Stereotypes About Enterotype:the Old and New Ideas
Mingyue Cheng ,Kng Ning*
Key Laboratory of Molecular Biophysics of the Ministry of Education,Hubei Key Laboratory of Bioinformatics and Molecular-imaging,Department of Bioinformatics and Systems Biology,College of Life Science and Technology,
Huazhong University of Science and Technology,Wuhan 430074,China
KEYWORDS
Abstract In 2011,theterm‘‘enterotype”f irst appeared to thegeneral public in Nature,which refers to stratif ication of human gut microbiota.However,with more studies on enterotypes conducted nowadays,doubts about the existence and robustness of enterotypes have also emerged.Here we reviewed current opinions about enterotypes from both conceptual and analytical points of view.We f irstly illustrated the def inition of the enterotype and various factors inf luencing enterotypes,such as diet,administration of antibiotics,and age.Then we summarized linesof evidencethat pose the concept against the enterotype,and described the current methods for enterotype analysis.Finally,we showed that the concept of enterotype has been extended to other ecological niches.Based on current studies on enterotypes,it has been clear that more studies with larger samplesizes are needed to characterize the enterotypes.Improved computational methods are also required to build sophisticated models,ref lecting the dynamics and resilience of enterotypes.
The def inition of enterotypes
Each individual isdifferent not only dueto his/her own genetic materials but also the gut microbiome.The human gut microbiome consists of at least 1800 genera and approximately 15,000-36,000 species of bacteria[1],with the total number of bacterial cells ranging from 1013to 1014,which is of the same order as the number of human cells(3.0×1013)[2].Gut microbiome also containsmorethan100 times more genes,compared with 25,000 genes in humans[3].As for the functions,gut microbiome has played a vital role in human body.For example,they can degrade a variety of otherwise indigestible dietary polysaccharidesand synthesize essential amino acids and vitamins[4].Furthermore,the dysbiotic microbiota can lead to the loss of regulatory immune effects on the gut mucosa,which is associated with a number of inf lammatory and immune-mediated diseases[5,6].
Gut microbiota vary largely among individuals in time and space scale[7],which has been regarded as an obstacle to the gut microbiome-based medical applications.The enterotype concept raised in 2011 might make this obstacle possible to be coped with.Arumugam et al.[8]analyzed 33 qualif ied samples from different populations and found that these samples can be stratif ied into three distinct robust clusters driven by discriminative genera.These include Bacteroides(enterotype 1),Prevotella(enterotype 2),and Ruminococcus(enterotype 3),with each cluster designated as an enterotype subsequently[8].The essence of enterotyping is to stratify human gut microbiome,serving as a process of dimensionality reduction to collapse global microbiome variation into a few categories.These categories,named‘‘enterotypes”,were reported originally as‘‘densely populated areasin a multidimensional spaceof community composition,”which means that they are not sharply delimited as human blood types[8].
Following the concept of enterotype,subsequent studies have demonstrated that enterotypes are quite robust among populations.For instance,Arumugam et al.[8]apply enterotype analysis to two large published gut microbiome datasets(85 metagenomes of Danish individuals from a published Illumina dataset and 154 pyrosequencing-based 16S sequences from American individuals)and have detected three enterotypes.Moreover,Liang et al.[9]have observed three enterotypes when investigating 181 human fecal samples from adultsin Taiwan,China.Besidesthetwo enterotypesidentif ied by Bacteroides and Prevotella as described before,a third enterotype is identif ied by family Enterobacteriaceae,suggesting that there might be a new enterotype in the Asian population.Nevertheless,whether it can be regarded as a feature of Asian microbiome or it is produced by chance needs further investigations.
However,with morestudiesfocusing on the stratif ication of human gut microbiome,it hasbeen noticed that thenumber of enterotypes varies when different methods are employed even on the same samples[9-11].For example,Wu et al.[11]used different distance matrices including weighted/unweighted UniFrac,Euclidean,the Bray-Curtis,and the Jensen-Shannon divergence(JSD)to stratify the gut microbiota samples of 98 healthy volunteers and have found that most analyses reveal two enterotypes with stronger support,whereas only the analysisusing weighted UniFrac distanceclearly showsthreeenterotypes.Hence,they claimed that Bacteroides enterotype is fused with the less well-distinguished Ruminococcus enterotype.Liang et al.[9]performed 9β-diversity matriceson enterotype analysis using three clustering methods and obtained inconsistent numbers of enterotypes based on various evaluation scores.Moreover,several other studies have conf irmed that according to the microbiome prof iles,samples can only be stratif ied into two enterotypes represented by Bacteroides and Prevotella,respectively[12-15].Therefore,it remains to be considered whether the Ruminococcus enterotype or other so-called‘‘the third enterotypes”should be abandoned and,instead,fused with the Bacteroides or/and Prevotella enterotypes,due to their less signif icance among gut microbiome samples[11,16]and the analytical bias[10,11].
