiary, Manita Daimari, Mritunjoy Roy, Dipanjali Haloi, Bijit Ramchiary
1Department of Zoology, Bodoland University, Kokrajhar, Assam, India
2Department of Zoology, Kokrajhar Govt. College, Kokrajhar, Assam, India
Keywords:Larvicide Glutathione S-transferase Acetylcholinesterase Udalguri district Aedes aegypti
ABSTRACT Objective: To investigate the phytochemical, antioxidant and larvicidal property of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula against Aedes aegypti.Methods: Antioxidant capacity of methanolic extract of the plants was studied by 2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay, ferric reducing antioxidant power assay,2,2’-azinobis-(3-ethylbenzothiazoline-6-sulfonate) assay (ABTS), thiobarbituric acid reactive substance (TBARS) assay, superoxide anion scavenging activity and total antioxidant activity assay following standard protocol. Total phenolic content, total flavonoid content,carbohydrate, and plant protein were also estimated following standard protocols. Larvicidal property of plant extracts were determined following World Health Organization standard protocol. Additionally, glutathione-s-transferase (GST) and acetylcholinesterase (AchE)inhibitory property was also tested biochemically.Results: Phytochemically, high protein, carbohydrate and phenolic were found in Terminalia chebula, while Cynodon dactylon showed high flavonoid contents. Similarly, high antioxidant activity was found in Terminalia chebula with IC50 values at 13.7, 2.9, 45.2 and 46.0 μg/mL in DPPH, ABTS, TBARS and superoxide anion scavenging activity, respectively. Larvicidal study showed strongest activity in Spilanthes acmella followed by Cynodon dactylon, and Clerodendrum viscosum and Terminalia chebula. GST and AchE of Aedes aegypti larvae showed reduced enzyme activity when pre-incubated with Cynadon dactylon and Spilanthes acmella.Conclusions: The methanolic crude extracts of Cynodon dactylon, Clerodendrum viscosum,Spilanthes acmella and Terminalia chebula possess strong antioxidant and larvicidal property against Aedes aegypti and therefore, may be further investigated for the molecular mode of action.
1. Introduction
Mosquito borne diseases (MBD) such as malaria, dengue, etc.are among the major health problems that account more than 17%of all the infectious diseases causing millions of death globally[1].Dengue is among the most common MDBs causing huge economic losses. According to World Health Organization, more than 3.9 billion people from over 128 countries are at risk of dengue infection. With about 100 million cases, dengue virus is the most prevalent mosquito-borne human virus worldwide[2]. In Indian subcontinent, the epidemics of dengue have been increased over the last few decades. Today it has emerged as a new threat to the people of Assam and other North eastern states of India[3,4]. According to the Directorate of Health Service of Assam, major outbreak of dengue was in 2010 with 237 cases including 2 deaths. Dengue was reported highest in 2013 (4 526 cases) followed by 92 cases in 2015 and 85 cases in 2014. Currently, the use of insecticide is the most common practice of mosquito control. Starting from dichlorodiphenyltrichloroethane to several other insecticides such as dieldrin, malathion, fenitrothion, carbamates etc. are introduced to control mosquito vectors. However, over and frequent use of the same insecticide for a long period of time has developed resistance capacity among the mosquito populations. It has also caused undesirable effects to non-target organisms[5]. Many studies have reported that the increased insecticide resistance developed by mosquito populations is due to increased insecticide-detoxification capacity and decreased sensitivity of target receptors[6].
Progress of medical healthcare system has never been adequate for controlling mosquito borne diseases. Meanwhile, development of insecticide resistance among the mosquito populations has increased the complexity of mosquito borne diseases and its control measures.Traditionally, most of the rural people believe in herbal medicine and choose plant-based treatment for many diseases including MBDs.As an alternative or supplementary to the commercial insecticides,plant-based herbal insecticides have become a priority. Many studies around the world have reported the effectiveness of plants against many mosquito vectors[7]. The State of Assam is one of the most populous north-eastern states of India with more than 70% people living in rural areas and about 36% living below poverty line.Udalguri is a district administered by Bodoland Territorial Council(BTC), an administrative privilege given by the Government of India[8]. There is a rich knowledge of traditional medicine system in Udalguri district of Assam, practiced mostly by economically deprived tribal communities. Several medicinal plants are used as mosquitocidal and larvicidal agent in this part of India. Despite of rich natural vegetations, very little work has been conducted to investigate the larvicidal and mosquitocidal property of medicinal plants in Assam[9]. Therefore, the present study has been designed to study 4 mosquitocidal plants traditionally used by the local people of Udalguri district of Assam, namely Clerodendrum (C.) viscosum,Cynodon (C.) dactylon , Spilanthes (S.) acmella and Terminalia (T.)chebula.
