Disseminated nocardiosis due to Nocardia otitidiscaviarum: A case report and literature review
Shu-Wei Zheng
Department of Infectious Diseases, Singapore General Hospital, Singapore
Keywords:Disseminated nocardiosis Nocardia otitidiscaviarum Corticosteroids
ABSTRACT Rationale: Disseminated nocardiosis due to Nocardia otitidiscaviarum is rarely reported in immunocompetent hosts.Patient concerns: A 59 year old male patient complained of painful soft tissue swellings and fever for two days.Diagnosis: Disseminated nocardiosis due to Nocardia otitidiscaviarum.Interventions: Initial antimicrobial therapy with imipenem and trimethoprim/sulfamethoxazole was switched to 6 weeks of trimethoprim/sulfamethoxazole, linezolid and tigecycline after sensitivity test results were available. Thereafter, the patient was switched to maintenance trimethoprim/sulfamethoxazole and moxifloxacin. Prednisolone was gradually tapered.Outcomes: Soft tissue swelling and pain disappeared and the patient was discharged uneventfully.Lessons: Disseminated nocardiosis due to Nocardia otitidiscaviarum should be suspected in immunocompetent hosts with risk factors such as medication with prednisolone. Early identification of the causative species and susceptibility results is crucial given the diverse resistance patterns amongst various Nocardia species.
1. Introduction
Nocardia spp. is a Gram-positive, weakly acid-fast microorganism that is ubiquitous in the environment with a worldwide distribution. Common factors include long term corticosteroid use, malignancy, human immunodeficiency virus infection and chronic obstructive pulmonary predisposing to Nocardia infectionsdisease[1]. Various Nocardia species differ in their pathogenicity, geographical distribution and antimicrobial susceptibility. Historically, before the advent of molecular techniques and mass spectrometry, biochemical methods were used in the identification of Nocardia species, resulting in only a few known Nocardia species[2]. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)has emerged as a useful tool for the accurate identification of Nocardia species[2-4].
First recognized in 1924 from a guinea pig with ear disease,Nocardia otitidiscaviarum (N. otitidiscaviarum) has been described as an opportunistic pathogen causing local and disseminated infection in both immunocompetent and immunocompromised hosts[1]. Herein, we describe a case of disseminated N.otitidiscaviarum predisposed by corticosteroid use and a literature review of published case reports and case series of N.otitiscaviarum infections.
2. Case report
This case report was approved by the local ethics committee of the author’s affiliated institution. The patient provided consent to the use of photographs in the report. Identifying information of the patient was removed.
A 59-year-old man presented with an acute history of painful soft tissue swellings and fever. Upon further questioning, he denied any history of trauma and had been perfectly well 2 d before. He had no cough, hemoptysis, or chest pain. He had a history of hypertension,dyslipidemia, and stage Ⅳ chronic kidney disease (baseline serum creatinine ranging 284-313 μmol/L, reference range, 54-101 μmol/L),secondary to presumptive glomerulonephritis. Two months ago,he had been started on a prednisolone dose of 60 mg daily by his nephrologist for suspected immune-mediated glomerulonephritis.Clinical examination revealed a febrile middle-aged man with discreet tender, warm, and erythematous swellings over his left elbow (Figure 1), right thigh, and right buttock, consistent with cutaneous abscesses. There was no palpable lymphadenopathy.Cardiorespiratory and abdominal examination was unremarkable.He had no focal neurological deficit.
Laboratory findings included the following: serum sodium 140 mmol/L (reference range, 136-146 mmol/L), serum potassium 4.5 mmol/L (reference range, 3.6-5.0 mmol/L), serum bicarbonate 24.6 mmol/L (reference range, 19.0-29.0 mmol/L), serum creatinine 332 umol/L, haemoglobin 12.5 g/dL (reference range,14.0-18.0 g/dL), white blood cell count 15.02×109/L (reference range, 4.0-10.0×109/L), C-reactive protein 86.6 mg/L (reference range, 0.2-9.1 mg/L), serum albumin 31 g/L (reference range,40-51 g/L), bilirubin 5 μmol/L (reference range, 7-32 μmol/L),alkaline phosphatase 51 U/L (reference range, 39-99 U/L), alanine aminotransferase 6 U/L (reference range, 6-66 U/L), and aspartate aminotransferase 17 U/L (reference range, 12-42 U/L). Blood cultures were non-yielding.