Enterotype is inf luenced by various factors
Since enterotypes are def ined based on gut microbiota,which changes rapidly in response to interventions[11,17-20],it is conceivable that enterotypes are not constant for individuals,but rather dynamically affected by various factors as well.However,the alternation of microbiota composition in a short term might not be suff icient to switch the enterotype[11],due to the reversibility and relative stability of gut microbiota[21-24].Dietary intake and administration of antibiotics are known to signif icantly impact our gut microbiota[11,25,26].In addition,many factors such as the diet,the life style,and environmental stress vary during different age stages[27,28],making age a combination of these factors that largely impacts both enterotype patterns and identif ication.
The effects of diet
The diet has been reported to affect gut microbiota composition in multiple studies[11,19,29-34].Although the shortterm diet adjustment might not be able to change the enterotype,the long-term diet has been observed to signif icantly associate with the enterotype patterns[11].
The short-term diet adjustment(usually lasting less than a month)can cause a rapid and signif icant change in microbiota composition[26,35],which,however,might not lead to stable switches between enterotypes[11].One controlled-feeding study involving 10 subjects has shown that the variation of gut microbiota composition is observed in 24 h after intake of high-fat/low-f iber or low fat/high-f iber diet,but enterotypes remain stable during the 10 days of dietary intervention[11].Only one subject switches from Bacteroides enterotype to Prevotella enterotype,which then reverts the next day[11].Exceptions have been found in another 6-week controlled feeding trial,when investigating the effects of dietary capsaicin on gut microbiota.Two subjects of Prevotella enterotype switch to Bacteroides enterotype during the high-capsaicin period and at the end of washout period,respectively.Nevertheless,their relative abundance of Prevotella is still much higher than that in the subjects of Bacteroides enterotype.Additionally,enterotypes of the other 10 subjects remain stable during this 6-week controlled feeding trial[15].These observations indicate that the impact of short-term dietary intervention on gut microbiota is not strong enough to change the enterotype.
During long-term environmental changes,the composition of an individual's gut microbiota is predominantly determined by dietary habits[11,36]and such dynamics is highly variable among individuals[37,38].Wu et al.[11]claimed that Bacteroides enterotype favors protein and animal fat,characterized by meat consumption as in a Western diet,whereas Prevotella enterotype prefers carbohydratesand simplesugars,which are typical of the carbohydrate-based diet in agrarian societies[11,14].Whether long-term dietary interventions(usually lasting morethan several months)can stably changeenterotypes still remains unknown.Interestingly,a recent study by Liu et al.[26]has revealed that even after half a year,theenterotypesof the Chineseindividualscan bereverted after thesubjects shift back to their routine diets.Therefore,the impact of long-term dietary shift needs to be further investigated.
The effects of antibiotics
Antibiotics have been widely used over the world nowadays,which can have both temporary and permanent effects on our gut microbiota[39,40]through various mechanisms[41,42].In a recent study,by administering cefprozil,a second-generation cephalosporin,to 18 healthy volunteers,Raymond et al.[43]have observed the increased abundance of several bacterial genera such as Flavonifractor,Lachnoclostridium,and Parabacteroides,but a decrease in the abundances of several bacterial families such as Bif idobacteriaceae and Coriobacteriaceae.Six of these 18 exposed participants have their level of Enterobacter cloacae complex bacteria increased from an average of 0.0001%to 0.1%after a 7-day course of cefprozil.It is worth noting that f ive of these six participants initially contain a Bacteroides enterotype with lower bacteria diversity[43].This f inding demonstratesthat the effect of cefprozil exposure may be linked to a Bacteroides enterotype.Therefore,not only the antibiotics themselves can have effects on our gut microbiota,but also these effects are likely inf luenced by the enterotypes of subjects.Thus,enterotype might be taken into account when considering therapeutic antibiotic intervention.