2. Materials and methods
2.1. Collection, identification and preparation of plant extracts
Four most common and traditionally used mosquitocidal plants namely, C. dactylon (L.) Pers. (Family Poaceae, BUBH2018032),C. viscosum Vent. (Family Lamiaceae, BUBH0000047), S. acmella vr. paniculata (Wall. Ex DC.) (Family Asteraceae, BUBH2018007)and T. chebula Retz. (Family Combretaceae, BUBH0000062) were collected from Udalguri district of Assam (geographical coordinates 26°46’N to 26°77’N and 92°08’E to 95°15’E) with the help of village traditional healer (Kuberaj). Sample plants were identified by Dr. S. Baruah, Department of Botany, Bodoland University.Fresh samples of C. dactylon (whole plant), C. viscosum (leaves), S.acmella (whole plant) and T. chebula (fruit flesh) were washed with distilled water, and dried completely in hot air oven at 45℃-50 ℃.Preparation of methanolic crude extract was carried out following the method as described in our earlier publication[10]. Dry, semisolid crude extracts of C. dactylon, C. viscosum, S. acmella and T.chebula were kept at -20 ℃ in an airtight container for further use.
2.2. Qualitative phytochemical analysis
The presence of protein, reducing sugar, phenols, flavonoids,saponins, steroids and terpenoids in the plant extracts was detected following the methods of Trease and Evans[11] and Sofowora[12].
2.3. Quantitative phytochemical study
2.3.1 Carbohydrate (Glucose)
The presence of total carbohydrate content in plants were estimated following Anthrone method[13]. Values were expressed as μg sugar/mg plant extract.
2.3.2 Protein The protein content of all the plant extracts was estimated following the Lowry method[14]. Values were expressed as μg protein/mg plant extract.
2.3.3 Total phenolic content (TPC)TPC was estimated following the method as described by Iloki et al.[15]. Gallic acid was used as reference chemical and the values were expressed as μg gallic acid equivalent (GAE)/mg plant extract.
2.3.4 Total flavonoid content (TFC)
TFC was determined following the method of Ordonez et al.[16]using quercetin as reference chemical. Values were represented as μg quercetin equivalent (QE)/mg plant extract.
2.4. Antioxidant activity study
2.4.1 Total antioxidant activity (TAA)TAA of the plant extract was done following Phosphomolybdate method[17]. TAA was expressed as μg ascorbic acid equivalent(AAE)/mg plant extract.
2.4.2 2,2-Diphenyl-1-picryl-hydrazyl-hydrate assay(DPPH)
The DPPH scavenging activity of methanolic plant extracts were estimated following the method of Mamta et al.[18] with slight modifications.
The scavenging activity of plant extract was calculated using the formula:
DPPH scavenging activity(%)=×100
Where Abs control=absorbance of DPPH and methanol; Abs sample=absorbance of DPPH and plant extract or ascorbic acid.
IC50(concentration at 50% inhibition) was calculated by plotting the % inhibition in ‘X’-axis and plant extract in ‘Y’-axis.
2.4.3 Ferric reducing antioxidant power assay (FRAP)
FRAP assay was performed following the method of Iloki Assanga et al.[19]. The FRAP activity of plant extracts were compared with the standard ascorbic acid. The values were expressed as μg Fe2+equivalent (FE)/mg plant extract.
2.4.4 Thiobarbituric acid reactive species assay (TBARS)
Lipid peroxidation inhibitory activity was studied following the modified thiobarbituric acid reactive species assay to measure the lipid peroxide formation using egg yolk homogenates as lipid-rich media[20]. Ascorbic acid was used as standard reference.
2.4.5 2,2’-Azinobis-(3-ethylbenzothiazoline-6-sulfonate)assay (ABTS)
The ABTS activity was measured following the method of Re et al.[21] using gallic acid as standard reference.
2.4.6 Superoxide anion scavenging activity
Scavenging activity for superoxide anion of plant extract was estimated according to the method of Robak and Gryglewski[22]using gallic acid as standard reference.
2.5. Larvicidal activity study
The larvicidal bioassay was done following World Health Organization standard protocol with little modifications[23]. In a series of test doses (50, 100, 200, 500, 1 000 and 2 000 μg/mL for C.dactylon, C. viscosum and T. chebula, and 10, 20, 50, 100, 200 and 500 μg/mL for S. acmella), 20 numbers of 3rd-4th instar larvae of Ae.aegypti was exposed separately for 24 h under standard experimental condition. Larval mortality was recorded after 24 h of treatment and lethal concentration (LC50) was calculated. Three replicates were done for all the treatments.