A chest radiograph showed a left upper lobe opacity, which was new compared to a previous radiograph taken three months before (Figure 2A-2B). A computed tomography scan of his thorax revealed a spiculated left upper lobe mass (Figure 2C)corresponding to the opacity detected on the chest radiograph.
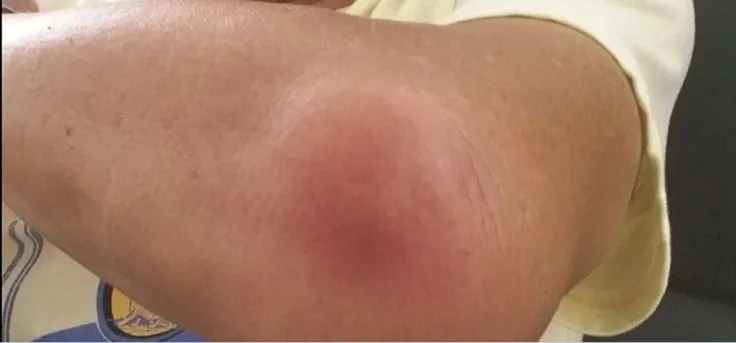
Figure 1. A cutaneous abscess seen over the left elbow of a febrile 59-year old man with disseminated nocardiosis.
He initially declined surgical drainage. Magnetic resonance imaging (MRI) to delineate the soft tissue abscesses and revealed subcutaneous collections over the left elbow joint, right gluteus maximus, and was right sartorius and iliacus muscle, together with an intramuscular abscess within the right gracilis muscle (Figure 3A-3H).
He did subsequently undergo a diagnostic transthoracic needle aspiration of the lung lesion as well as an incision and drainage of the multiple subcutaneous abscesses. Histology of the lung specimen showed acute-on-chronic pneumonitis with microabscess formation. No granulomas or infective organisms were identified and there was no evidence of malignancy. Gram stain, fungal microscopy, acid-fast bacilli stain, and nucleic acid amplification test for Mycobacterium tuberculosis complex were negative.
Eventually, chalky colonies were seen on the blood agar plate several days later (Figure 4A) from both the lung and subcutaneous tissue specimens. On microscopy, beaded Gram-positive rods were seen (Figure 4B), suspicious for nocardiosis. Antimicrobial therapy was empirically switched to a combination of imipenem and trimethoprim/sulfamethoxazole. The causative microorganism was identified to be N. otitidiscaviarum by the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry.
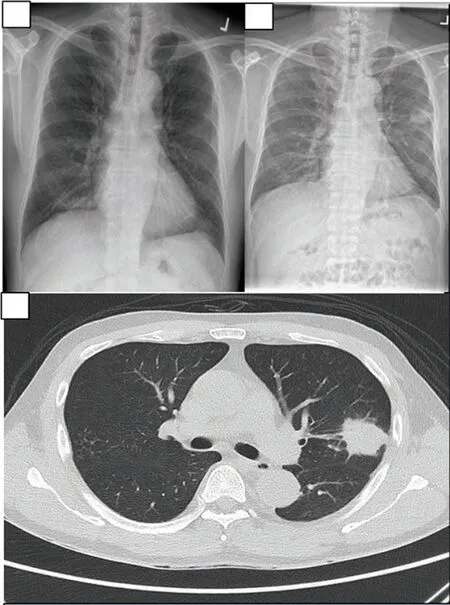
Figure 2. Radiographic images including previous and presenting chest radiographs, as well as a coronal view on CT scan of the pulmonary Nocardial lesion of the febrile middle-aged man. A: Chest radiograph 3 months prior to presentation, prior to commencement of corticosteroids. B:Left middle zone opacification at presentation. C: Spiculated mass in the left upper lobe corresponding to the opacity detected on chest radiograph.

Figure 3. Skin and soft tissue abscesses at the right thigh, groin, buttock and the left elbow demonstrated on MRI on the febrile middle-aged man with disseminated nocardiosis. A/B: An 4 intramuscular abscess collection within the right gracilis muscle with surrounding edema. C/D: Small collection is noted within the subcutaneous fat overlying the right sartorius and iliacus muscle at the right groin region. E/F: A small collection within the subcutaneous fat overlying the postero-lateral aspect of the right gluteus maximus. G/H: Extensive area of subcutaneous hyperintense theta signal seen in the posterior aspect of the elbow joint; focal thick wall fluid collection with layering noted in the subcutaneous layers.

Figure 4. Culture morphology and microscopic (magnified at 40×) images of the Nocardia spp. isolates cultured from the patient. A: Chalky white colonies on blood agar plate. B: Microscopy slides of beaded Gram positive bacilli of Nocardia spp.
