Weaning methods affect ruminal methanogenic archaea composition and diversity in Holstein calves
DONG Li-feng, MA Jun-nan, TU Yan, DlAO Qi-yu
Feed Research Institute, Chinese Academy of Agricultural Sciences/Beijing Key Laboratory for Dairy Cow Nutrition/Key Laboratory of Feed Biotechnology, Ministry of Agriculture, Beijing 100081, P.R.China
Abstract The objective of the present study was to examine the effect of different weaning methods on the ruminal methanogenic archaea composition and diversity in Holstein calves. Thirty-six newborn Holstein bull calves were assigned to 1 of 3 treatments: (1) conventional weaning (d 56) and fed a high proportion of solid feed (CWS); (2) conventional weaning (d 56) and fed a high proportion of liquid feed (CWL); (3) early weaning (d 42) and fed with a high proportion of solid feed (EWS). High-throughput sequencing of the methyl coenzyme M reductase (mcr A) gene, which encodes the α-subunit of methyl coenzyme M reductase - the enzyme that catalyzes the f inal step in methanogenesis was used to determine the composition and diversity of rumen methanogens. No signif icant difference (P>0.05) was observed for operational taxonomic units (OTUs) or richness indices, but diversity indices increased (P<0.05) for calves fed high dietary solids. Predominant families across the three treatments were Methanobacteriaceae, Thermoplasmataceae and Methanomassiliicoccaceae. Calves in the EWS treatment had a higher (P<0.05) relative abundance of Methanobrevibacter sp. strain AbM4 and Methanosphaera stadtmanae, while calves in the CWL treatment had a higher (P<0.05) abundance of Methanosphaera sp. strain SM9. A positive (P<0.05) relationship was identif ied between butyrate and Methanobrevibacter sp. strain AbM4. In conclusion, the composition and diversity of methanogens in the rumen of Holstein calves varied under the different weaning methods. This study identif ied a positive relationship between butyrate and Methanobrevibacter sp. strain AbM4, potentially ref lecting correlations between ruminal fermentation variables and methanogenesis function. These in-depth analyses provide further understanding of weaning methods for intensif ied production systems.
Keywords: calf, methyl coenzyme M reductase (mcr A) gene, methanogenic archaea diversity, rumen fermentation, weaning methods
1. lntroduction
Atmospheric methane (CH4) is one of the most signif icant greenhouse gas (GHG) from agricultural activities and responsible for around 20% of global warming effect (EPA 2009). Livestock sector is responsible for a substantial proportion of global anthropogenic GHG emissions, e.g., CH4emissions from enteric fermentation represented approximately 26% of total CH4emissions from human activities in 2016 (EPA 2018). Enteric CH4is a nature byproduct from dairy and beef production, which is normally produced during anaerobic fermentation of feeds by ruminal methanogenic archaea that use hydrogen (H2) and carbon dioxide (CO2) as substrates (Martin et al. 2010). Because this process represents a waste of 2 to 12% of ingested gross energy (Johnson and Johnson 1995), reducing enteric CH4emission could be directed into milk or meat production and improve production eff iciency (Dong et al. 2015). Therefore, understanding the diversity and role of methanogenic archaea will have ecological and economic implications for mitigating enteric CH4emissions in ruminant livestock production systems.
Weaning is a critical period for young ruminants and entails physiological and metabolic changes to allow the initiation of solid feed intake. During this period, calves start to consume solid feed and their rumens are gradually colonized by methanogenic archaea and bacteria (Yáñez-Ruiz et al. 2010). A range of weaning methods have been proposed to optimize growth performance, rumen development, and health condition, including a change in the duration of the weaning period (Terré et al. 2006; Hill et al. 2012), step-down milk reduction (Khan et al. 2007), and provision of different proportions of liquid and solid feed (Khan et al. 2011; Guzman et al. 2015). Among them, reducing the duration of the weaning period (or early weaning) is widely adopted in commercial dairy production systems. It is well reported that early weaning can increase feed intake and average daily body weight (BW) gain during the preweaning period, and enhance milk yield in the f irst lactations by f irst-calf heifers (Mirzaei et al. 2018). However, provision of different proportions of liquid and solid feed to calves would result in different performances when early weaning method was used. Generally, either feeding liquid milk or milk replacer is more expensive than feeding solid feed to calves. Diao et al. (2017) found high death rates of pre-weaned calves and increased rearing cost on most of China's dairy farms. The reason for that was mainly attributed to the adoption of traditional weaning methods with relatively high fresh milk consumption of calves (around 400-500 kg per calf). Eckert et al. (2015) found that rumen development and postweaning growth were compromised when a large amount of milk was offered to calves. This result agreed with a previous report that excessive provision of liquid feed might delay initiation of solid feed intake and hinder rumen development (Hill et al. 2012). Therefore, transitioning calves from liquid feed to solid feed as early as possible is of great signif icance to improve performance and reduce feed and labor expenditures.
Early intervention in the rumen can markedly inf luence microbial colonization and rumen fermentation, which provided a great opportunity to manipulate the microbiome of the adult ruminants and potentially reduce CH4production (Abecia et al. 2014; Yáñez-Ruiz et al. 2015). Yáñez-Ruiz et al. (2010) showed that diet composition at weaning modif ied the rumen bacterial population and CH4emission of lambs, and this effect can last for more than 4 mon. Morvan et al. (1994) found that methanogenic archaea were present in the undeveloped rumen of lambs before they started solid feed. Abecia et al. (2014) reported that dietary regulation during early life can change the colonization of archaeal population in the rumen, which consequently affects enteric CH4emissions in lambs. Moreover, Khan et al. (2011) reported changes in microbial colonization in the rumen depending on the amount and nature of solid feed offered to calves. However, little is known about the effect of weaning methods on the composition and diversity of methanogenic archaea in Holstein calves. Previous research demonstrated an association between CH4production and the fermentation pattern (Bodas et al. 2012; Cabezas-Garcia et al. 2017), but the relationships between ruminal fermentation characteristics and methanogen community composition are not yet fully understood.
