The chemical treatments combined with antagonistic yeast control anthracnose and maintain the quality of postharvest mango fruit
SHAO Yuan-zhi, ZENG Jiao-ke, TANG Hong, ZHOU Yi, LI Wen
1 College of Food Science, Hainan University, Haikou 570228, P.R.China
2 College of Horticulture, Hainan University, Haikou 570228, P.R.China
Abstract During the storage and transportation of the mango fruit, the major source of disease is anthracnose, caused by the fungus Colletotrichum gloeosporioides. The objective of this study is to f ind an appropriate method that not only reduces mango decay but also maintains its postharvest quality. The effects of chemicals, the use of the yeast species Metschnikowia pulcherrima and their combination on storage quality, focusing on the enzyme activity related to disease of Tainong mangos was studied. By immersing the mangoes in M. pulcherrima suspension (1.0×108 cfu mL-1), salicylic acid (SA) solution (50 mg L-1) or calcium chloride (CaCl2) solution (1.0 g L-1), the lesion expansion and decay of the mango fruit caused by C. gloeosporioides could be signif icantly delayed. These treatments also delayed the changes in quality traits (a* value, f irmness, contents of total soluble solids (TSS) and vitamin C (Vc), while the activities of anti-disease enzymes such as polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL), chitinase (CHT) and β-1,3-glucanase (GUN) were enhanced as compared to the control treatment. Furthermore, the combination of SA solution, CaCl2 solution and M. pulcherrima suspension presented an additive effect, increasing the eff icacy in controlling the disease and maintaining the storage quality of mango fruits. Taken together, our data suggest that the integration of chemical treatments combined with M. pulcherrima could be an alternative to the use of fungicides in postharvest treatment of the mango fruit, specif ically for improving storage quality as well as the control of the anthracnose.
Keywords: mango fruit, Metschnikowia pulcherrima, Colletotrichum gloeosporioides, storage quality, enzyme activity
1. Introduction
Postharvest disease is one of the most important factors in the serious decay and loss of horticultural products worldwide. Particularly, the mango (Mangifera indica L.) is one of the most perishable fruits and is often subject to softening, senescence, deterioration and fungal decay.
Anthracnose, caused by the fungus Colletotrichum gloeosporioides, is a major disease of the mango fruit, and can lead to considerable losses in the mango industry (Xu et al. 2017). Among the various methods of control of anthracnose, chemical fungicides have been routinely used to treat mangoes before storage (Freeman et al. 2014).
However, this method has been increasingly challenged and is now under scrutiny due to the adverse effects on the environment as well as food safety (Usall et al. 2016).
Therefore, exploring alternatives that are more effective as well as more convenient storage methods are needed for mango industry. Currently, there are various postharvest treatments that have been proven to prevent decay and extend the shelf-life of a number of fruits including salicylic acid (SA), the use of calcium chloride (CaCl2) and the application of biocontrol yeast.
SA is a simple phenol compound with various known roles in plant growth and developmental processes (Zhang et al. 2008). It can not only mediate the host response against pathogen (Pichia membranaefaciens) but can also prevent deterioration of orange fruit (Zhou et al. 2014). SA has numerous positive effects, including maintenance of total soluble solids (TSS), vitamin C (Vc) levels, phenols and anthocyanins, reducing respiration and ethylene biosynthesis as well as preventing weight loss and chilling injury. These effects have been noted in a number of fruits, vegetables and f lowers such as mangosteen (Mustafa et al. 2018), sponge gourd (Han et al. 2017) and anthurium cut f lowers (Aghdam et al. 2016).
Mineral nutrition is one of the main factors for quality and growth of horticultural crops. Among the m ineral nutrients, calcium (Ca) is known to play an important role in plant cell functions and is considered a key determinant in both fruit quality and their shelf life. The postharvest application of Ca has been reported to have numerous benef its to the fruit, including reduc ing physiological disorders, maintenance of membrane permeability, prevention of fruit softening, slow ing ripening processes as well as reducing the decay of fruits and making them more acceptable (Gago et al. 2016; Liu et al. 2017; Mansourbahmani et al. 2017). Previously, it has been noted that the use of CaCl2could be used as food additives to improve the texture of the meat in goose meat processes (Li X et al. 2017).
The application of biocontrol yeast is yet another promising strategy to control postharvest disease in fruits (Perez et al. 2017), due to the genetic stability of yeast, the effectiveness of yeast at low concentrations and the ability of yeast to act on a broad spectrum of pathogens (Liu et al. 2013). In particular, antagonistic yeasts have been proven to control postharvest pathogens, suppress disease incidence and reduce postharvest loss in numerous fruits, including winter jujube fruit (Guo et al. 2015), avocado (Campos-Martínez et al. 2016) and stone fruit (Grzegorczyk et al. 2017).
However, there are both limits and disadvantages of the use of a single substance or a single method (Dessalegn et al. 2013). Therefore, current research has been exploring multiple technologies and methods to improve the effects of a single chemical or antagonistic yeast. The application of chemical treatments in combination with biocontrol agents for the control of postharvest fungi has attracted signif icant research attention. There have been a number of technologies and approaches highlighted that could be used to develop and manage resistance against crop diseases and pests (Hartman et al. 2016) with fruits such as kiwifruit (Tang et al. 2015), mandarin fruit (Guo et al. 2014) and pineapple fruit (Ou et al. 2016). These studies show that the use of antagonistic yeast integrated with physical, chemical and/or other biological methods may have either an additive or synergistic effect on preventing pathogens in fruit.