2.6. Enzyme assays
2.6.1 Acetylcholinesterase
Larval Ae. aegypti acetylcholinesterase (AchE) activity was measured following the method of Ellman et al.[24] with necessary modifications as described by Pontual et al.[25]. AchE activity was calculated by using the extinction coefficient of 5,5’-Dithiobis-(2-nitrobenzoic acid and absorbance changes per minute was read at 405 nm. Enzyme activity was expressed as nM product formed/min/mg tissue protein.
2.6.2 Glutathione S-transferase (GST)
GST activity was measured following the method of Habig et al.[26].The enzyme activity was expressed as μM product formed/min/mg tissue protein. Enzyme inhibitory activity was carried out by preincubating the larvae tissue homogenate with crude extracts of all the 4 plants.
2.7. Statistical analysis
Statistical calculations were carried out in MS. Excel 2007,OriginPro 8.5 software (OriginLab Corp., USA) and IMB SPSS 21 (SPSS® 10.0 Syntax, SPSS Inc, Chicago) software. All data are presented as mean±standard deviation for at least 3 replications for each experiment. The results are considered to be significant at P<0.05 level.

Table 1. Qualitative phytochemical test of methanolic crude extracts of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula.
3. Results
3.1. Qualitative and quantitative study
The methanolic extract of all the 4 plants, C. dactylon, C. viscosum,S. acmella and T. chebula were tested for the presence of protein,carbohydrate, phenols, flavonoids, saponins, steroids and terpenoids.The test result and methodology used is shown in Table 1. Qualitative study showed high content of tested phytochemicals. T. chebula showed strong blue-green colouration indicating the presence of high phenolic content. However, C. dactylon showed negative result in steroid test. Similarly, quantitative study showed high content of protein, carbohydrate and TPC in T. chebula (Figure 1). Meanwhile,C. dactylon showed low content in protein and TPC while S. acmella showed lowest in carbohydrate and TFC. Protein content ranged from (204.75±78.72) to (701.60±18.27) μg/mg plant extract,carbohydrate from (7.45±0.28) to (17.37±0.32) μg/mg plant extract,TPC from (35.79±3.32) to (373.48±37.08) μg GAE/mg plant extract and TFC from (2.76±0.21) to (20.92±0.41) μgQE/mg plant extract,respectively.
3.2. Antioxidant assay
The methanolic crude extract of all the 4 plants were also tested for its antioxidant property. Six different antioxidant tests were conducted such as DPPH, ABTS, TBARS, FRAP, superoxide anion scavenging and TAA. IC50values of antioxidant tests are presented in Table 2.
The IC50values ranged from 13.7-950.0 μg/mL for DPPH, 2.9-115.0 μg/mL for ABTS, 45.2-2 882.0 μg/mL for scavenging activity,and 46.0-353.0 μg/mL for TBARS assay, respectively. For all the antioxidant assays T. chebula was found to possess stronger activity followed by C. viscosum, S. acmella, and C. dactylon, respectively(Figure 2).

Table 2. IC50 values of different antioxidant activity assays of methanolic plant extracts (μg/mL).

Figure 1. Graph showing the phytochemical content of plant extracts. A: Protein, B: Carbohydrate, C: Total phenolic content and D: Total flavonoid content. CdME, CvME, SaME and TcME stands for methanolic extract of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula, respectively.
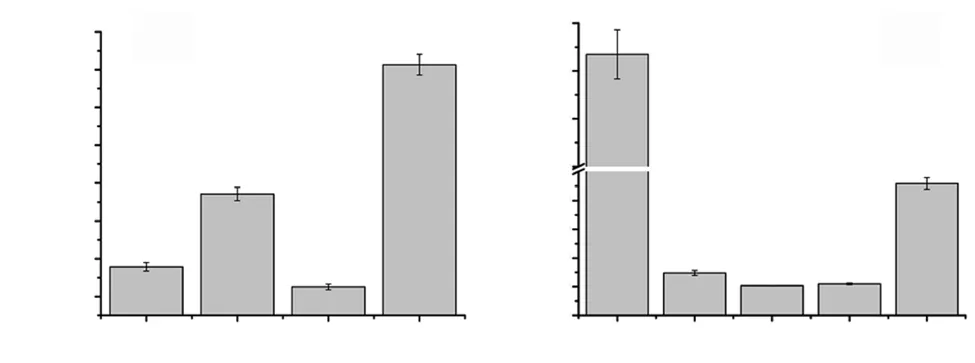
Figure 2. Comparative antioxidant activity. A: Total antioxidant activity by phosphomolybdate assay and B: FRAP activity. CdME, CvME, SaME and TcME stands for methanolic extract of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula, respectively. Total antioxidant activity was expressed as μg ascorbic acid equivalent (AAE)/mg plant extract. FRAP values were expressed as μg Fe2+ equivalent (FE)/mg plant extract.