Methanogens are strictly anaerobic archaea whose diversity and phylogeny can now be determined by a range of molecular technologies. Unlike the traditional method involving Sanger sequencing of 16S rRNA, new approaches now target a functional gene encoding the α-subunit of methyl coenzyme M reductase (mcr A) as an alternative strategy to detect the presence, abundance, and/or activity of methanogens under various conditions (Luton et al. 2002; Tomkins et al. 2015). The mcr A gene is highly conserved phylogenetically and encodes methyl coenzyme M reductase (mcr), which catalyzes the terminal step of methanogenesis in all methanogenic archaea (Friedrich 2005). Therefore, we hypothesized that Holstein dairy calves under early and conventionally weaning methods would have different ruminal fermentation characteristics and methanogenic archaea community composition. The objective of the present study was to examine the effects of different weaning methods on fermentation variables and the composition and diversity of methanogens.
2. Materials and methods
2.1. Animals and managements
This study was conducted at the Experimental Station of the Chinese Academy of Agricultural Sciences (CAAS), Beijing, China. All animal procedures were reviewed and approved by the Animal Ethics Committee of CAAS. Thirtysix newborn Holstein bull calves were selected from a commercial dairy herd and transported to the experimental station of CAAA in Beijing. An individual automatic ventilation hutch (3.0 m×1.2 m, 3.6 m2/calf; Black and White Animal Husbandry Technology Ltd., Beijing, China) with an outside feeding station was provided for each calf. The rear shelter was bedded with clean straw, which was cleaned and replaced 3 times per week, while the outdoor yard had concrete slatted f looring that was cleaned once a week. Three metal supports were attached to the side of the fence (1.1 m height) to hold pails containing ad libitum water, milk replacer, or feed starter.
2.2. Feeding, weaning methods, and sample collections
The initial average BW of the calves was (36±2.5) kg. The newborns were given 2 L of colostrum, ensuring total intake of 5 L within the f irst 12 h after birth. After provision of colostrum to calves in the f irst 3 d, all animals were offered whole milk for the following 4 d until the experiment started (Dong et al. 2017a). From d 7 onward, the calves were fed with standard milk replacers (22% crude protein, 12.17% crude fat, and 18.49 MJ kg-1; dry matter (DM) basis; Beijing Precision Animal Nutrition Research Center, China) from a pail in two meals at 08:00 in the morning and 16:00 in the afternoon. The nutrient composition of milk replacer was formulated by our research group and can be obtained from the State Intellectual Property Off ice in China (CN 1494832 A). A common textured starter was introduced on d 21. To avoid the inf luence of different nutrient levels in the milk replacer and starter provided to the calves at the different growth phases, the respective milk replacer and starter contained the same ingredients and same nutrient maintained either in milk replacer or starter. The powdered milk replacer was moistened and steamed at 50°C to retain nutrients. An 8-mm diameter starter pellet (22% crude protein and 50% ether extracts on a DM basis; Beijing Precision Animal Nutrition Research Center, China) was selected for easy digestion and offered ad libitum (Dong et al. 2017b). The volume of milk replacer was fed at the same percentage (as fed basis) of BW (1.2%), and then adjusted weekly based on calf BW. The milk replacer was reconstituted at the rate of 125 g of powder per 1 L of warm water.
During the experimental period from d 21 to 70, 36 newborn Holstein bull calves ((36±2.5) kg BW, mean±SD) were randomly assigned to 1 of 3 weaning treatments with 3 replicates of 4 calves per replicate for each treatment in a completely randomized design. These 3 treatments included: (1) Treatment 1 (n=12) consisted of conventionally weaned calves (d 56) given a high proportion of solid feed (calf starter) in the diet (CWS). The starter offering was increased by 400 g per week from the start of the experiment, and reached 1.00 kg d-1on d 56. (2) Treatment 2 (n=12) consisted of conventionally weaned calves (d 56) given a high proportion of liquid feed (milk replacer) in the diet (CWL). The starter offered to these calves was increased by 200 g per week and reach 0.60 kg d-1on d 56. (3) Treatment 3 (n=12) consisted of the early-weaned calves (d 42) given a high proportion of solid feed in the diet (EWS). The amount of starter provided to these calves was increased by approximately 400 g per week and reached 1.00 kg d-1on d 42. During the experiment, the total DM intake offered to calves in all 3 treatments remained the same through the adjustment of the amounts of milk replacer.
Six animals with a similar BW were selected from each treatment at the end of the experiment, and their rumen contents were then collected using an oral stomach tube connected to a 60-mL syringe after the morning feeding (within 2 h). The oral stomach tube used in the present study was designed and manufactured by Anscitech Co. Ltd. (Wuhan, China) and was a modif ied type of that used by Geishauser (1993) with a probe head length of 15 cm, probe head mass of 435 g, probe head diameter of 3.1 cm, 160 holes (hole diameter of 2.5 mm) in the probe head and spiral spring of 18 mm diameter. After collection, rumen contents from all calves in the same treatment were mixed and strained through 4 layers of cheesecloth. The p H value of the strained ruminal f iltrate was measured immediately using a portable p H meter (Starter 300; Ohaus Instruments Co. Ltd., Shanghai, China), which was calibrated before each sampling using standard buffers (p H 4.00 and 6.86).
The rumen f iltrate was thawed and centrifuged at 20 000×g for 10 min. The concentrations of volatile fatty acids (VFAs) were analyzed at the Nankou Experimental Station, Beijing, China, using a Shimadzu GC-8A gas chromatograph (Shimadzu Corp., Kyoto, Japan) with a 50-m (0.32 mm, i.d.) silica-fused column (Dalian Zhonghuida Scientif ic Instrument, Beijing, China). The initial column temperature was 100°C and was gradually increased to 130°C for 1 min, and then increased to 180°C for 5 min. The detector and injector temperatures were set at 230 and 220°C, respectively. Nitrogen was used as the carrier gas, and f low rates of hydrogen and air were 30 and 300 mL min-1. One 5-mL sample of each stored ruminal f luid sample was thawed and centrifuged at 3 500×g for 10 min, and the supernatants were used to determine the ammonia concentrations using the method described by Chaney and Marbach (1962). Rumen samples were acidif ied and analyzed for VFAs, as described above. Another 30-mL sample was thawed and centrifuged at 4 000×g for 10 min, the supernatants were withdrawn, and the pellet was immediately stored at -80°C for DNA extraction and Illumina sequencing analyses.