Previously, two strains of biocontrol yeast, Debaryomyces nepalensis and Metschnikowia pulcherrima, were isolated from a mango orchard (Luo et al. 2015). To date, there has been no comprehensive investigation into the effects of combining the antagonistic yeast M. pulcherrima with SA and CaCl2on the storage of mango fruits. Therefore, the aim of this study was to investigate the effects of M. pulcherrima, SA solution and CaCl2solution alone as well as combined, on the storage quality of mango fruit, and the eff iciency on pathogen severity and enzyme activities related to the disease process was studied.
2. Materials and methods
2.1. Fruits
Mango fruits (M. indica L. cv. Tainong) were harvested at 80% maturity from an orchard in Changjiang City, Hainan Province, China. The fruits were put into plastic bags and transported by the car to the laboratory within 3 h in ambient condition, and t he fruits were selected based on uniform size as well as the absence of disease and mechanical wounding.
2.2. Antagonistic yeast
The antagonistic yeast M. pulcherrima was previously identif ied in our laboratory (Luo et al. 2015). It was isolated and screened from the rhizosphere soil of a mango tree in Changjiang City, Hainan Province, China. Before being used, it was cultured in potato dextrose broth (PDB) shaking at 28°C for 48 h. The yeast cells were diluted to the desired concentration and purif ied using the f lat line method (Huang et al. 2012). The f inal yeast preparation was stored at 4°C until use.
2.3. The pathogen
The pathogen C. gloeosporioides was provided by the Institute of Environment and Plant Protection of the Chinese Academy of Tropical Agricultural Sciences.
2.4. Testing of postharvest chemical treatments, antagonist yeast and their combination on severity of C. gloeosporioides disease
The treatment solutions were prepared as follows, distilled water (CK), M. pulcherrima suspension (1.0×108cfu mL-1), 50 mg L-1SA solution, 1.0 g L-1CaCl2solution and a combination treatment consisting of 50 mg L-1SA with 1.0 g L-1CaCl2and M. pulcherrima suspension (1.0×108cfu mL-1) solution. They were recorded as CK, MS, SA, CaCl2and CBT, respectively. A total of 20 µL d istilled water was used for control fruits.
Then a uniform wound that was 3 mm in diameter and approximately 4 mm deep was made at the equator of each mango fruit using the tip of a bacteriological loop (100 fruits total). Mangoes were divided into 5 groups and 20 µL of each solution (CK, MS, SA, CaCl2and CBT) was pipetted into the wound side of the corresponding group.
Two hours after inoculation, 10 µL of the C. gloeosporioides suspension (106spores mL-1) was inoculated into all wounds. All treated fruits were air-dried at room temperature and kept at 25°C and 90-95% relative humidity (RH). Lesion diameters were recorded every 4 days.
2.5. Testing of postharvest chemical treatments, antagonist yeast and their combination on storage quality of mango fruit
Mango fruits were randomly divided into 5 groups, each consisting of 150 fruits. Each group was immersed in CK, MS, SA, CaCl2or CBT solutions for 30 min. All treated fruits were air-dried at room temperature and kept at 25°C and 90-95% RH. For determination of storage quality with the testing methods described below, fruits were picked randomly to be analysed at 4-day intervals. All assays were performed 3 times each with 3 replicates (3 fruits for 1 replicate) and at ambient temperature (approximately 25°C).
Determination of d isease ind exFruit decay was determined for 30 fruits per treatment group according to a 5-point scale, where 0=no decay, 1=very slight decay, covering <10% of the fruit surface, 2=slight decay, covering>10% but <25% of the fruit surface, 3=moderate decay, covering>25% but <40% of the fruit surface and 4=severe decay, covering>40% of the fruit surface. The decay index was calculated using the following formula:
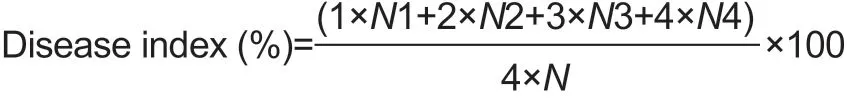
Where, N is the total number of fruit measured and N1, N2, N3 and N4 are the numbers of fruit showing the different severities of decay.
Determination of quality traits of mango fruit(1) Skin colour. Nine individual fruits for each treatment were selected for the determination of peel colour. This was measured at 3 points around the equatorial zone of the fruit by a Minolta CM-700d Chronometer (Konica Minolta Sensing Inc., Osaka, Japan), using the CIE (Commission International del'Eclairage) colour space L, a* and b* values. Values of lightness L* (ranging from 0, black to 100, white) and a* (positives values for red, negative values for green) were measured (here, only a* value was presented). The average of 3 readings was taken as a measure of a* for each fruit.
(2) Firmness. Flesh f irmness was measured on two opposite sides of each fruit, after peel removal, with a handheld penetrometer (FMH-1, Takemura Motor Manufacturing, Matsumoto, Japan), f itted with a conical probe (12 mm in diameter) and recorded as N (Newtons). Results were presented as the mean maximum force required to push the probe 7 mm into the fruit f lesh. The average of 3 readings was taken as a measure of f irmness for each fruit.
(3) Total soluble solids (TSS). TSS content was determined by measuring the refractive index of each fruit with a hand-held refractometer (N-1α, Atago Ltd., Tokyo, Japan) and the results expressed as percentages (%).
(4) Ascorbic acid (vitamin C). Vc content was determined using the method of 2,6-dichlorophenol indophenol (Shao et al. 2013). Results were expressed as mg of ascorbic acid per 100 g of fresh weight (FW).
(5) Assay of enzyme activities related to the disease process. Enzyme extraction was performed as described previously (Stadnik and Buchenaue 2000). In summary, mango f lesh tissues (2 g) were ground on ice, in a mortar using 5 mL of 100 mmol L-1Tris-HCl buffer (p H 5.5) c ontaining 4% (w/v) polyvinylpyrrolidon. The homogenate was centrifuged at 18 000×g for 30 min at 4°C. Supernatants were collected for the determination of the activities of polyphenol oxidase (PPO), phenylalanine ammonia lyase (PAL), chitinase (CHT) and β-1,3-glucanase (GUN) in the mango samples.