Correlation with age
In the original paper about enterotypes[8],age has been described as not associated with enterotypes.This conclusion is obtained based on the observation that the distribution of age seems not signif icantly different among different enterotypes.However,the stability and composition of human gut microbiota vary largely across different age stages in human life(infancy,childhood,adulthood,and elderhood)[28,44-46].That is to say,the results of enterotype analysis would bedifferent when using gut samplesof subjectsat different age stages.A recent study has reported three enterotypes of children at school age,driven by Bacteroides,Prevotella,and Bif idobacterium,respectively[47].They are different from the enterotypes reported in the adults.Moreover,the gut microbiota of infants and the elderly is very dynamic[19,27,28],making it unsuitable for analysis of a‘‘stable status”.
Gut microbiota of infants has been reported to be quite unstable[28,46].Firstly,their gut microbiota can be inf luenced by the mode of delivery:vaginally-delivered infants differ from those delivered by caesarean section,both in terms of the timing of colonization and the composition of their microbiota[24,48-50].Additionally,the gut microbiota prof ile of preterm babies differs from that of full-term babies[50,51].In the f irst year of life,the gut of infants is quite sterile initially and gradually becomes extremely densely colonized with bacteria.Finally,the gut microbiota of infants ends with a prof ile largely similar to that in adults[28,52].This process of evolution of the gut microbiota of infants is still poorly understood.Not to mention that many key events,such as the solid food intake and administration of antibiotics,can have effects on gut microbiota of infants as well[48].
The gut microbiota composition of the elderly(>65 years)varies extremely between individuals[19].Additionally,the core microbiota of the elderly is quite different from that of young adults,with a greater proportion of Bacteroides spp.and distinct abundance patterns of Clostridium groups[27].
Gut microbiota prof iles are quite distinct among different stages of human life[28,44-46].Along with aging,changes of life-style happen,such as the dietary habits,the frequency and variety of administration of antibiotics,as well as the human activities.Asa result,theagecan beregarded asa combination of multiple factors affecting human enterotypes.Age can also be a confounding factor when choosing samples for enterotype analysis.It might not be appropriate to mix the unstable microbiota samples of the infants and elderly with those of adults when performing enterotype analysis.Therefore,the investigations should be conducted separately for these age groups.
The concept against enterotypes
Enterotype might be continuous
In principle,enterotypes can clearly separate samples,and such separation can beclearly observed in principal coordinate analysis(PCoA),as isshown in Figure1A.However,a number of studies especially with larger sample size[10,16,53,54]have indicated that samples from different enterotypes cannot be separated clearly into distinct clusters(Figure 1B).Moreover,when looking at the gradient of log ratio of Bacteroides to Prevotella,the discrete enterotypes are expected to show an obvious gap of the gradient,thus stratifying these samples into clusters(Figure 1C).Nevertheless,in most cases,a continuous gradient rather than a gap is observed[11,55](Figure 1D).Therefore,doubts about the presence of discrete enterotypes emerge[16,55].Instead of discrete enterotypes,this kind of distribution is deemed as a continuum changing from Bacteroides-driven to Prevotella-driven microbiota types[55].
Enterotype is not always stable
After the concept of enterotype was initially proposed[8],the enterotypes of subjects are considered to be stable during a long time,with their microbiota composition varying but within limits.Figure 2A shows that three cohorts of subjects contain three different enterotypes at the beginning.As time evolves,their gut microbiota composition changes,but the extent of this change is not enough to switch the enterotypes.These samples still belong to the groups of their original enterotypes in PCoA,even after many years(Figure 2B and C).