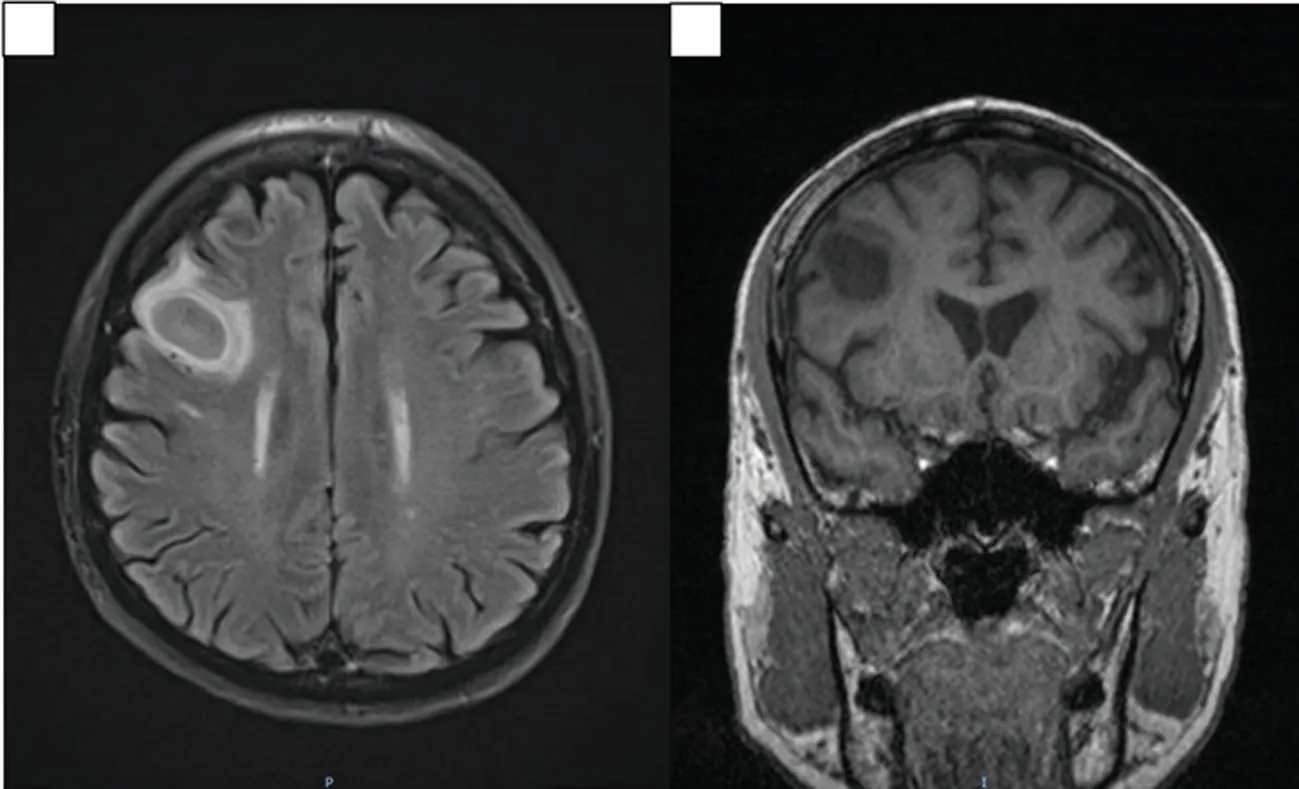
Figure 5. MRI brain images demonstrating presumptive Nocardial brain abscess at the right frontal lobe on coronal and sagittal views. A: Coronal view of MRI brain showing 2.3 cm×2.1 cm×1.3 cm solitary, well-circumscribed, lesion demonstrating restricted diffusion, T2-weighted hyperintensity and T1-weighted hypointensity that is consistent with a cerebral abscess. Mild surrounding vasogenic edema without significant mass effect is seen. B: Sagittal view showing the same lesion in the frontal lobe.
An MRI of his brain showed a solitary lesion in the right frontal lobe, consistent with a cerebral abscess (Figure 5A-5B).Hence, this patient has disseminated nocardiosis resulting in subcutaneous,intramuscular, lung and brain abscesses, predisposed by cortico steroids.