PPO activity was measured using the method previously described by Yuan et al. (2013) with the appropriate modif ications in the value of catechol. Using spectrophotometry, PPO activity was measured with 0.1% catechol (w/v) as the substrate at 398 nm. One unit of PPO activity was def ined as a change of 0.01 per min in OD398and results were expressed as U g-1FW min-1.
PAL activity was determined as previously described with modif ications (Assis et al. 2001) in the enzyme reaction system. Using spectrophotometry, PAL activity was measured with 0.02 mol L-1L-phenylalanine as the substrate at 290 nm. One unit of PAL activity was def ined as a change of 0.001 per min in OD290, and results were expressed as U g-1FW min-1.
CHT and GUN activities were assayed using the previously described methods with the needed modif ications (Bautista-Rosales et al. 2014) in the enzyme reaction system. For CHT activity, every 1×10-9mol of N-acetylglucosamine produced per second by CHT per gram of fruit weight that decomposed colloidal chitin, was considered one enzyme activity unit (U) and expressed as enzyme activity with U g-1FW s-1. For GUN activity, for every 1×10-9mol of glucose produced per second by GUN per gram of fruit weight that decomposed laminarin was considered one enzyme activity unit (U) and expressed as enzyme activity with U g-1FW s-1.
2.6. Statistical analysis
All statistical analysis was performed using SPSS Software (Version 17.0, SPSS Inst., Chicago, Ill., USA). The data were analysed using one-way analysis of variance (ANOVA). A comparison of the means was performed using Duncan's multiple range test and P<0.05 was considered statistically signif icant.
3. Results
3.1. Effects of different treatments against C. gloeosporioides pathogen
The lesion diameter is known to effectively ref lect the growth and reproduction of the pathogen within the fruit. The varying lesion diameters of each of the wounded fruits treated by the different solutions are shown in Fig. 1. When stored for 12 days at 25°C, the lesion diameter of the CBT treatment was the smallest at 10.4 mm, 15.7 mm smaller than the CK (26.1 mm). The lesion diameters of the other treatments, MS (14.8 mm), SA (18.3 mm) and CaCl2(22.4 mm) were also lower than that of the CK by 11.3, 7.8 and 3.7 mm, respectively (P≤0.05). These results suggest that the CBT treatment had a much more signif icant eff icacy against the growth of the pathogen C. gloeosporioides and inhibited the further development of anthracnose in the mango fruit.
3.2. Effects of different treatments on the disease index of mango fruits
The disease index (DI) of each of the treated mango fruits (stored at 25°C) is shown in Table 1. After 20 days of storage at 25°C, the DI of each treatment, CK, MS, SA, CaCl2and CBT were 37.7, 17.8, 22.2, 28.2 and 4.7, respectively. The lowest DI was that of the MS, followed by the SA treated (P≤0.05). After a further 4 days of storage at 25°C, the DIs of MS, SA, CaCl2and CBT treatments were lower by 60.75, 29.88, 11.16 and 80.28%, respectively, when compared with the CK control. Fig. 2 presented the control eff icacy of the different treatments on disease incidence of mango fruits stored at 25°C. Overall, these results suggest that all treatments, MS, SA, CaCl2and CBT could inhibit the disease incidence in the mango fruits, however, once again, the effect of the CBT treatment was the most pronounced.

Fig. 1 The lesion diameters of wounded mangoes incubated for 12 days at 25°C (top) and control eff icacy of different solutions against mango anthracnose (bottom). CK, sterile distilled water; MS, Metschnikowia pulcherrima suspension (1.0×108 cfu mL-1); SA, 50 mg L-1 salicylic acid solution; CaCl2, 1.0 g L-1 calcium chloride solution; CBT, 50 mg L-1 SA+1.0 g L-1 CaCl2+M. pulcherrima (1.0×108 cfu mL-1). Each value is the average of 3 experiments. Bars are SE.
3.3. Effects of different treatments on storage quality of mango fruits
The storage quality of the mango fruits was analysed in a number of ways, using skin colour, f irmness of the fruit, the levels of TSS and Vc along with activities of a number of enzymes known to be involved in the disease incidence of the fruit.
The changes in peel colour and f irmness of treated fruits can be seen in Fig. 3. The effect of each of the different treatments was found to be highly signif icant for the change in peel colour (P≤0.05) (Fig. 3-A). In regard to the peel color of the fruits, the a* value of the control treatment (CK) fruits was higher than that of the other treatments throughout the storage time. The change in the a* value seen for the CBT-treated fruits was the slowest, followed by SA treatment. However, compared with the CK fruits, all treatments could delay the change in f irmness (Fig. 3-B), and the effects of CBT and SA treatments were most obvious. On the 16th day of storage, the highest and lowest fruit f irmness were observed in CaCl2-treated fruits (7.33 N) and the CK fruits (4.23 N), respectively. After 24 days in storage, the highest f irmness was noted in the CBT-treated fruits (5.23 N) suggesting that both the CaCl2and CBT treatments could signif icantly inhibit the softening of mango fruits during storage.