2.3. DNA extraction and lllumina MiSeq sequencing
DNA was extracted using the QIAamp Fast DNA Stool Mini Kit (Qiagen, CA, USA) according to the manufacturer's instructions, and stored at -25°C until further analysis. The average DNA concentration, determined using a Nano Drop 2000 Spectrophotometer (Thermo Fisher Scientif ic Inc., USA), was 188.1 ng µL-1for the 72 samples. The mcr A-specif ic primers used in the present study was 5´-GGTGGTGTMGGATTCACACARTAYGCWACAGC-3´ (forward) and 5´-TTCATTGCRTAGTTWGGRTAGTT-3´ (reverse), which was from Luton et al. (2002). PCR amplif ication was performed using the TaKaRa rTaq DNA polymerase system and 2 µL of 10× buffer, 2 µL of d NTPs mixture (2.5 mmol L-1), 0.2 µL of rTaq polymerase (5 U µL-1), and 0.8 µL of each primer (forward and reverse) with a concentration of 5 µmol L-1. The PCR protocol was 95°C for 3 min; 30 cycles at 95°C for 30 s, 55°C for 30 s, 72°C for 45 s; and 72°C for 10 min. PCR products were separated in a 1% agarose gel in Tris-acetate-EDTA buffer and visualized with ethidium bromide staining.
Amplicons were extracted from the agarose gels, purif ied using an Axy Prep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer's instructions, and quantif ied using QuantiFluor-ST (Promega, USA). Purif ied amplicons were pooled in equimolar amounts and paired-end sequenced (2×300) on an Illumina MiSeq PE300 platform according to standard protocols using an MiSeq Reagent Kit v2 (300 cycle; Illumina Inc., USA).
2.4. Processing of sequencing data
Raw FASTQ f iles were demultiplexed and quality-f iltered using QIIME (version 1.17) with the following criteria: (i) The 300-bp reads were truncated at any site that had an average quality score <20 over a 50-bp sliding window, and truncated reads <50 bp were discarded; (ii) exact barcode matching was required, and any 2-nucleotide mismatch in primer matching and reads containing ambiguous characters were removed; and (iii) only sequences that overlapped by more than 10 bp were assembled according to their overlap sequences. Reads that could not be assembled were discarded, and the f inal average length of the 72 samples was 435.6 bp.
Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1, http://drive5.com/uparse/), and chimeric sequences were identif ied and removed using UCHIME. The taxonomy of each mcr A gene sequence was analyzed by the RDP Classif ier (http://rdp.cme.msu.edu/) against the FunGene Database using a conf idence threshold of 70% (Amato et al. 2013). The data obtained in the present study were submitted to NCBI (submission ID: SUB2657791, BioProject ID: PRJNA386396).
2.5. Growth performance
As the calves were kept individually in each treatment, the daily feed intake during pre- and post-weaning stages was determined. Growth performance at different growing stages (d 28, d 42, and d 56, respectively) was also assessed throughout the experiment.
2.6. Statistical analyses
The diversity and richness indices of the ruminal methanogens were analyzed using a one-way ANOVA in SPSS statistics version 22 software (IBM, Armonk, NY, USA). The replicate served as the experimental unit. Relative abundance data are presented as percentages/proportions, and the correlation between ruminal fermentation variables and methanogens was examined using Pearson's correlation coeff icient analysis. The effect was signif icant at P<0.05, and the differences between treatments were compared with the post-doc test (Fisher's least signif icant difference procedure). The data are presented as least squares means±standard errors.
3. Results
In this study of the effect of weaning methods on enteric methanogenic archaea of Holstein calves, feed intake and growth performance were also measured to assess their relationship to the composition and diversity of methanogens in the rumen (results are presented in Appendix A). There was no signif icant difference (P>0.05) in feed intake for calves within the CWS, CWL, and EWS treatments (887.5 g d-1vs. 900.4 g d-1vs. 859.4 g d-1) during the pre-weaning period, whereas feed intake after weaning was signif icantly higher (P>0.05) for CWS (2 043.5 g d-1) and EWS (1 959.0 g d-1) treatments when compared with CWL (1 798.8 g d-1) treatment. During the whole experiment, calves in the EWS and CWS treatments had higher body masses than those in the CWL treatment, but there was no signif icant difference (P>0.05) among these three treatments.
3.1. Characteristics of methanogenic archaea in rumen samples from Holstein calves
A total of 540 012 high quality sequences, ranging from 20 360 to 39 701 per sample, were obtained. The Good's coverage indices obtained for each treatment were all over 0.999, indicating a high integrity of sampling and sequencing. Table 1 shows the OTUs, methanogen richness, and diversity indices from Holstein calves subjected to the different weaning methods. No signif icant differences (P>0.05) were noted among the CWS, CWL, and EWS treatments in terms of richness indices (Ace and Chao) and OTUs. The Shannon value was higher (P<0.05) for the CWS treatment than for the EWS treatment, but it was similar (P>0.05) between the CWL and either the CWS or the EWS treatment. By contrast, the Simpson value was lower (P<0.05) for the CWS treatment than for the EWS, while there was no signif icant difference (P>0.05) between the CWL treatment and either the CLS or EWS treatment. The EWS and CWL treatments did not differ signif icantly (P>0.05) for either the Shannon or the Simpson index.

