Wild chive oil is an extremely effective larvicide against malaria mosquito vector Anopheles stephensi
Alireza Sanei-Dehkordi, Moussa Soleimani-Ahmadi✉, Yaser Salim Abadi, Azim Paksa
1Department of Medical Entomology and Vector Control, School of Health, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
2Infectious and Tropical Diseases Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
3Department of Health Services and Health Promotion, School of Health, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
4Tabriz University of Medical Sciences, Tabriz, Iran
Keywords:Larvicide Allium schoenoprasum Chive Essential oil Gas chromatography-mass spectrometry Anopheles stephensi
ABSTRACT Objective: To assess the chemical composition and mosquito larvicidal potentials of essential oil of wild chive (Allium schoenoprasum L.) against Anopheles stephensi.Methods: In the search for an environmentally safer alternative mosquitoes control, the larvicidal efficacy of essential oil obtained from the leaves of Allium schoenoprasum L. against Anopheles stephensi was determined. The composition of chive essential oil was analyzed by gas chromatography-mass spectrometry.Results: In toxicity assays, the essential oil demonstrated substantial larvicidal activity against larvae of Anopheles stephensi with LC50 and LC90 values of 2.60, and 7.31 ppm after 24 h of exposure, respectively. Gas chromatography-mass spectrometry analysis of chive essential oil identified 35 components representing more than 97.31% of the total essential oil. The main constituents were sulfur compounds, including diallyl trisulfide (13.72%), 2-ethyl[1,3]dithiane(8.93%), allyl methyl trisulfide (8.77%), and trimethylene trisulfide (6.64%), respectively.Conclusions: This study demonstrated that wild chive essential oil has a rich source of eco-friendly bioactive compounds for use as a mosquito larvicide. The main reason for its extraordinary properties may be related to the high percentage of sulfur compounds.
1. Introduction
Mosquitoes are dangerous vectors of numerous serious human pathogens, causing millions of deaths every year[1]. Some species within the genera Anopheles, Aedes, and Culex are responsible for the most deadly and debilitating mosquito-borne diseases[2].
Malaria is one of the major diseases that transmitted by Anopheles mosquitoes[1]. According to the latest World Health Organization(WHO) estimates, at least 216 million new cases of malaria in 2016 occurred worldwide resulting in more than 445 000 deaths[3]. The Anopheles (Cellia) stephensi Liston 1901 is one of the most important malaria vectors[2,4].
A more efficient strategy to reduce mosquito populations is to target the larvae. Currently, the control of mosquito larvae relies upon on using synthetic insecticides[2]. Continued use of such insecticides for mosquitoes control programs has brought on disruption of natural biological systems and has sometimes caused the development of resistance in mosquitoes[5,6].
Based on this view, new compounds must be found in vector control that will replace synthetic insecticides. Secondary metabolites of plants are candidates for the discovery of new products to fight mosquitoes[7,8]. Botanical pesticides appear to have no adverse effect on non-target organisms. Insecticide-based on plants has the advantage of presenting novel modes of action against mosquito vectors that could lessen the risk of cross-resistance[7]. In this context, essential oils (EOs) have received much attention as a potential source of bioactive compounds against mosquitoes[9].
Plants belonging to Allium genus possess a very long tradition of ethnobiological uses, as foods, spices, medicinal herbs, and food preservatives. Allium schoenoprasum L.( A. schoenoprasum)(chive), is one of the members of this genus. The species of the genus Allium are characterized by the presence of their rich content in sulfur compounds[10]. The chive leaves contain some sulphur compounds[11]. In recent studies, the EO obtained from A. schoenoprasum has been evaluated for antimicrobial[12],antioxidant[12,13], antifungal[14], anthelminthic[15] and anticancer[16]activities.
In this study, we examined the toxicity of A. schoenoprasum EO against Anopheles stephensi(An. stephensi). In addition, the constituents of EO were identified and quantitatively determined by gas chromatography-mass spectrometry (GC-MS) analyses.
2. Materials and methods
2.1. Plant material
Fresh leaves of A. schoenoprasum L. were collected before flowering stage from different areas of naturally growing areas in May 2017 from Marghmalek region, Chaharmahal and Bakhtiari Province, Iran(50o25´ E, 32o25´ N elevation: 2 490 m). The plant was identified and sample voucher was deposited at the Department of Biology,Shahrekord University (SKU), Shahrekord, Iran. The leaves were dried in a dry and shady place and kept in a light protected container.