Table 1 Disease index (%) of treated mango fruits stored at 25°C
The next indicator of fruit quality in storage tested was the TSS content. It was found that the TSS content increased during the f irst stages of storage but then declined with increasing storage duration (Fig. 4-A). However, the TSS content increased more drastically in both the CK and SA-treated fruits when compared to the MS-, CaCl2- and CBT-treated fruits. Of interest, the maximum TSS content was observed in the CK and CBT-treated mangoes on days 12 and 20. As shown in Fig. 4-B, the next indic ator of fruit quality, Vc content, reduced gradually throughout the time in storage for all treatments. The Vc content in CK and MS-treated fruits decreased drastically from the f irst day of storage, however, the Vc content of both the SA- and CBTtreated fruits remained quite high in comparison throughout the entire storage period. According to the analysis of variance, the effects of all treatments compared to CK on the Vc content during storage, were highly signif icant (P≤0.01). Nonetheless, at the end of storage, the Vc content in the CBT-treated fruits was higher than that found in the other treatments. Overall, these data suggest that all treatments had increasing effects on the maintenance of both TSS and Vc levels in comparison to the CK fruits, with the greatest effect being noted in the CBT-treated fruits once again.
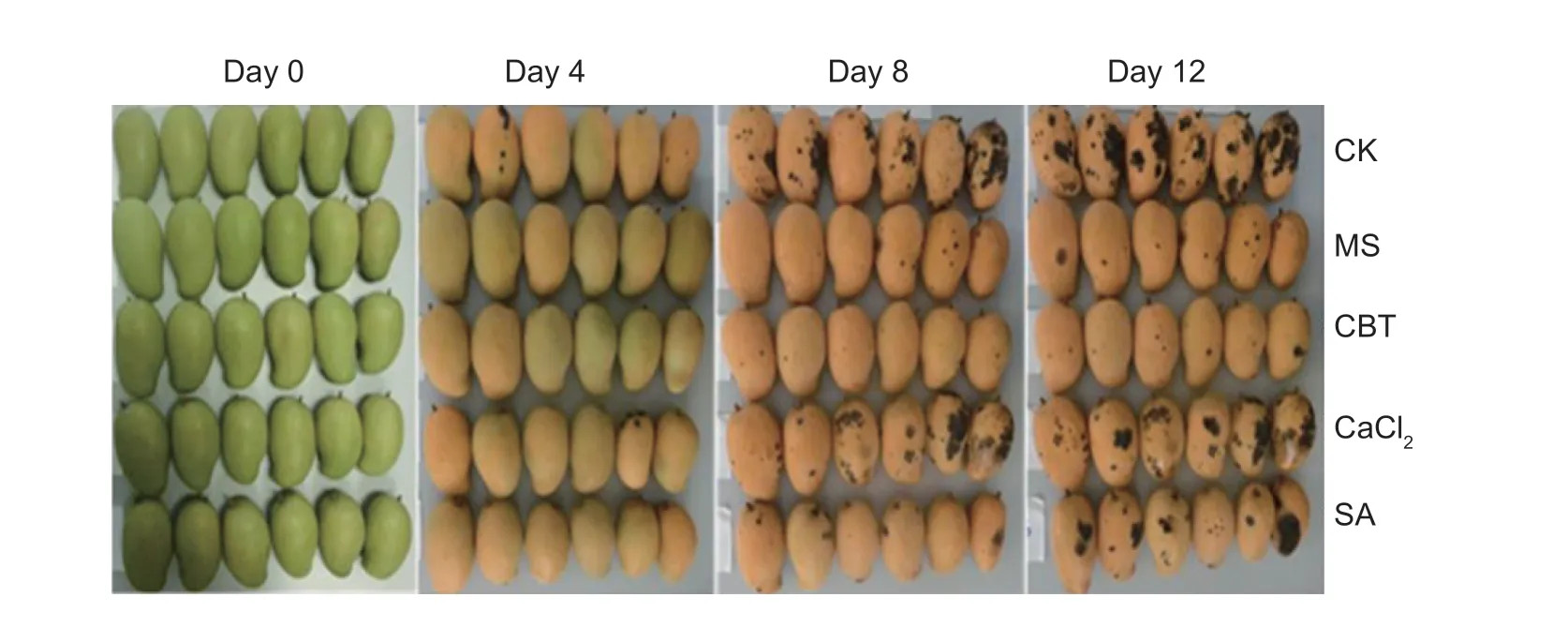
Fig. 2 The control eff icacy of the different treatments on disease incidence of mango fruits stored at 25°C. CK, sterile distilled water; MS, Metschnikowia pulcherrima suspension (1.0×108 cfu mL-1); SA, 50 mg L-1 salicylic acid solution; CaCl2, 1.0 g L-1 calcium chloride solution; CBT, 50 mg L-1 SA+1.0 g L-1 CaCl2+M. pulcherrima (1.0×108 cfu mL-1).

Fig. 3 The effects of different treatments on peel colour (a* value) (A) and t he f irmness (B) of mango fruits stored at 25°C. CK, sterile distilled water; MS, Metschnikowia pulcherrima suspension (1.0×108 cfu mL-1); SA, 50 mg L-1 salicylic acid solution; CaCl2, 1.0 g L-1 calcium chloride solution; CBT, 50 mg L-1 SA+1.0 g L-1 CaCl2+M. pulcherrima (1.0×108 cfu mL-1). Bars are SE.

Fig. 4 The effects of different treatments on total soluble solids (TSS) content (A) and vitamin C (Vc) content (B) of mango fruits stored at 25°C. CK, sterile distilled water; MS, Metschnikowia pulcherrima suspension (1.0×108 cfu mL-1); SA, 50 mg L-1 salicylic acid solution; CaCl2, 1.0 g L-1 calcium chloride solution; CBT, 50 mg L-1 SA+1.0 g L-1 CaCl2+M. pulcherrima (1.0×108 cfu mL-1). Bars are SE.