Table 1 Diversity indices of methanogenic archaea based on methyl coenzyme M reductase (mcr A) gene sequences from Holstein calves in 3 weaning treatments
A Venn diagram was constructed for the species level from the sequences obtained from Holstein calves (Fig. 1). The shared and unique OTUs were represented at a 97% similarity level in the three treatments. In general, 15, 19, and 14 OTUs were obtained in the EWS, CWS, and CWL treatments, respectively, and 10 OTUs (43% of the total OTUs) were common to all 3 treatments. Specif ically, 10 OTUs were shared between the EWS and CWL treatments, 14 between the EWS and CWS treatments, and 15 between the CWS and CWL treatments. Only 4 OTUs were exclusive to the EWS and CWS treatments, whereas no unique OTU was found for the CWL treatment.
The relative abundance of the various methanogenic archaea obtained using the mcr A gene sequencing approach is shown in Table 2 and Fig. 2. Taxonomy-based analysis revealed 1 phylum, 2 classes, 3 orders, 3 families, 4 genera, and 9 species. The predominant archaeal family in the order Methanobacteriales was Methanobacteriaceae, which belongs to the class Methanobacteria. At the genus level, Methanobrevibacter was the most abundant, followed by Methanosphaera, Thermoplasma and Candidatus Methanomethylophilus. Nine species were detected across the 18 samples. The most abundant species were Methanobrevibacter sp. strain AbM4 and Methanosphaera stadtmanae, followed by Methanobrevibacter sp. strain SM9 and Candidatus Methanomethylophilus alvus, whereas the relative abundances of Methanosphaera sp. strain ISO3-F5 and Methanobrevibacter ruminantium were below 5%. Methanobrevibacter smithii and Thermoplasmatales archaeon strain BRNA1 were only observed in some treatments. Among the species, uncultured rumen bacterium accounted for approximately 15% of the total sequences.

Fig. 1 Venn diagram representation of the shared and exclusive operational taxonomic units (OTUs) at 97% similarity level of 3 treatment groups (the conventionally weaned calves (d 56) with a high proportion of solid feed (calf starter) treatment (CWS), or liquid feed (milk replacer) treatment (CWL), and the early weaned calves (d 42) with a high proportion of solid feed treatment (EWS).