Susceptibility testing using the broth microdilution method suggested that this isolate was susceptible to amikacin [minimum inhibition concentration (MIC)≤8 μg/mL], tobramycin (MIC≤4 μg/mL),trimethoprim/sulfamethoxazole (MIC≤2/38 μg/mL), and linezolid(MIC≤8 μg/mL), intermediate to moxifloxacin (MIC=2 μg/mL),doxycycline (MIC=2 μg/mL), and minocycline (MIC=2 μg/mL),and resistant to amoxicillin/clavulanic acid (MIC≥32/16 μg/mL), ceftriaxone (MIC≥64 μg/mL), cefepime (MIC≥32 μg/mL), imipenem (MIC≥16 μg/mL), ciprofloxacin (MIC≥4 μg/mL), and clarithromycin (MIC≥8 μg/mL). Tigecycline MIC was tested to be 0.5 μg/mL but without interpretive criteria from the Clinical&Laboratory Standards Institute. Antimicrobial therapy was further switched to trimethoprim/sulfamethoxazole,linezolid and tigecycline. Aminoglycosides were not used in view of his underlying renal disease. He completed 6 weeks of this combination therapy before switching to maintenance trimethoprim/sulfamethoxazole and moxifloxacin. His prednisolone was gradually tapered and ceased and all his cutaneous lesions were drained surgically. A summary of his clinical progress is shown in Figure 6.
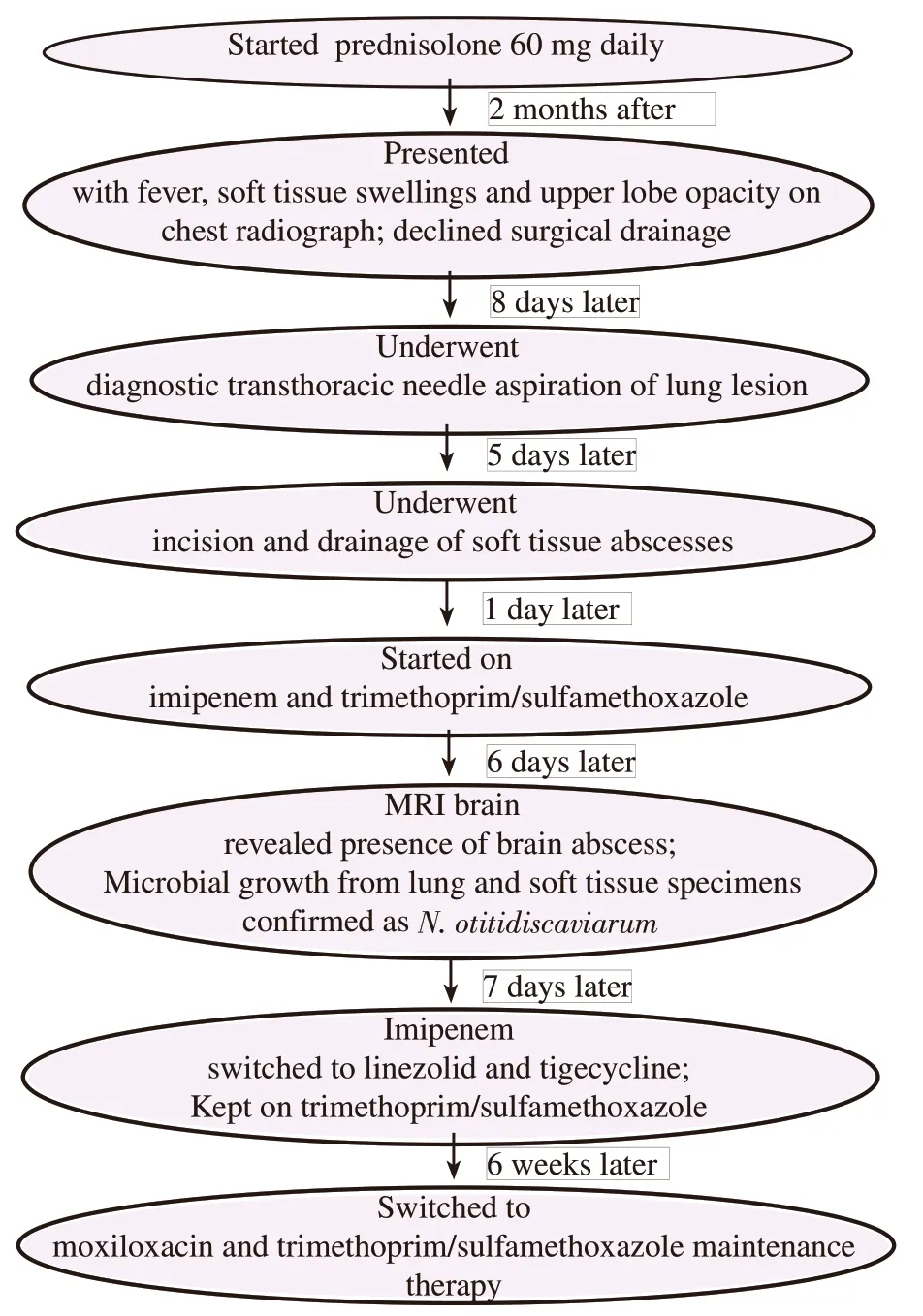
Figure 6. Timeline of clinical events summarizing the progress of this patient from immunosuppression, presentation to eventual treatment regimen.