However,subsequent short-term and long-term studies have revealed that such stability is subject to environmental changes,and is not strong in the long run[11,15,16,26].Dan Knights et al.[16]projected a dense time series of daily gut microbiome samples from a single individual in a year[56]onto the published putative enterotype clusters[8].Based on the trajectories of microbiome prof ile from these consecutive daily samples,they have found a switch from one putative enterotype to another over the course of several days[16].Although validation in a largecohort iswarranted,their investigations demonstrate that a certain number of healthy subjects might switch their enterotypes over time,suggesting that the enterotype might not be always stable[16].As described in Figure 2D and E,as time evolves,a number of subjects change their enterotypes.Two trajectories of switches between enterotypesare shown as examples.The gut sample of a subject represented by the yellow square switches from the original Ruminococcus enterotype to the Bacteroides enterotype,whereas gut sample of another subject indicated by the yellow triangle switches from the Bacteroides enterotype to the Prevotella enterotypes.After a certain period of time,enterotypes of these subjects changed completely.As a result,most gut samples of these subjects do not belong to the group that they originally comefrom(Figure2F).Therefore,it seems inappropriate to classify people merely according to their enterotypes,due to the instability of enterotypes.Monitoring of enterotypevariation of individualsat frequent timeintervals is required for further investigations on enterotypes.

Figure 1 Enterotypes might be continuous rather than discrete Simulated microbiota samples are indicated using dots,triangles,or squares with different shapes representing different patterns of microbiota composition that these samples initially contain.The simulated samples are grouped into three enterotypes(Prevotella,Bacteroides,and Ruminococcus)according to their microbiota composition,plotted in thescenario showing what isexpected to see(A)and what is actually observed(B).The log ratio of the relative abundance of Prevotella to Bacteroides of these simulated samples is plotted against the f irst principal coordinates in panels A and B,to show the expected scenario(C)and the observed scenario(D).
Enterotype might not serve as the biomarker
Given enterotypes aredef ined to indicateclusterscomposed of gut microbial communities that share similar bacteria composition[8],enterotypes can be used to collapse the highlymultidimensional human microbiome variation into just a few categories.It seems a good idea to use enterotypes as biomarkers that correlate gut microbiome with phenotypes such as diseases.For instance,assuming that the occurrence of certain diseases is observed to signif icantly correlate with an enterotype,patients then can be grouped according to the enterotypes.Subsequently,a personalized enterotype-based diagnostics and therapeutics would be readily pursued for them.However,enterotypesmight not haveenough resolution for specif ic disease-related taxa[16].In Figure 3,taxon related to a particular disease is depicted in the same color as the risk for thisdisease.It isshown that theabundanceof an‘‘orange”disease-related taxon is correlated directly with the risk of the‘‘orange”disease.In addition,the gut sample that contains dominant‘‘orange”taxon is classif ied as‘‘orange”enterotype,while the other samples are classif ied as‘‘pink”or‘‘blue”enterotypes.When determining the disease risk based on taxon,the proportion of‘‘orange”taxon would directly ref lect the actual risk of‘‘orange”disease.When determining the disease risk based on enterotypes,the‘‘orange”enterotype would signify high risk and other enterotypes would be the sign as low risk.As shown in Figure 3A,if the‘‘orange”taxon is only present in‘‘orange”enterotype,the enterotype-based risk of the‘‘orange”disease would be consistent with the actual risk.However,as shown in Figure 3B,if the‘‘orange”taxon is present in all enterotypes,enterotype-based risk of the‘‘orange”disease would be likely misleading.Such misleading observation could be explained by the attributes of enterotypes,that enterotypes are not sharply delimited but rather exist as a‘‘broad region”,with even unclear boundary between enterotypes.Hence,if a sampleof‘‘blue”enterotypecontainsa high abundance of‘‘orange”taxon,while this abundance is not high enough to designate this sample as‘‘orange”enterotype,the enterotype-based risk would be low,although the actual risk is high(Figure 3B).Therefore,using enterotypes as the biomarker might mask the real disease risk.
Despite their signif icant correlation with some diseases,enterotypes might not be appropriate for predicting disease risk due to the masking effects[16].More investigations are needed to test the feasibility of using enterotypes as the biomarkers.
Methods and assessments for enterotype analysis
Methods for enterotype analysis
Metagenomic data or 16S rRNA gene sequencing data are required for enterotype analysis.Accordingly,the built phylogenetic annotation can be used to obtain relative abundances of taxa in gut microbiota at different taxonomic level.
Enterotype analysis was initially conducted at the genus level[8].First,the abundances of classif ied genera are used to produce a JSD matrix between samples.Partitioning around medoids (PAM) clustering algorithm is then employed to stratify samples using the distance matrix and the putative number of clusters(designated as‘k')as input.To decide the most optimal‘k',the Calin´ksi-Harabasz(CH)index[57]is applied to display how good performance in picking different‘k'as the input to PAM.Subsequently,silhouette index(SI)[58]is adopted to assess the statistical validation of their clustering results.Finally,between-class analysis(BCA)and PCoA are performed to visualize the enterotypes.