3.2. Comparison of methanogenic archaea from Hol-stein calves in different weaning treatments
At the order level, no signif icant difference in the structure and composition of the methanogen populations was found among the three weaning treatments in terms of Methanobacteriales and Methanomassiliicoccales, whereas the Thermoplasmatales were more abundant (P<0.05) in the CWS treatment (8.93%) than in the CWL (4.34%) and EWS (5.83%) treatments (Table 2). The species abundances of Methanobrevibacter sp. strain AbM4, and M. stadtmanae were signif icantly higher (P<0.05) in the EWS treatment (16.71 and 15.29%, respectively) than in the CWS treatment (12.61 and 10.15%, respectively), but not between the CWL and EWS treatments (P>0.05). The relative abundance of Methanobrevibacter sp. strain SM9 was signif icantly higher (P<0.05) in CWL (13.62%) than in CWS (8.15%) or EWS treatment (4.76%). The relative abundance of Methanosphaera sp. strain ISO3-F5 was signif icantly higher (P<0.05) in the CWL treatment (12.23%) than in the CWS (3.47%) or EWS treatment (4.45%). By contrast, relative abundance of M. ruminantium, Ca. Methanomethylophilus alvus, and T. archaeon BRNA1 did not differ among the 3 treatments (P>0.05) and was below 10% in the 3 treatments.
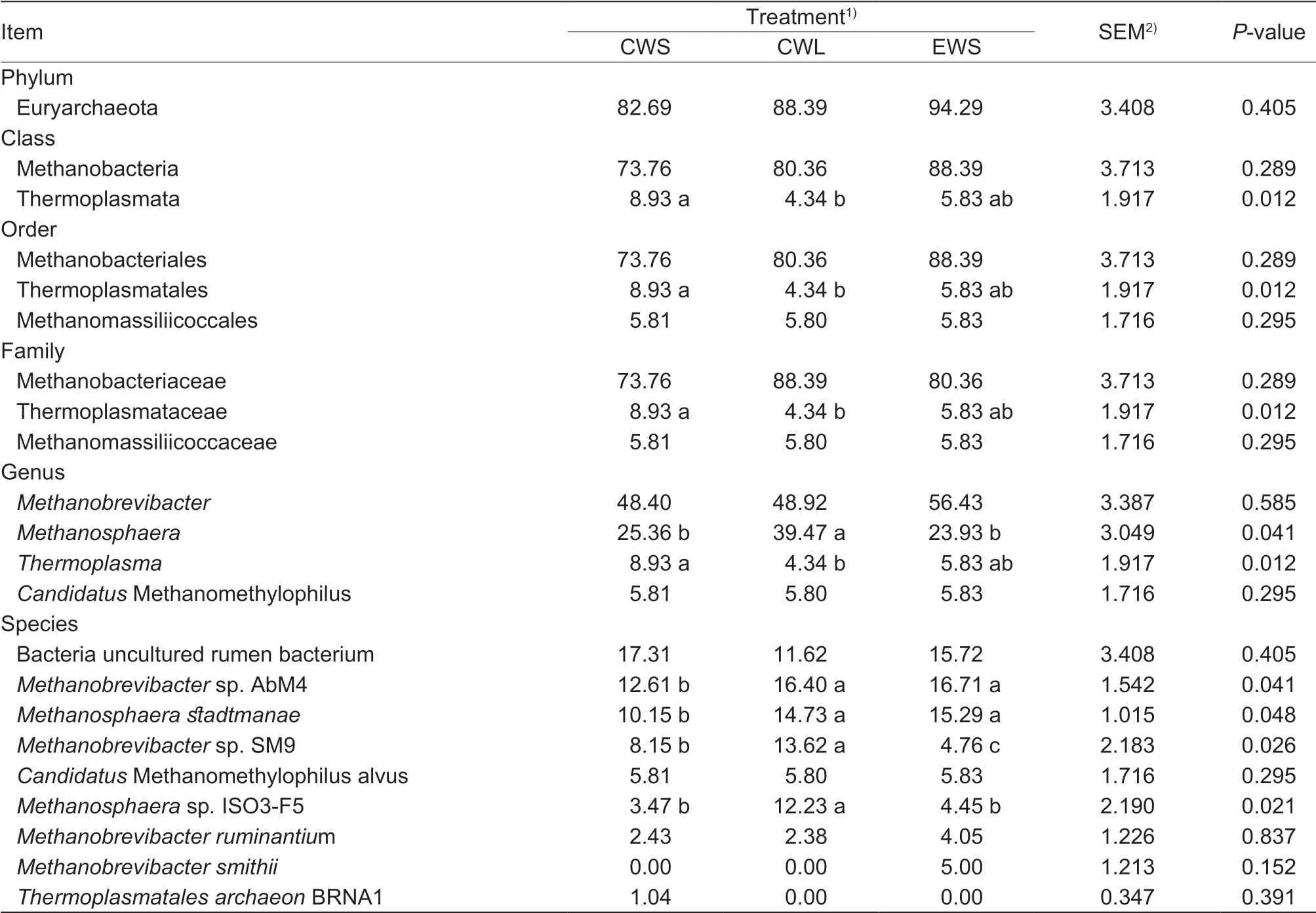
Table 2 Relative abundances of methanogenic archaea at different classif ication levels based on methyl coenzyme M reductase (mcr A) gene sequences from Holstein calves in 3 weaning treatments
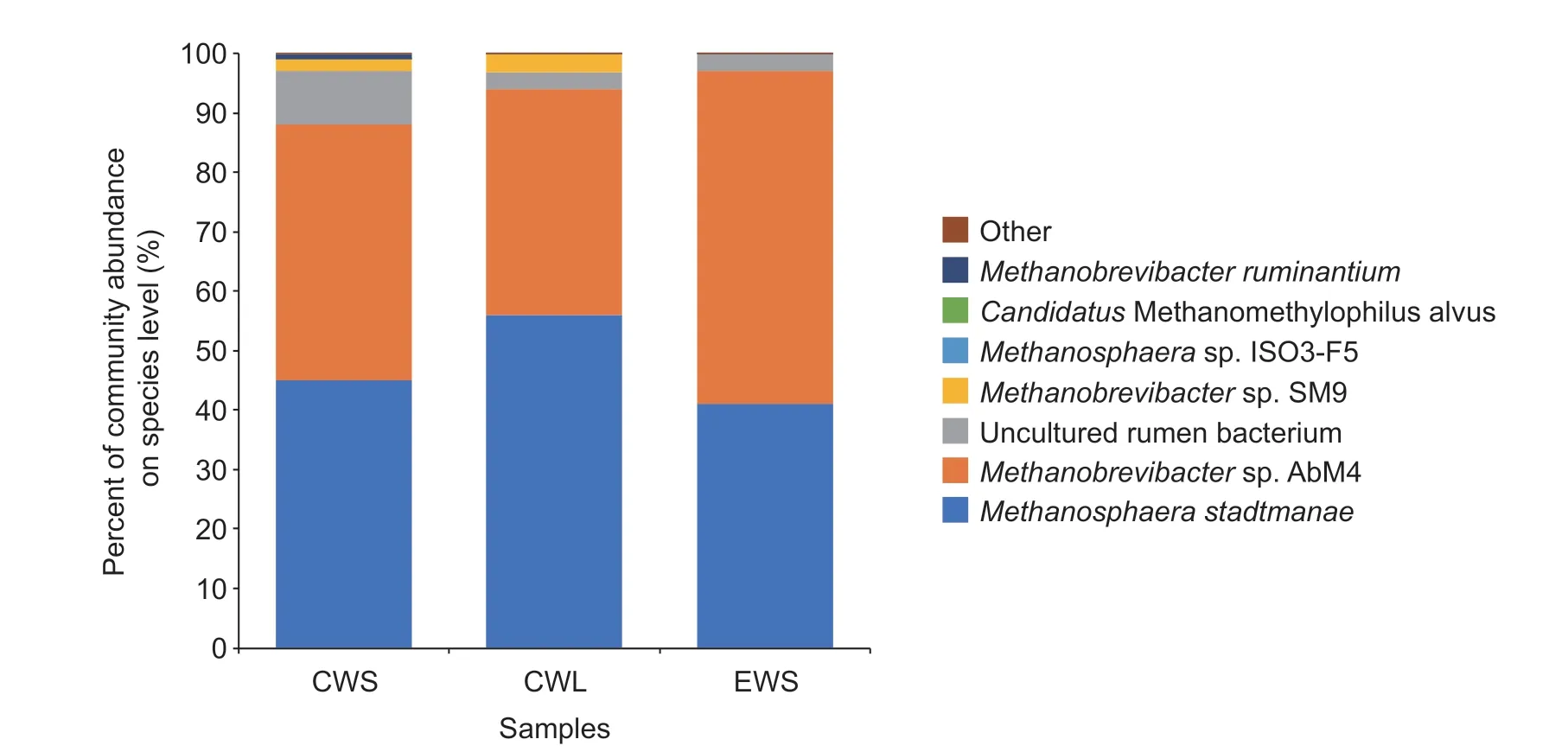
Fig. 2 Relative abundance (percentage of sequences) under the archaeal species level in the conventionally weaned calves (d 56) with a high proportion of solid feed (calf starter) treatment (CWS), or liquid feed (milk replacer) treatment (CWL), and the early weaned calves (d 42) with a high proportion of solid feed treatment (EWS) based on methyl coenzyme M reductase (mcr A) gene sequences of Holstein calves.