3. Discussion
A computerized search for publications on N. otitidiscaviarum in the Medline database of the National Library of Medicine was conducted for the literature review. Only reports available in English, published in the last 25 years, were included. Articles were identified through screening of titles and their abstracts to determine final eligibility. Key words used include “Nocardia otitidiscaviarum”.
The search terms yielded a total of 106 articles, in which 49 articles were included[5-53]. One article was excluded in view of inconsistently reported results. One article[43] was reviewed from its abstract only, as the full manuscript was not available.A total of 141 cases of N. otitidiscaviarum infections, including our case, were collated. Their clinical features and antimicrobial susceptibilities were shown in Table 1. The median age was 57 years, and 69.2% were males. The commonest risk factors reported included corticosteroids use (n=11), chronic lung disease (n=12),solid organ transplant (n=8), underlying human immunodeficiency virus (n=4) and solid organ malignancy (n=3). Eighteen patients did not appear to have any underlying medical conditions.Mortality occurred in 8 out of 49 cases reported (16.3%). Seventytwo cases had their sites of infection stated. The commonest clinical infective syndrome included pneumonia (n=35, 48.6%),skin and soft tissue infection (n=25, 34.7%), brain abscess (n=8,11.1%), parapneumonic effusion/empyema (n=7, 9.72%), lymph nodal involvement (n=3, 4.17%), septic arthritis (n=3, 4.17%),ocular infection (n=3, 4.17%), osteomyelitis (n=1, 1.39%),extraperitoneal mass (n=1, 1.39%) and bacteremia (n=1, 1.39%). 7 patients (9.72%) presented with disseminated disease.

Table 1. Clinical and microbiological characteristics of patients with reported Nocardia otitidiscaviarum.

?

AMC: amoxicillin clavulanic acid; AMS: ampicillin/sulbactam; CRO: ceftriaxone; CTX: cefotaxime; FEP: cefepime; IMI: imipenem; MEP: meropenem;GEN: gentamicin; AMK: amikacin; TOB: tobramycin; STR: streptomycin; SXT: trimethoprim/sulfamethoxazole; GAT: gatifloxacin; MOX: moxifloxacin;LEV: levofloxacin; CIP: ciprofloxacin; CLR: clarithromycin; AZI: azithromycin; ERY: erythromycin; LZD: linezolid, DOX: doxycycline; MIN:minocycline, RIF: rifampin; TIG: tigecycline; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; HIV: human immunodeficiency virus;NM: not mentioned.
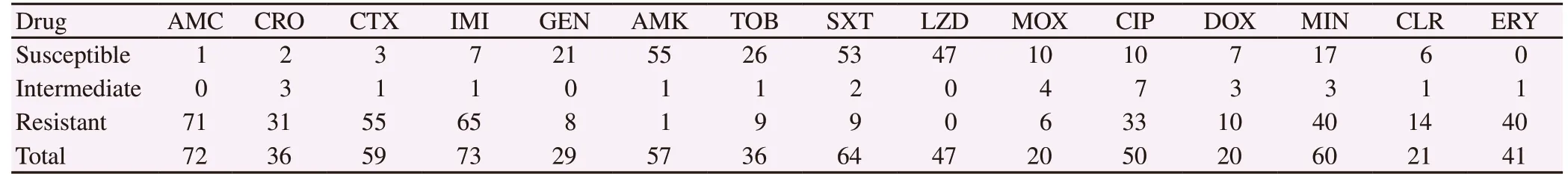
Table 2. Antimicrobial susceptibility of Nocardia otitidiscaviarum reported in the literature.