Finally, the activity levels of a number of enzymes known to be involved in the disease process seen in fruit storage, PPO, PAL, CHT and GUN were investigated. The changes noted for these enzymes in the mango fruits stored at 25°C are exhibited in Fig. 5. In the CK fruits, the activities of all the monitored enzymes showed the tendency to increase and then decrease overtime in storage. On the other hand, all other treated fruits showed the higher activities of these enzymes on days 4 and 16 when compared to the control. Additionally, the activities of each of the enzymes tested, PPO, PAL, CHT and GUN in the CBT-treated fruits were the highest among all the treatments. The activities of PPO, CHT and GUN in the CBT-treated fruits reached a maximum at day 16, with the maximum for PAL activity noted at day 12. The activities for all enzymes in the SA-treated fruit were the second highest among all the treatments. These results suggest that the MS, SA, CaCl2and CBT treatments could all greatly accelerate the activities of the known enzymes important in storage, PPO, PAL, CHT and GUN. Finally, with all the observations made in this study taken together, the effect of the CBT treatment on the mango fruit health and quality was the greatest.
4. Discussion
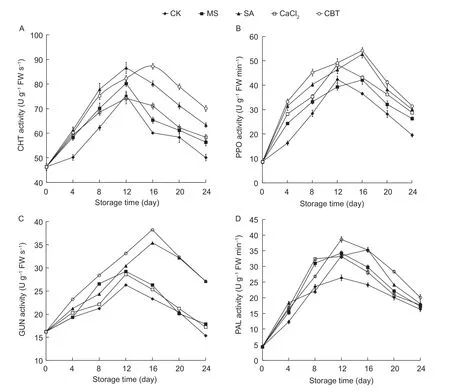
Fig. 5 The effects of different treatments on changes in enzmaytic activities of polyphenol oxidase (PPO, A), phenylalanine ammonia lyase (PAL, B), chitinase (CHT, C) and β-1,3-glucanase (GUN, D) of mango fruits stored at 25°C. CK, sterile distilled water; MS, Metschnikowia pulcherrima suspension (1.0×108 cfu mL-1); SA, 50 mg L-1 salicylic acid solution; CaCl2, 1.0 g L-1 calcium chloride solution; CBT, 50 mg L-1 SA+1.0 g L-1 CaCl2+M. pulcherrima (1.0×108 cfu mL-1). Bars are SE.
The control of disease during both storage and transportation of horticultural products is of upmost importance. Therefore, f inding safe and effective alternatives to synthetic fungicides for reducing postharvest losses of these commodities has been a focus of much research especially over the past three decades. Although the application of certain chemicals can have unwanted residual effects on human safety, SA and CaCl2have been proven to be not only safe but also effective methods for controlling postharvest disease and reduce fruit decay (Madani et al. 2014; Shen and Yang 2017). In agreement with previous research, immersion treatments of the fruit with SA and CaCl2alone could lower the incidence of disease in the fruit and reduce decay of stored mangoes. The effects of SA and CaCl2on the control of fruit disease could likely be because they are signal response compounds and cause the activation of downstream responses, like the synthesis of antimicrobial compounds and cell wall reinforcement mechanisms (Kumar 2014).
In this study we demonstrated that M. pulcherrima could signif icantly inhibit the growth of C. membranifaciens as well as reduce the decay index of treated stored mango fruits. Previously, there have been numerous studies also highlighting the effectiveness of yeast in reducing fungal disease in postharvest fruits, further supporting these results (Mekbib et al. 2011; Oro et al. 2014; Mahunu et al. 2016). According to Sharma et al. (2009) and Li Q F et al. (2017), the inhibition of disease in fruits by treatment with yeasts such as M. pulcherrima, may be achieved through a number of mechanisms, including competition between the yeast and the pathogen for both the nutrients and space in the fruit, by producing a biof ilm and adhering to the fruit as well as the pathogen to stunt further growth or by producing hydrolytic enzymes such as chitinase and PAL. However, it is most likely that the combination of these different actions of the yeast that increases their ability to control postharvest disease. Previously, it has been established that yeast in combination with other synergists, such as chitosan (Meng et al. 2010), SA (Qin et al. 2003) and CaCl2(Yu et al. 2012) greatly enhanced the known inhibitory effects of yeast against the fungal pathogen and achieved greater synergistic control of the disease in fruits while in storage. In this work, we also observed that same control of disease in postharvest fruit, which was greatly enhanced by the combination of SA, CaCl2and M. pulcherrima. This has previously been noted in both pear fruits (Yu et al. 2007) and Chinese cabbage (de Silva Felix et al. 2017).
It is well established that PPO, PAL, CHT and GUN are enzymes related with disease process in plant, important in the interaction between the host and pathogen, by improving the resistance of the host to various stresses, such as disease, salt and cooling (Romanazzi et al. 2016). We observed that SA, CaCl2and M. pulcherrima signif icantly enhanced the activities of these disease process enzymes in mango fruits with the effect of the combination of all three being the greatest. This observation has been made previously in both the cherry fruit (Dokhanieh et al. 2013) and the strawberry fruit (Chalfoun et al. 2016). Also, we found that the activities of these enzymes were negatively correlated with the infection diameter, meaning that higher enzyme activities were found in fruits with lower infection diameters. This suggests that the inhibition of disease in the SA, CaCl2and M. pulcherrima treatments may be due to accelerated activities of these defence enzymes during mango storage. Research has focused on elucidating the role of SA, CaCl2and yeast in the control of disease in fruit from a molecular standpoint, however, the role of the defence enzymes PPO, PAL, CHT and GUN against the anthracnose disease is yet to be understood (Cova et al. 2017).