3.3. Weaning-induced changes in enteric fermentation prof iles
No difference was noted among the 3 treatments in terms of the concentration of total VFAs (Table 3). The proportion of propionate in the CWL and EWS treatments was similar, but signif icantly higher (P<0.05) than in the CWS treatment. The acetate to propionate ratio was signif icantly higher in the CWS treatment than in the CWL and EWS treatments, but did not differ between CWL and EWS (P>0.05). Similarly, the concentration of ruminal NH3-N was similar (P>0.05) between the EWS and CWS treatment and between the CWS and CWL treatment, with the highest (P<0.05) value found in the CWS treatment.
3.4. Correlation between methanogenic community structure and fermentation variables
In the correlation heatmap based on Pearson's correlation coeff icient for the relationship between the methanogen community (at the genus and species level) and ruminal fermentation variables (Figs. 3 and 4), the presence of Methanobrevibacter was positively correlated with the presence of acetate (P<0.01) and butyrate (P<0.05). The p H value was signif icantly (P<0.01) related to the presence of Methanosphaera, but negatively related to the presence of Methanobrevibacter. Total VFAs were negatively related to Methanosphaera, and propionate content was negatively related to Methanomethylopilus, but neither was statistically signif icant (P>0.05). Further analysis at the species level revealed a positive correlation between butyrate and Methanobrevibacter sp. strain AbM4, and a positive correlation (P<0.05) between the p H value and uncultured rumen bacteria. A negative relationship (P>0.05) was observed between acetate and both M. stadtmanae and M. smithii and between butyrate and both Methanobrevibacter sp. strain SM9 and Methanosphaera sp. strain IOS3-F5.
4. Discussion
4.1. Characteristics of methanogenic archaea in Holstein calves
In the present study, the predominant methanogenic archaeal species identif ied in the rumens of the Holstein calves varied among the different treatments. Methanobrevibacter sp. strain AbM4 was the highest for the CWL and EWS groups, accounting for 16.40 and 16.71% of the archaea, respectively, while the proportion of M. stadtmanae in the CWS group was 12.61%. Yu et al. (2008) detected M. stadtmanae in sheep but did not report its relative abundance. The presence of Methanobrevibacter sp. strain AbM4 was also reported by Zhou et al. (2009) in the bovine rumen, where Methanobrevibacter sp. strain AbM4 and M. stadtmanae represented 10.8 and 5.7% of the total sequences, respectively. Zhou et al. (2014) examined the methanogenic community throughout the gastrointestinal tract of pre-weaned calves using the 16S rRNA approach and found Methanobrevibacter sp. strain AbM4 and M. stadtmanae across the gastrointestinal tract in most of the experimental animals, with a density of 1.0 and 1.5×107(log10transformation of 16S rRNA gene copy numbers per gram of ingesta), respectively, in the rumen. In accordance with the present study, Zhou et al. (2010) detected Methanobrevibacter sp. strain SM9 in cattle steers using polymerase chain reaction-denaturing gradient gel electrophoresis (PCR-DGGE) analysis but did not consider its relative abundance in the rumen. Methanosphaera sp. strain ISO3-F5, was found at a low relative abundance in the current study, similar to the report of Seedorf et al. (2015), who reported a mean abundance of (8.2±6.7)% for Methanosphaera sp. strain ISO3-F5 in the rumen of sheep and cattle. To the best of our knowledge, Ca. Methanomethylop hilus alvus and T. archaeon BRNA1 that we found in the present study have not been previously reported in the bovine rumen. These species were occasionally detected among the samples, but their function and interaction with the community of methanogenic archaea need further investigation.

Table 3 The ruminal fermentation variables obtained from of Holstein calves subjected to different weaning methods
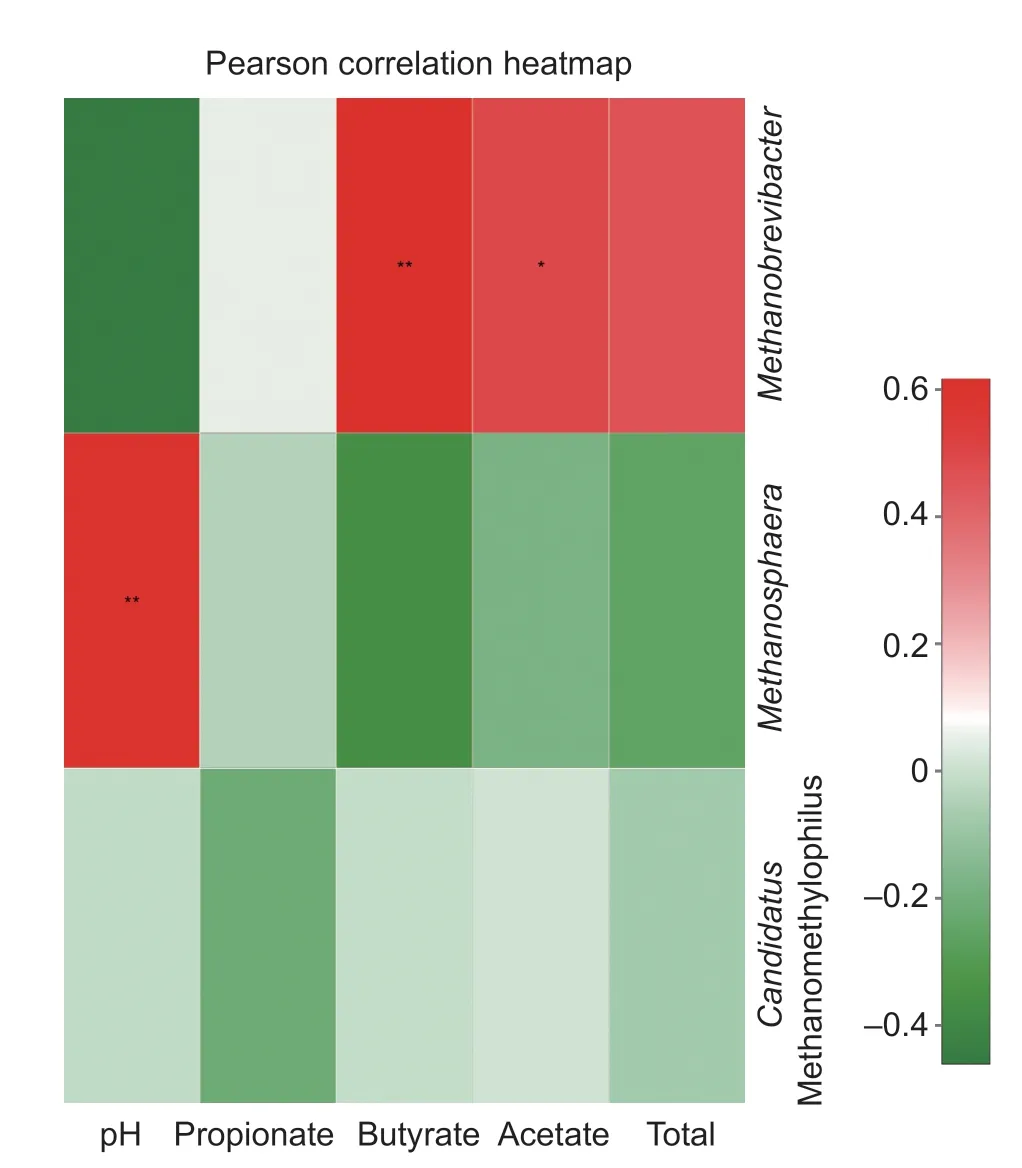