2.2. Essential oil extraction
The dried leaves (400 g) were extracted by hydro-distillation for 3 h, using a Clevenger-type. The yields were measured according to dry weight. The obtained EO was dried over sodium sulfate anhydrous and kept in a refrigerator at 4 ℃ till required.
2.3. Essential oil analysis
The EO obtained from A. schoenoprasum was analyzed using an Agilent 6890/5973 N GC-MS equipped with an Agilent 19091S-433 HP-5 MS capillary column. The carrier gas was helium (1.2 mL/min).In split mode, a 1 μL volume of each sample has been injected and the split ratio was 1: 20. The GC oven program was as follows: initial temperature of 50 ℃ for 3 min, programmed rate 3 ℃/min up to 300 ℃. The temperatures of the injector and detector were 250 ℃ and 280 ℃, respectively. An ionizing energy of 70 eV has been used for MS detection. The Kovat´s indices (KI) for the detected compounds were determined in relation to a homologous series of n-alkanes(C8- C24) running under the same operating conditions. Tentative identification of the compounds was based on computer matching with the Wiley and NIST libraries, as well as by comparing the mass spectrum and retention indices (RI) with those reported within the literature[12,17,18].
2.4. Mosquito rearing
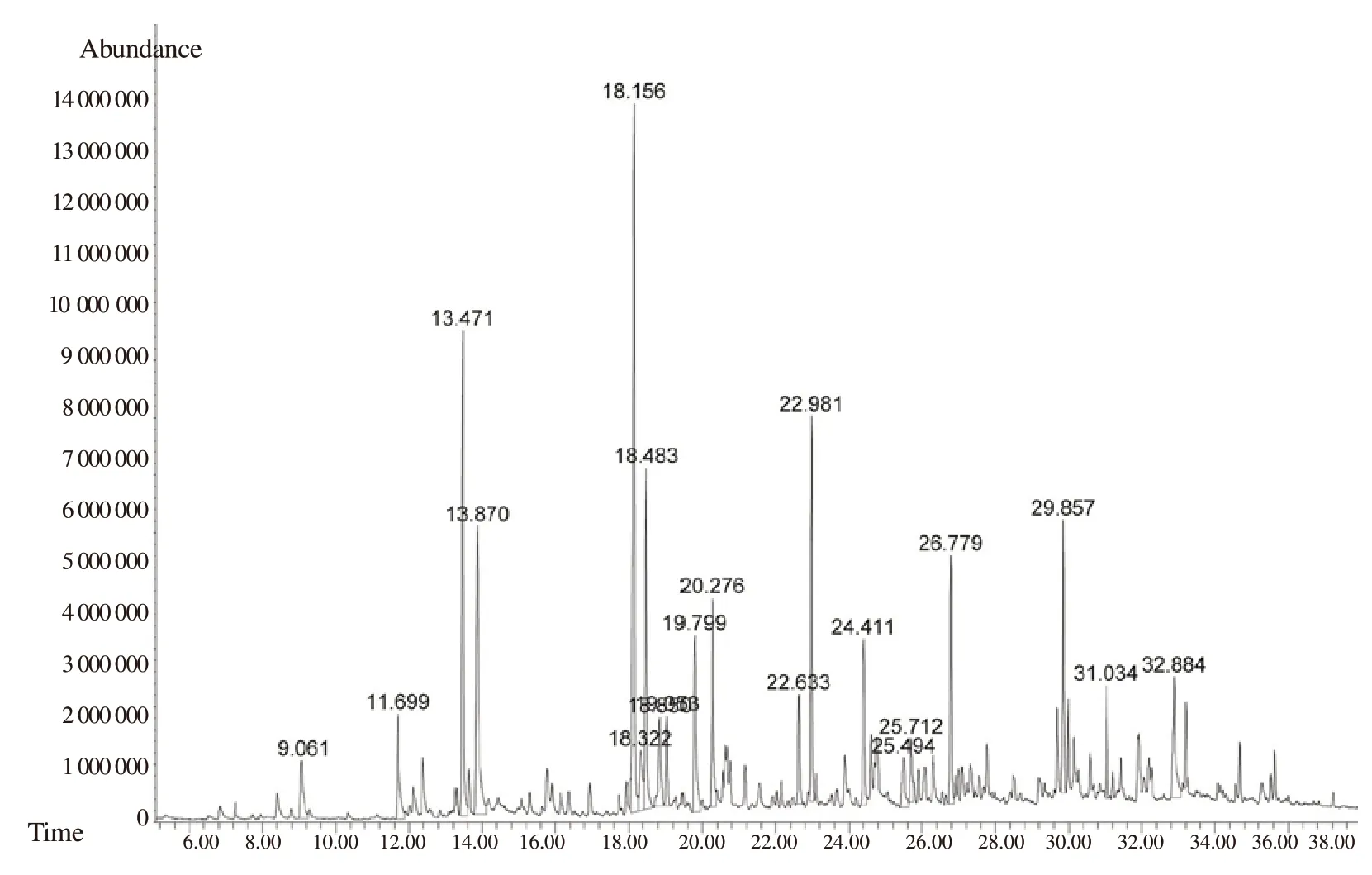
Figure 1. Total ion chromatogram of GC-MS analysis of the essential oil from leaves of Allium schoenoprasum.