The maintenance of fruit quality in both storage and transportation is very important in improving the commercial value of the fruit as well as meeting consumer demands. In this work, during storage of the differently treated mango fruits, a number of observations, in accordance with previous literature, were noted (Gill et al. 2017; Tian et al. 2018). In the control (untreated) fruit, the peel colour (a*) and the TSS content increased and the f irmness and Vc content decreasing gradually. However, when the fruits were treated with SA, CaCl2and yeast individually or in combination, the increase in peel colour (a*) and the TSS content was delayed, and the decrease in f irmness and Vc content was signif icantly suppressed. Similarly, in previous work conducted by Gimenez et al. (2014) and Lo'ay (2017), it was reported that SA treatment increased TSS, Vc, phenols and anthocyanins while reducing weight loss, softening and decay of fruit. The benef icial effects of SA treatment in the prevention of fruit decay in storage may be attributed to the preservation of membrane integrity, enhancement of antioxidant systems and suppression of the activities of PAL and CHT enzymes (Zhu et al. 2013). Another indicator of fruit quality during storage and transportation is fruit softening. In this work, consistent with previous observations where fruits treated by CaCl2had greater f irmness due to the breakdown of hemicellulose and pectin suppressed by calcium, we showed that the application of CaCl2alone or combined with SA or yeast signif icantly inhibited the softening of the fruits, as well as maintaining the f irmness throughout the storage time (Khaliq et al. 2016; Belge et al. 2017).
Many previous studies have reported that the combination of physical treatments, chemical treatments and biocontrol methods present an additive effect, by increasing the eff icacy in controlling disease as well as extending the fruit shelf-life. It has been shown that SA application with chitosan signif icantly decreased respiration rate, weight loss, pericarp browning and decay incidence, while improving levels of total soluble solids, anthocyanins and titratable acidity of litchi and citrus during storage (Zhou et al. 2014; Kumari et al. 2015). Additionally, it has been reported that the implementation of a pre- and postharvest application of calcium delayed the reduction in the total soluble solids in persimmon fruit when it was combined with a 45°C hot water treatment (Shaf iee et al. 2010; Terao et al. 2017; Naser et al. 2018). In this study, we demonstrated that the fruits treated by the combination of SA, CaCl2and M. pulcherrima had the lower decay index and lightness value for the peel colour, while maintaining the higher f irmness, TSS and Vc contents as well as the enzyme activities. It is very possible that there is a synergy between SA, CaCl2and antagonism yeast that leads to the combination treatment having the most signif icant effects on maintaining the fruit quality while also extending the storage life of the mango fruits.
5. Conclusion
The study was conducted to investigate appropriate treatments for the mango fruit that not only reduced decay in storage and transportation, but also maintained their postharvest quality. It was found that the applications of SA, CaCl2and M. pulcherrima yeast prevented the growth of the fungal pathogen C. gloeosporioides, reduced the decay index, inhibited the softening of the fruit, suppressed changes to the TSS and Vc contents and enhanced the activities of important defensive enzymes such as PAL, PPO, CHT and GUN. Furthermore, the combination of SA, CaCl2and M. pulcherrima yeast was found to be more effective than each treatment alone. Thus, we suggest that the combination of SA, CaCl2and M. pulcherrima yeast has the potential for utiliz ation as a safe and effective postharvest tool to control anthracnose while also maintaining and extending the postharvest life of mangoes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31660587 and 31660586).
References
Aghdam M S, Jannatizadeh A, Sheikh-Assadi M, Malekzadeh P. 2016. Alleviation of postharvest chilling injury in anthurium cut f lowers by salicylic acid treatment. Scientia Horticulturae, 202, 70-76.
Assis J S, Maldonado R, Muñoz T, Escribano M I, Merodio C. 2001. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biology and Technology, 23, 33-39.
Bautista-Rosales P U, Calderon-Santoyo M, Servín-Villegas R, Ochoa-Álvarez N A, Vázquez-Juárez R, Ragazzo-Sánchez J A. 2014. Biocontrol action mechanisms of Cryptococcus laurentii on Colletotrichum gloeosporioides of mango. Crop Protection, 65, 194-201.
Belge B, Goulao L F, Comabella E, Graell J, Lara I. 2017. Refrigerated storage and calcium dips of ripe ‘Celeste' sweet cherry fruit: Combined effects on cell wall metabolism. Scientia Horticulturae, 219, 182-190.
Campos-Martínez A, Velazquez-del Valle M G, Flores-Moctezuma H E, Suarez-Rodríguez R, Ramírez-Trujillo J A, Hernandez-Lauzardo A N. 2016. Antagonistic yeasts with potential to control Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. and Colletotrichum acutatum J. H. Simmonds on avocado fruits. Crop Protection, 89, 101-104.
Chalfoun N R, Castagnaro A P, Ricci J C D. 2016. Induced resistance activated by a culture f iltrate derived from an avirulent pathogen as a mechanism of biological control of anthracnose in strawberry. Biological Control, 58, 319-329.
Cova V, Paris R, Toller C, Patocchi A, Velasco R, Komjanc M. 2017. Apple genes involved in the response to Venturia inaequalis and salicylic acid treatment. Scientia Horticulturae, 226, 157-172.
Dessalegn Y, Ayalew A, Woldetsadik K. 2013. Integrating plant defense inducing chemical, inorganic salt and hot water treatments for the management of postharvest mango anthracnose. Postharvest Biology and Technology, 85, 83-88.
Dokhanieh A Y, Aghdam M S, Fard J R, Hassanpour H. 2013. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Scientia Horticulturae, 154, 31-36.
Freeman S, Maymon M, Biton A, Levin A G, Shtienberg D. 2014. Management of mango malformation disease based on a novel strategy of timing of fungicide applications combined with sanitation. Crop Protection, 61, 84-91.