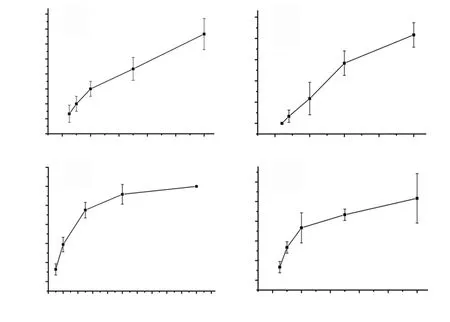
Figure 3. Larvicidal activity of methanolic plant extracts against Aedes aegypti. A: Clerodendrum viscosum, B: Cynodon dactylon, C: Spilanthes acmella and D:Terminalia chebula.
3.3. Larvicidal activity
The present study also tested the larvicidal effect of all the plants on Ae. aegypti. Figure 3 showed the larvicidal property of all the plants. On exposure to different concentrations of plant extracts, Ae.aegypti showed dose-dependent mortality after 24 h of treatment.S. acmella was found to possess strongest larvicidal property with LC50=27.5 μg/mL followed by C. dactylon (LC50=640.0 μg/mL), C.viscosum (LC50=885.0 μg/mL) and T. chebula (LC50=2 360.0 μg/mL).
3.4. GST and AchE inhibitory study
The crude extract of C. viscosum, C. dactylon, S. acmella and T.chebula were also tested for its GST and AchE inhibitory property. The tissue protein content of Ae. aegypti larvae was (21.13±2.77) mg/g wet tissue. Under standard laboratory conditions, the control, untreated larvae showed high AchE and GST activity (Figure 4). The AchE enzyme activity was (88.95±6.85) nM/min/mg tissue protein and GST (118.23±24.79) μM/min/mg tissue protein. When larvae tissue was pre-incubated with plant extracts, decreased enzyme activities were observed in both the enzymes (Figure 4). The reduction in AchE enzyme activity was (26.93±8.96) nM/min/mg protein, (17.92±5.33)nM/min/mg protein, (33.16±6.92) nM/min/mg protein and(22.42±7.28) nM/min/mg protein compared to control on exposure to the extracts of C. dactylon, C. viscosum, S. acmella and T. chebula,respectively. The crude extract of S. acmella showed strongest AchE inhibitory activity followed by C. dactylon, T. chebula and C.viscosum. Statistically, C. dactylon, S. acmella and T. chebula showed significant reduction in AchE activity compared with the control group (Figure 4A).

Figure 4. AchE (A) and GST (B) enzyme inhibitory activities of plant extracts. *indicates significant difference (at P<0.05 level) between control and plant extract treated enzyme activities. CdME, CvME, SaME and TcME stands for methanolic extract of Cynodon dactylon, Clerodendrum viscosum, Spilanthes acmella and Terminalia chebula, respectively.
Similarly, S. acmella and C. dactylon showed stronger inhibitory activity against GST. The reduction in GST enzyme activity was(46.86±8.59) μM/min/mg protein, (24.69±7.63) μM/min/mg protein,(103.56±3.36) μM/min/mg protein and (15.07±7.37) μM/min/mg protein on treatment with C. dactylon, C. viscosum, S. acmella and T. chebula, respectively. S. acmella and C. dactylon plant extract showed significant reduction in GST activity while C. viscosum and T. chebula did not show significant reduction (Figure 4B).