Figure 2 Enterotype might not always be stableSimulated microbiota samples are indicated using dots,triangles,or squares with different shapes representing different patterns of microbiota composition that these samples initially contain.In the expected scenario,the simulated samples stay in the region of their original enterotypes during a long period of time ranging from 5 to 10 years(A-C).In the observed scenario,the microbiota composition of the simulated samples changes a lot over the time,so that most of them do not stay in the region of enterotypes where they originally belong(D-F).
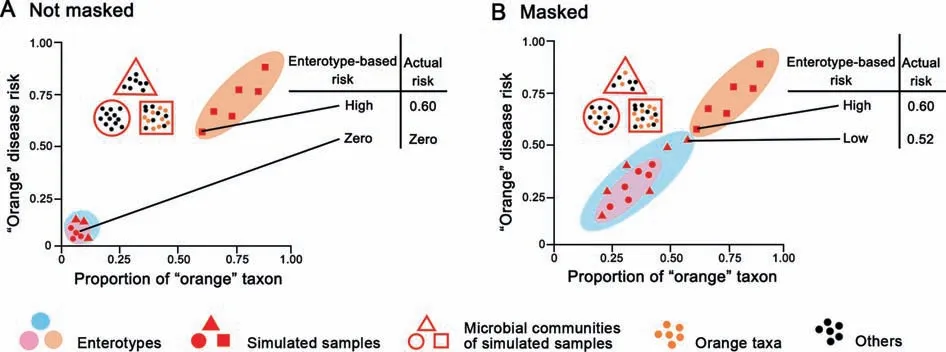
Figure 3 Enterotype might not be a good biomarker Simulated gut microbiota samples are plotted as triangles,circles and squares based on their enterotypes.The microbiota composition within these samples determines their enterotypes(e.g.,‘‘triangle”samples of‘‘blue”enterotype,‘‘circle”samples of‘‘pink”enterotype,and‘‘square”samples of‘‘orange”enterotype,respectively).These samples disperse on the plots according to the proportion of‘‘orange”taxon(horizontal axis)in their microbiota composition.The‘‘orange”disease risk(vertical axis)is directly associated with theabundance of‘‘orange”taxon.A.Under the condition that‘‘orange”taxon is present only in the‘‘square”microbiota,the enterotype-based risk is consistent with actual risk based on the taxon.B.Once the‘‘orange”taxon is present in all microbiota samples,the enterotype-based risk might be misleading,which masks the actual risk.
Assessments for enterotype analysis
Several factors strongly affect the outcome of enterotype analysis as reported by Koren and colleagues[10].Firstly,different cluster scoring methods,such as prediction strength(PS)[59],SI[58],and CH[57],could produce inconsistent decisions on the optimal number of clusters when using the same distance matrix.SI and PS provide absolute measures to assess the likelihood of cluster structure emerging from a dataset,whereas CH index provides a relative measure to indicate the optimal number of clusters.Thus,at least one absolute measure(PS for a large-scale of samples and SI for few samples)is recommended to be included in enterotype analysis.
Moreover,using the same cluster scoring method on different distance matrices,such as the JSD,root JSD(rJSD),Bray-Curtis,and weighted/unweighted UniFrac distances,could produce inconsistent results.That means,the detection of enterotype is sensitive to the distance metrics employed.Furthermore,when using different taxonomic levels such as genus level and species level to calculate the distance matrices,inconsistent number of clusters might also be produced in enterotype analysis.
Therefore,various methods should be tested in enterotype analysis to f igure out the discrepancies.Due to the lack of a unif ied standard for methods of enterotype analysis,users should justify their methods of choice.
Application of theenterotypeconcept to other organisms
In general,the term‘‘enterotype”refers to our microbiota typeswithin thegut.Interestingly,recently it hasbeen adopted to describe microbiota types across different human body sites[10],even in insects[60]and animals[38].