Gago C M L, Guerreiro A C, Miguel G, Panagopoulos T, Silva da M M, Antunes M D C. 2016. Effect of calcium chloride and 1-MCP (SmartfreshTM) postharvest treatment on ‘Golden Delicious' apple cold storage physiological disorders. Scientia Horticulturae, 211, 440-448.
Gill P P S, Jawandha S K, Kaur N, Singh N. 2017. Physicochemical changes during progressive ripening of mango (Mangifera indica L.) cv. Dashehari under different temperature regimes. Journal of Food Science and Technology, 54, 1964-1970.
Gimenez M J, Valverde J M, Valero D, Guillen F, Martinez-Romero D, Serrano M, Castillo S. 2014. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chemistry, 160, 226-232.
Grzegorczyk M, Żarowska B, Restuccia C, Cirvilleri G. 2017. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiology, 61, 93-101.
Guo D Q, Zhu L X, Hou X J. 2015. Combination of UV-C treatment and Metschnikowia pulcherrimas for controlling Alternaria rot in postharvest winter jujube fruit. Journal of Food Science, 80, 137-141.
Guo J, Fang W W, Lu H P, Zhu R Y, Lu L F, Zheng X D, Yu T. 2014. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biology and Technology, 88, 72-78.
Han C, Zuo J H, Wang Q, Dong H Z, Gao L P. 2017. Salicylic acid alleviates postharvest chilling injury of sponge gourd (Luffa cylindrica). Journal of Integrative Agriculture, 16, 735-741.
Hartman G L, Pawlowski M L, Chang H X, Hill C B. 2016. Successful technologies and approaches used to develop and manage resistance against crop disease and pests. Emerging Technology Promoting Food Section, 16, 43-66.
Huang R, Che H J, Zhang J, Yang L, Jiang D H, Li G Q. 2012. Evaluation of Sporidiobolus pararoseus strain YCXT3 as biocontrol agent of Botrytis cinerea on post-harvest strawberry fruits. Biological Control, 62, 53-63.
Khaliq G, Mohamed M T M, Ghazali H M, Ding P, Ali A. 2016. Inf luence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biology and Technology, 111, 362-369.
Kumar D. 2014. Salicylic acid signalling in disease resistance. Plant Science, 228, 127-134.
Kumari P, Barman K, Patel V B, Siddiqui M W, Kole B. 2015. Reducing postharvest pericarp browning and preserving health promoting compounds of litchi fruit by combination treatment of salicylic acid and chitosan. Scientia Horticulturae, 197, 555-563.
Li Q F, Li C L, Li P X, Zhang H Y, Zhang X Y, Zheng X F, Yang Q Y, Apaliya M T, Boateng N A S, Sun Y W. 2017. The biocontrol effect of Sporidiobolus pararoseus Y16 against postharvest diseases in table grapes caused by Aspergillus niger and the possible mechanisms involved. Biological Control, 113, 18-25.
Li X, Sun Y Y, Pan D D, Wang Y, Cao J X. 2017. The effect of CaCl2marination on the tenderizing pathway of goose meat during conditioning. Food Research International, 102, 487-492.
Liu H, Chen F S, Lai S J, Tao J R, Yang H S, Jiao Z G. 2017. Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chemistry, 225, 97-116.
Liu J, Sui Y, Wisniewski M, Droby S, Liu Y S. 2013. Review: Utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. International Journal of Food Microbiology, 167, 153-160.
Lo'ay A A. 2017. Preharvest salicylic acid and delay ripening of ‘Superior seedless' grapes. Egyptian Journal of Basic and Applied Sciences, 4, 227-230.
Luo S S, Wan B, Feng S H, Shao Y Z. 2015. Biocontrol of postharvest anthracnose of mango fruit with Debaryomyces nepalensis and effects on storage quality and postharvest physiology. Journal of Food Science, 11, 2555-2563.
Madani B, Mohamed M T M, Biggs A R, Kadir J, Awang Y, Tayebimeigooni A, Shojaei T R. 2014. Effect of pre-harvest calcium chloride applications on fruit calcium level and postharvest anthracnose disease of papaya. Crop Protection, 55, 55-60.
Mahunu M, Zhang H Y, Yang Q Y, Zhang X Y, Li D D, Zhou Y X. 2016. Improving the biocontrol eff icacy of Pichia caribbica with phytic acid against postharvest blue mold and natural decay in apples. Biological Control, 92, 172-180.
Mansourbahmani S, Ghareyazie B, Kalatejari S, Mohammadi R S, Zarinnia V. 2017. Effect of post-harvest UV-C irradiation and calcium chloride on enzymatic activity and decay of tomato (Lycopersicon esculentum L.) fruit during storage. Journal of Integrative Agriculture, 16, 2093-2100.
Mekbib S B, Regnier T J, Korsten L. 2011. Eff icacy and mode of action of yeast antagonists for control of Penicillium digitatum in oranges. Tropical Plant Pathology, 36, 233-240.
Meng X H, Qin G Z, Tian S P. 2010. Inf luences of preharvest spraying Cryptococcus laurentii combined with postharvest chitosan coating on postharvest diseases and quality of table grapes in storage. LWT-Food Science and Technology, 43, 596-601.
Mustafa M A, Ali A, Seymour G, Tucker G. 2018. Delayed pericarp hardening of cold stored mangosteen (Garcinia mangostana L.) upon pre-treatment with the stress hormones methyl jasmonate and salicylic acid. Scientia Horticulturae, 230, 107-116.
Naser F, Rabiei V, Razavi F. 2018. Effect of calcium lactate in combination with hot water treatment on the nutritional quality of persimmon fruit during cold storage. Scientia Horticulturae, 233, 114-123.