4. Discussion
Plants have been a source of medicines since ancient times.In the present study, four most commonly used mosquitocidal plants namely C. viscosum, C. dactylon, S. acmella and T. chebula collected from Udalguri district of Assam were studied to test its ethnomedicinal value as well as phytochemical and antioxidant property. Phytochemical study revealed high content of protein in all the plants. T. chebula showed highest protein content with
(701.60±18.27) μg/mg plant extract indicating rich source of plant proteins. Unlike protein, highest carbohydrate content was found to be (17.37±0.32) μg/mg plant extract in T. chebula followed by
C. dactylon, C. viscosum and S. acmella. The protein content of the plants ranged from 20% to 70% by weight of crude extract.Literature survey revealed similar pattern of plant proteins[27].Similar to our study, Akter et al.[28] estimated dry crude plant extract of Terminalia ferdinandiana contains 32% protein content. Spilanthes uliginosa is reported to contain 41% protein by dry weight[29].However, unlike protein, quantitative phytochemical analysis revealed variations in the quantity of TPC and TFC. Higher content of TPC can be correlated with the higher antioxidant activity and has been supported by many recent publications[30]. Polyphenols are among the most widespread class of metabolites in nature and their antioxidant property can be attributed to the ability to chelate metal ions involved in the production of free radicals. Our study also showed similar kind of correlation between TPC and the antioxidant property.
In the present study, we also assessed the larvicidal property of the crude extracts of C. viscosum, C. dactylon, S. acmella and T.chebula against Ae. aegypti larvae. Our study revealed strongest larvicidal activity in S. acmella followed by C. viscosum, C.dactylon and T. chebula. The LC50of 4 plants were found to range from (27.50 to 2 360.0) μg/mL. Several research findings also showed similar kind of larvicidal property against Ae. aegypti.Chore et al.[31-33] studied the larvicidal property of 3 plants from Kenya and the LC50were found to range from 50.00 μg/mL to 3.89 mg/mL. Similarly, Rios et al.[34] investigated the larvicidal activity of 10 medicinal plants from Colombia against Ae. aegypti and LC50were found to ranging from (45.73 to 114.65) mg/mL. Based on our results, we attempted to see whether there is any correlation between antioxidant and larvicidal activity of the plants. No such positive correlation was observed between antioxidant and larvicidal property of the plants. Studies conducted by several other researchers found positive correlation between antioxidant and larvicidal property of the plants[35]. T. chebula showed strongest antioxidant property while weakest in larvicidal activity among all the plants. The antioxidant and larvicidal properties of plants can be written as T. chebula>C.viscosum>S. acmella>C. dactylon and S. acmella> C. viscosum> C.dactylon >T. chebula, respectively.
GST and AchE are two of the most important enzymes that confer resistance capacity to mosquitoes. From our study high activity of AchE and GST was observed in control Ae. aegypti. Similarly,Lima et al.[36] investigated the resistance capacity of Ae. aegypti and found that the AchE and GST values ranged from (65-131) nM/min/mg protein and (51-116) μM/min/mg tissue protein, respectively. Larvae tissue homogenates when pre-incubated with plant extracts, both the enzyme showed reduced activity. S. acmella showed maximum reduction in both the enzyme activities among all the plants while C.viscosum and T. chebula showed lowest inhibitory activity.
The methanolic crude extracts of C. dactylon, C. viscosum, S.acmella and T. chebula were tested for its phytochemical, antioxidant and larvicidal property against dengue vector, Ae. aegypti under laboratory conditions. The results clearly showed highest phenolics and antioxidant activity in T. chebula followed by C. viscosum, S.acmella and C. dactylon. Larvicidal study demonstrated highest mortality with S. acmella followed by C. dactylon, C. viscosum and T. chebula. However, the larval mortality profile of plant extracts showed no positive correlation to the antioxidant property.Meanwhile, enzyme activities were inhibited in a similar pattern of larvicidal profile of plants. From our study it has also been seen that plants having strong larvicidal property also possess strong GST and AchE inhibitory property. Therefore, the presence of any correlation between the larvicidal property and enzyme inhibitory property of plant extracts cannot be denied. However, further study need to be carried out to isolate and purify the bioactive compounds from the plants and also to study the molecular mode of action.
Conflict of interest statement
We declare that there is no conflict of interest.
Acknowledgments
Authors acknowledge Science and Engineering Research Board,Govt. of India for providing instrumentation facility under DSTSERB project sanctioned to AS. Authors also like to acknowledge all the elderly village people and kuberaj who provided necessary information as and when required during the survey period.
 Asian Pacific Journal of Tropical Medicine2019年5期
Asian Pacific Journal of Tropical Medicine2019年5期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Parsonage-Turner syndrome following chikungunya virus infection:A case report
- Seroprevalence of Cryptosporidium and risks of cryptosporidiosis in residents of Sothern Egypt: A cross-sectional study
- Phylogeny of Culex theileri virus flavivirus in Spain, Myanmar, Portugal and Turkey
- Climate change and potential distribution of zoonotic cutaneous leishmaniasis in Central Iran: Horizon 2030 and 2050
- Antimalarial activity of a novel series of artemisinin-derived 1, 2,3-triazole dimers