Fig. 3 Environmental factors associated with the methanogenic archaea community at the genus level. Total was the group of volatile fatty acid (VFA) detected in the rumen fermentation. The unit for propionate, acetate, butyrate, and total was mmol L-1. Signif icant difference between any two parameters within this f igure was represented by using * (P<0.05) or ** (P<0.01), respectively.
4.2. Effects of weaning methods on methanogenic archaea community in the rumens of Holstein calves
Enteric methanogenesis is the f inal step of ruminal fermentation and considered to occur in the ruminant. A range of exp eriments have d emonstrated that weaning methods have an impact on nutrition utilization, gastrointestinal development, and related welfare issues (Bach et al. 2010; Eckert et al. 2015; Nemati et al. 2015). For calves transitioning from milk to solid feed, the developing rumen has been identif ied as a unique environment for manipulating methanogen colonization and development. However, as calves around the time of weaning produce minimal CH4, little consideration has been given to the effect of weaning methods on CH4emission and methanogenic archaea composition and diversity.
The diversity indices (Shannon and Simpson) observed in the present study were signif icantly inf luenced by the weaning treatment, which ref lected the fact that the ruminal fermentation prof ile induced by weaning methods affected the methanogenic bacterial diversity in the rumen. In previous studies, the introduction of solid feed and initiation of weaning led to the production of VFAs, which stimulate the anatomical and physiological development of rumen (Roth et al. 2009; Eckert et al. 2015). In the present study, the calves given the CWS treatment had higher levels of propionate, butyrate, and total VFAs when compared with the calves given the other two treatments. This result was consistent with previous research showing that the end products of microbial fermentation (butyrate and propionate) contribute to rumen growth (Baldwin et al. 2004). Furthermore, calves in the CWS treatment had relatively higher BW body than in the other treatments, which might be attributed to enhanced ruminal fermentation and consequently improved energy utilization eff iciency.
The early-weaned calves also had higher abundances of Methanobrevibacter sp. strain AbM4, M. stadtmanae, and M. ruminantium and lower abundances of Methanobrevibacter sp. strain SM9 and Methanosphaera sp. strain ISO3-F5 when compared with conventionally weaned calves. Traditionally, early weaning has been encouraged to decrease feed costs by encouraging consumption of solid feed and restricting milk feed levels. Kay et al. (1969) reported that calves receiving different forms of feed exhibited similar microbial development, but early-weaned animals tended to have high ruminal microbial activity at an earlier age when compared with conventionally weaned calves. By contrast, Eckert et al. (2015) found that calves had a higher starter intake when weaned at 8 weeks than at 6 weeks, which may contribute to a higher diversity of the rumen microbiome. The authors attributed this discrepancy to differences in gastrointestinal and metabolic development due to higher levels of solid feed intake before weaning. The changes in the relative abundances of the methanogen population under different weaning methods may also be due to a substrate utilization preference for methanogenesis. For example, M. ruminantium, the relatively more abundant species detected in the rumen of early-weaned calves, produces CH4utilizing CO2as the substrate, and CO2is the main end product of solid feed (Miller et al. 1986). However, some studies have shown that providing starter feeds containing a high proportion of grains may increase lactic acid production, lower rumen p H, and reduce rumen microbial diversity (Greenwood et al. 1997). The structure and diversity of methanogenic archaea and fermentation prof ile in the rumen during weaning therefore require further exploration.