The mosquito, An. stephensi was reared in the Culicidae insectary,Hormozgan University Medical Sciences. The mosquito colonies were kept up at (28 ± 1) ℃ with 12:12 light and dark photoperiod in(70 ± 5)% relative humidity. The larval stage of An. stephensi were continuously accessible for the bioassay tests.
2.5. Toxicity of essential oil against mosquito larvae
The larvicidal bioassays of the A. schoenoprasum EO were carried out according to procedures prescribed by the World Health Organization[19]. In brief, twenty-five larvae (in a 250 mL beaker)at the late 3rd and early 4th instars of An. stephensi were exposed to 0.625, 1.25, 2.5, 5 and 10 ppm of EO in water for 24 h. Four replicates were performed for each concentration (100 larvae).Larval mortality at 24 h after exposure was recorded.
2.6. Data analysis
The LC50and LC90values, regression equation and the 95%confidence limit of upper confidence limit (UCL) and lower confidence limit (LCL) and Chi-square values were determined by using probit analysis (Finney’s method)[20, 21].
3. Results
3.1. Yield and chemical components of the essential oil
The yield of EO from A. schoenoprasum was 0.005% (v/w) based on dry weight. The chemical components and their relative percentage of A. schoenoprasum EO were shown in Table 1.
GC-MS identified 35 constituents representing about 97.31% of the EO (Figure 1). The main constituents were sulfur compounds,including diallyl trisulfide (13.72%), 2-ethyl[1,3]dithiane (8.93%),allyl methyl trisulfide (8.77%) and trimethylene trisulfide (6.64%),respectively. Other minor components were found to be β-ionone(5.97%), β-eudesmol (4.53%), and allyl methyl tetrasulfide (4.22%).
3.2. Mosquito larvicide effect
The EO of wild chive exerted significant larvicidal activity against An. stephensi after exposure for 24 h. A positive correlation between EO concentration and mosquito mortality has been observed (Figure 2). EO induced (5.00±0.96)% , (24.00±0.82)%,(37.00±1.89)%, (76.00±2.16)% and 100% larval fatality at 0.625,1.25, 2.5, 5 and 10 ppm, respectively. The obtained values for LC50and LC90were 2.6 (Lower: 1.69-Upper: 4.01) and 7.31(Lower:4.57-Upper: 21.89) ppm, respectively; the Probit equation was Y =-1.1832 + 2.8532X. The Chi-square value is significant at P<0.05 level (χ2: 13.08, df: 3). The Chi-square value in the bioassay tests indicated probably the heterogeneity of the test population.

Table 1. Chemical constituents of the essential oil from leaves of Allium schoenoprasum
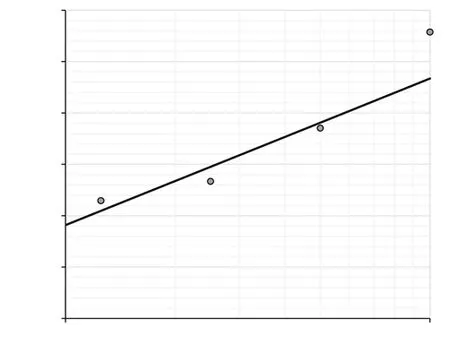
Figure 2. Probit regression line of Anopheles stephensi exposed to different interval concentrations of essential oils of Allium schoenoprasum.
4. Discussion
The Allium genus produces sulfur-based chemicals which are extremely potent in terms of aroma and physiological effects[22, 23].Forever previous investigations have also revealed that the sulfur compounds in EOs of Allium spp. could inhibit acetylcholinesterase enzyme activity in insects[24, 25]. Our results show that more than 66%of the compounds of the chive oil are comprised of sulfur volatiles.diallyl trisulfide (13.72%), 2-ethyl[1,3]dithiane (8.93%), and allyl methyl trisulfide (8.77%) which are known as the main components of the EO of wild chive. The presence of these mentioned compounds in chive oil may be responsible for the larvicide effect on An. stephensi.