Oro L, Feliziani E, Ciani M, Romanazzi G, Comitini F. 2014. Biocontrol of postharvest brown rot of sweet cherries by Saccharomyces cerevisiae Disva 599, Metschnikowia pulcherrima Disva 267 and Wickerhamomyces anomalus Disva 2 strains. Postharvest Biology and Technology, 96, 64-68.
Ou C, Liu Y, Wang W, Dong D. 2016. Integration of UV-C with antagonistic yeast treatment for controlling post-harvest disease and maintaining fruit quality of Ananas comosus. BioControl, 61, 591-603.
Perez M F, Ibarreche J P, Isas A S, Sepulveda M, Ramallo J, Dib J R. 2017. Antagonistic yeasts for the biological control of Penicillium digitatum on lemons stored under export conditions. Biological Control, 115, 135-140.
Qin G Z, Tian S P, Xu Y, Wan Y K. 2003. Enhancement of biocontrol eff icacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiology Molucular Plant, 62, 147-154.
Romanazzi G, Sanzani S M, Bi Y, Tian S P, Martínez P G, Alkan N. 2016. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biology and Technology, 122, 82-94.
Shaf iee M, Taghavi T S, Babalar M. 2010. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Scientia Horticulturae, 124, 40-45.
Shao Y Z, Xie J H, Chen P, Li W. 2013. Changes in some chemical components and in the physiology of rambutan fruit (Nephelium lappaceum L.) as affected by storage temperature and packing material. Fruits, 68, 15-24.
Sharma R R, Singh D, Singh R. 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control, 50, 205-221.
Shen Y F, Yang H Q. 2017. Effect of preharvest chitosan-gsalicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea-induced spoilage. Scientia Horticulturae, 224, 367-373.
da Silva Felix K C, da Silva C L, de Oliveira W J, de Lima Ramos Mariano R, de Souza E B. 2017. Calcium-mediated reduction of soft rot disease in Chinese cabbage. European Journal of Plant Pathology, 147, 73-84.
Stadnik M J, Buchenaue R H. 2000. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat to Blumeria graminis f. sp. tritici. Physiology and Molecular in Plant, 57, 25-34.
Tang J M, Liu Y Q, Li H H, Wang L M, Huang K, Chen Z X. 2015. Combining an antagonistic yeast with harpin treatment to control postharvest decay of kiwifruit. Biology Control, 89, 61-67.
Tian Y Q, Li W, Jiang Z T, Jing M M, Shao Y Z. 2018. The preservation effect of Metschnikowia pulcherrima yeast on anthracnose of postharvest mango fruits and the possible mechanism. Food Science and Biotechnology, 27, 95-105.
Terao D, de Nechet K L, Ponte M S, de Maia A H N, de Anjos V D A, de Halfeld-Vieira B A. 2017. Physical postharvest treatments combined with antagonistic yeast on the control of orange green mold. Scientia Horticulturae, 224, 317-323.
Usall J, Torres R, Teixido N. 2016. Biological control of postharvest diseases on fruit: A suitable alternative? Current Opinion in Food Science, 11, 51-55.
Xu X B, Lei H H, Ma X Y, Lai T F, Song H M, Shi X Q, Li J K. 2017. Antifungal activity of 1-methylcyclopropene (1-MCP)againstanthracnose (Colletotrichum gloeosporioides) in postharvestmango fruitand its possible mechanisms of action. International Journal of Food Microbiology, 241, 1-6.
Yu T, Chen J H, Chen R L, Huang B, Liu D H, Zheng X D. 2007. Biocontrol of blue and gray mold diseases of pear fruit by integration of antagonistic yeast with salicylic acid.
International Journal of Food Microbiology, 116, 339-345.
Yu T, Yu C, Lu H, Zun M, Chen F, Zhou T, Sheng K, Zheng X D. 2012. Effect of Cryptococcus laurentii and calcium chloride on control of Penicillium expansum and Botrytis cinerea infections in pear fruit. Biological Control, 61, 169-175.
Yuan L, Bi Y, Ge Y H, Wang Y, Liu Y Y, Li G L. 2013. Postharvest hot water dipping reduces decay by inducing disease resistance and maintaining f irmness in muskmelon (Cucumis melo L.) fruit. Scientia Horticulturae, 161, 101-110.
Zhang H Y, Ma L C, Wang L, Jiang S, Dong Y, Zheng X D. 2008. Biocontrol of gray mold decay in peach fruit by integration of antagonistic yeast with salicylic acid and their effects on postharvest quality parameters. Biological Control, 47, 60-65.
Zhou Y H, Ming J, Deng L L, Zeng K F. 2014. Effect of Pichia membranaefaciens in combination with salicylic acid on postharvest blue and green mold decay in citrus fruits. Biological Control, 74, 21-29.
Zhu F, Chen J J, Xiao X, Zhang M F, Yun Z, Zeng Y L, Xu J, Cheng Y J, Deng X X. 2013. Salicylic acid treatment reduces the rot of postharvest citrus fruit by inducing the accumulation of H2O2, primary metabolites and lipophilic polymethoxylated f lavones. Food Chemistry, 207, 68-74.
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Mulching with plastic f ilm improved the root quality of summersown sweet potato (Ipomoea batatas (L). Lam.) in northern China
- Impacts of Sletr1-1 and Sletr1-2 mutations on the hybrid seed quality of tomatoes
- Comparison of phenolic prof iles and antioxidant activities in skins and pulps of eleven grape cultivars (Vitis vinifera L.)
- Effect of grazing time and intensity on growth and yield of spring wheat (Triticum aestivum L.)
- Polyaspartic acid mediates the absorption and translocation of mineral elements in tomato seedlings under combined copper and cadmium stress
- lnteraction effect of nitrogen form and planting density on plant growth and nutrient uptake in maize seedlings