Several studies have reported the stratif ication of microbiota in other body sites.For instance,Klatt et al.[61]have successfully stratif ied 688 HIV-negative women into two clusters,using vaginal microbiota.The f irst cluster is dominated by Lactobacillus and the other one is dominated by non-Lactobacillus microbiota.Additionally,two clusters have been identif ied at oral sites[62],and two clusters have also been recognized in a lung microbiota[63].
As for stratifying microbiota of insects,Li et al.[60]have investigated the gut microbiota of 142 worker bees from 28 species of Chinese bumblebees,and have observed two robust clusters.Most samples(73%)are clustered into a subtype distinguished by abundant Gilliamella and Snodgrassella,with another subtype containing more Serratia and Hafnia.Both clusters share Lactobacillus.
For animal studies,Moeller et al.[38]have investigated the gut microbiota of 35 chimpanzees from the Gombe Stream National Park.They f ind that microbiota prof ilesof thesechimpanzeescan bestratif ied into threeclusters,with dominant Faecalibacterium,Lachnospiraceae,and Bulleidia,respectively.It is of notethat themicrobiota clustering isnot signif icantly related to the age,genealogy,or gender of their hosts.
Conclusion and perspectives
Enterotypehasremained a controversial concept asto whether human gut microbiome can be clustered into different types or just fall into a continuous gradient.
Owing to the extreme complexity of highly dimensional microbiota in human guts,it is really pragmatic for researchers to collapse them into a few categories.Nevertheless,existing studies cannot either substantiate or deny the enterotype concept.With more experiments conducted at a larger space scale to validate and improve the enterotype concept,it would be feasible to realize the categorization of human gut microbiota,using a unif ied enterotype method.This categorization would help usfurther understand the correlationsbetween gut microbiota and diseases to facilitate precision medicine based on gut microbiota.A recent work has already made a concrete step toward this goal[54].According to a classif ication procedure of enterotypes proposed by Costea et al.[54],if new gut samples cannot overlap with the enterotypes in the provided reference set,the enterotypes of these new samples will be computed de novo.Otherwise,these samples will be assigned to existing enterotypes of the reference set.This study provides the possibility for a unif ied standard of enterotypes analysis.
However,a number of technical issues would impact the enterotype analysis.For instance,the input datasets of enterotype analysis are directly inf luenced by multiple factors including the sample processing,DNA extraction,and the sequencing technology[64].Moreover,relative abundance,rather than the absolute abundance,is adopted in enterotype analysisdueto thetechnological hurdlesin obtaining theabsolute abundance,which might not describe real prof iles of microbiota.Using more advanced techniques such as f low cytometry,we can now perform enterotype analysis based on the absolute number of taxa[65].
As we have described,the concept of enterotype can be applied not only in human gut microbiota but also in microbiota samples from other human body sites.Thus,we expect in other ecological niches,microbiota might also be stratif ied into different subtypes designated as‘‘soiltype”(in soil),‘‘marinotype”(in marine),‘‘plantotype”(in plant),etc.Collapsing the highly multidimensional microbiota of ecological niches into a few categories might help us to better describe thecharacteristics of these microbiota,and then deal with environmental issues.
Finally,we have to admit that gut microbiota changes constantly in a dynamic status.Considering this,more studies are supposed to focus on the dynamic nature,using frequent sampling,with integrativecomparison of microbiota on time-series or among the changing conditions.This would be a critical step toward a comprehensive understanding of the ecology and evolution of any microbiota.
Competing interests
The authors have declared no competing interests.
Acknowledgments
This work was partially supported by the National Key R&D Program of China(Grant No.2018YFC0910502)and the National Natural Science Foundation of China(Grant Nos.61103167,31271410,and 31671374).
 Genomics,Proteomics & Bioinformatics2019年1期
Genomics,Proteomics & Bioinformatics2019年1期
- Genomics,Proteomics & Bioinformatics的其它文章
- Intestinal Microbiota in Early Life and Its Implications on Childhood Health
- Correlation of Gut Microbiome Between ASD Children and Mothers and Potential Biomarkers for Risk Assessment
- Antibiotic Treatment Drives the Diversif ication of the Human Gut Resistome
- Eあects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Ref lux Disease
- Inulin Can Alleviate Metabolism Disorders in ob/ob Miceby Partially Restoring Leptinrelated Pathways Mediated by Gut Microbiota
- Agricultural Risk Factors Inf luence Microbial Ecology in Honghu Lake