Previous research conducted by Mnayer et al.[12] showed that the main constituents in the EO of bulbs of chive collected from France were dipropyl disulfide, dipropyl trisulfide, and methyl propyl trisulfide which including about 19.49%, 15.21%, and 8.47%of total EO, respectively. In another report, the EO of the leaves of chive from Venezuela mainly contained bis-(2-hydroxyethyl)-disulfide[72.06], 2,4,5-trithiahexane (5.45%), and tris-(methylthio)-methane (4.01%)[18]. It is known that several factors such as genetic and environmental conditions, seasonal variation, plant age,phenological stages and isolation methods influence the composition of EO produced by same-species plants[26].
According to the bioassay tests, chive EO has exerted significant larvicidal potent against An. stephensi, so that 10 ppm of it was effective enough to cause 100 % larval mortality.
Chive EO possessed very strong larvicidal activity (LC50: 2.6 ppm)against An. stephensi compared with the other EOs using the same bioassay method in previous investigations, e.g. EOs of Zhumeria majdae (LC50: 61.34 ppm)[27], Citrus paradise (LC50: 35.71 ppm)[28], Bunium persicum (LC50: 27.72 ppm)[29], Tanacetum persicum(LC50: 48.64 ppm)[30], Achillea kellalensis (LC50: 35.42 ppm)[30],
Satureja bachtiarica (LC50: 24.27 ppm)[31], Achillea wilhelmsii (LC50:39.04 ppm)[32], Cionura erecta (LC50: 77.30 ppm)[33], Zanthoxylum armatum (LC50: 58 ppm)[34], Nigella sativa (LC50:53.9 ppm)[35],Clausena anisate (LC50: 119.59 ppm)[36]. Prajapati et al. reported the mosquito larvicidal efficacy of EOs from 10 medicinal herbs with LC50values ranging from 110.2 ppm to 387.8 ppm against An.stephensi[37]. In this paper, which is based on previous studies about the use of plants EOs to fight against mosquito larvae, there are eight ratings for mosquito larvicidal activity of plant EOs. And EO of A. schoenoprasum lies in the second group extremely active. If we consider Sedaghat et al. suggestion in the regard, this oil was thought to be a highly active larvicide[38].
It seems that the mosquito larvicidal properties of chive oil are related to their content of volatile sulfur compounds. Organosulfur compounds, such as diallyl sulfide and diallyl disulfide, have demonstrated insecticidal effects on pest insects[39, 40]. Kimbaris et al. studied the biological activity of several garlic oil ingredients;their results showed diallyl disulfide showed good larvicidal activity against Culex pipiens with LC50of 6.09 ppm, while the garlic EO had an LC50value of 8.01 ppm[41]. Plant EOs have favorable ecotoxicological properties, making them suitable to be used as pesticide in vector control programs in the future[7].
This study demonstrated that the EO obtained from wild chive has a rich source of eco-friendly bioactive compounds for use as a mosquito larvicide. The main reason for its extraordinary properties might be related to the high percentage of sulfur compounds,including diallyl trisulfide, 2-ethyl[1,3]dithiane, allyl methyl trisulfide, and trimethylene trisulfide, which can be used as a natural larvicidal agent for breeding-site management in the mosquito control programs.
In that way, the findings of the current investigation provide a possible way for further studies to find out the active molecule.However, further investigations must be conducted to describe the mode of action of each constituent separately and also its effects on non-target organisms.
Conflict of interest statement
The authors declare that no conflict of inerest exists.
 Asian Pacific Journal of Tropical Medicine2019年4期
Asian Pacific Journal of Tropical Medicine2019年4期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Disseminated nocardiosis due to Nocardia otitidiscaviarum: A case report and literature review
- Latent tuberculosis infection among medical students in Malaysia
- Misinformation on salt water use among Nigerians during 2014 Ebola outbreak and the role of social media
- Characterization of the salivary microbiome in healthy Thai children
- Morphometric discrimination between females of two isomorphic sand fly species, Phlebotomus caucasicus and Phlebotomus mongolensis (Diptera:Phlebotominae) in endemic and non-endemic foci of zoonotic cutaneous leishmaniasis in Iran
- Herbal remedies, vaccines and drugs for dengue fever: Emerging prevention and treatment strategies