Table 2 shows the commonest antimicrobial agents tested.We included agents with at least 20 isolates tested, where susceptibilities were reported specifically to be sensitive,intermediate or resistant only. The most susceptible agents included linezolid (n=47, 100.0%), amikacin (n=55, 96.5%),trimethoprim/sulfamethoxazole (n=53, 82.8%), gentamicin (n=21,72.4%) and tobramycin (n=26, 72.2%). Beta-lactam antimicrobial agents exhibited high rates of resistance against this species, with 98.6% for amoxicillin clavulanic acid, 93.2% for cefotaxime,89.0% for imipenem and 86.1% for ceftriaxone.
In one of the largest retrospective study of nocardiosis with 113 patients proven with Nocardial infections, the commonest implicated species included Nocardia brasilensis (n=54, 47.8%),
Nocardia asteroides (n=36, 31.8%), Nocardia farcinica (n=7, 6.2%),Nocardia flavorosea (n=4, 3.5%), and N. otitidiscaviarum (n=3,2.7%). Cutaneous infection was the commonest presentation in 64 patients (56.6%), followed by pulmonary infection in 38(33.6%) and brain abscesses in 7 patients (6.2%). Eight patients(7.1%) presented with disseminated infection, with 5 secondary to Nocardia asteroides and 3 secondary to Nocardia farcinica.Mortality in this series was 8.0%[19]. We report the largest series of cases of N. otitidiscaviarum infection reported over the course of 25 years, in which pulmonary involvement appear the commonest.The case fatality rate in our series appears much higher at 16.3%,likely due to more cases of pneumonia and brain abscesses reported. Indeed, amongst the eight cases of mortality in our series,six patients had pulmonary involvement only and two had brain abscesses.
Based on a Spanish series of 35 strains of N. otitidiscaviarum,beta-lactam resistance rates appeared to be high, which is consistent with our series. Among three beta-lactams tested,including amoxicillin/clavulanate, cefotaxime, and imipenem,resistance ranged from 82.9% to 100.0%. This contrasts with other commoner Nocardial species that are relatively more beta-lactam susceptible. For example, Nocardia cyriacigeorgica, which is the commonest Nocardial isolate in this series, exhibits resistance to cefotaxime and imipenem in only 2.8% of the cases[53]. Given the significant associated morbidity and mortality, and that susceptibility results take time to return, early identification of the causative Nocardial species plays a huge role in targeting an appropriate initial empiric therapy. In general, the treatment of disseminated nocardiosis entails a combination systemic antimicrobial therapy for a protracted duration. Currently, no prospective trials are available to guide effective therapy. For severe infections secondary to N. otitidiscaviarum, we suggest initial combination therapy with linezolid, amikacin and trimethoprim/sulfamethoxazole until susceptibility results return.
There are current no interpretative breakpoints from the Clinical&Laboratory Standards Institute for the use of tigecycline for nocardiosis. In a case series of fifteen cases of nocardiosis reported by Maraki et al[24] the minimal inhibitory concentration(MIC90) range of all isolates are at most 0.19 g/mL, including four isolates of N. otitidiscaviarum. In Lai et al’s study, tigecycline appears most effective for Nocardia brasiliensis and Nocardia puris, with MIC values of ≤8 μg/mL against all of the tested isolates[51]. Our patient received tigecycline together with linezolid and trimethoprim/sulfamethoxazole as part of a combination therapy for disseminated nocardiosis with clinico-radiological improvement. These emerging data potentially suggests tigecycline as a possible option in the treatment of nocardiosis.
We present an interesting case of disseminated nocardiosis secondary to a rare species of Nocardia, treated with combination tigecycline, linezolid and trimethoprim/sulfamethoxazole.Awareness of the possible risk factors for this infection is crucial to suspecting this disease, which can present in a widely diverse manner, virtually involving any organ. Early identification of the causative species and susceptibility results is crucial given the diverse resistance patterns amongst various Nocardia species.
Conflict of interest statement
We declare that we have no conflict of interest.
 Asian Pacific Journal of Tropical Medicine2019年4期
Asian Pacific Journal of Tropical Medicine2019年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Latent tuberculosis infection among medical students in Malaysia
- Misinformation on salt water use among Nigerians during 2014 Ebola outbreak and the role of social media
- Wild chive oil is an extremely effective larvicide against malaria mosquito vector Anopheles stephensi
- Characterization of the salivary microbiome in healthy Thai children
- Morphometric discrimination between females of two isomorphic sand fly species, Phlebotomus caucasicus and Phlebotomus mongolensis (Diptera:Phlebotominae) in endemic and non-endemic foci of zoonotic cutaneous leishmaniasis in Iran
- Herbal remedies, vaccines and drugs for dengue fever: Emerging prevention and treatment strategies