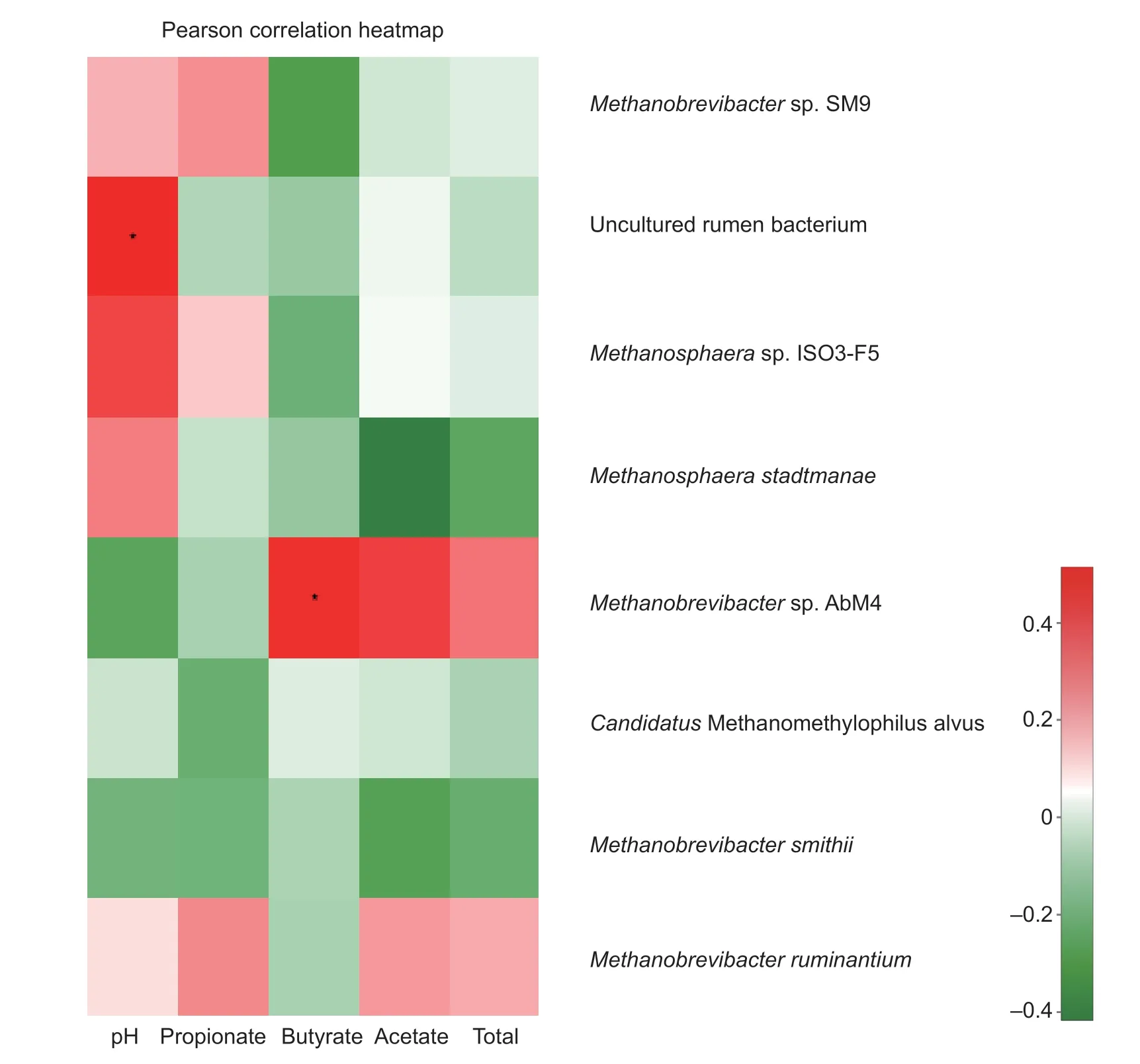
Fig. 4 Environmental factors associated with the methanogenic archaea community at the specie level. Total was the group of volatile fatty acid (VFA) detected in the rumen fermentation. The unit for propionate, acetate, butyrate, and total was mmol L-1. Signif icant difference between any two parameters within this f igure was represented by using * (P<0.05).
4.3. Correlation between methanogenic community structure and fermentation prof ile
Methane formation is closely related to the rumen fermentation pattern, so that degradation of f iber favored the production of VFAs to eliminate the excess H2. Variability in rumen fermentation is greatly inf luenced by the feed intake level and dietary composition. The rumen p H is also a consequence of feed degradation and microbial function. In the present study, the butyrate concentration was positively related with Methanobrevibacter sp. strain AbM4, while a negative relationship was observed between acetate and M. stadtmanae according to Pearson correlation analysis.
Methanobrevibacter sp. strain AbM4 is a strict anaerobe and characterized by its ability to produce CH4from H2, CO2, and formate (Leahy et al. 2013). Zhou et al. (2009, 2013) reported a correlation between the presence of Methanobrevibacter sp. strain AbM4 and two genera of protozoa (Entodinium and Polyplastron) using PCR-DGGE technique, indicating that Methanobrevibacter sp. strain AbM4 may be one of the protozoa-associated methanogens (PAM) that obtain H2for methanogenesis through H2transfer from protozoa (Finlay and Fenchel 1989; Tóthová et al. 2008). Yu et al. (2008) reported that feeding tallow to sheep depressed their levels of Methanobrevibacter sp. strain AbM4. Zhou et al. (2013) also found that fat lowered the occurrence of Methanobrevibacter sp. strain AbM4 within the rumen of beef cattle. High levels of dietary fat may reduce the abundance Methanobrevibacter sp. strain AbM4 by suppressing the protozoal population (H2transition) and associated methanogens (Methanobrevibacter sp. strain AbM4). One consequence might be that production of butyrate in the rumen is accompanied by H2production, which would contribute to the growth of protozoa and PAM, such as Methanobrevibacter sp. strain AbM4. Carberry et al. (2012) observed negative and positive correlations between the relative abundance of protozoa and the molar proportions of propionate and butyrate, respectively, in rumen VFAs. Li et al. (2015) found that the presence of Epidinium caudatum was negatively correlated with butyrate, whereas Eududiplodinium maggii was positively correlated with butyrate in the rumen of Sika deer (Cervus nippon). Michalowski et al. (2003) reported that E. maggii increased the butyrate production rate or concentration in the sheep rumen. E. caudatum and E. maggii are two representative protozoa of the B-type rumen ciliate community (Eadie 1962). Butyrate is an important end product of rumen ciliate metabolism, so the composition of protozoa may affect the energy supply to the host, as butyrate was one of the most important products for the daily metabolic energy of ruminants.
Zhou et al. (2009) reported a negative correlation between the population of Methanobrevibacter sp. strain AbM4 and acetate concentration (P<0.01), in accordance with the results of the present study. Extensive evidence has demonstrated that high acetate and butyrate production enhances CH4production, while high propionate production reduces CH4emissions (Moss et al. 2000). As substrates in the methanogenesis pathway of Methanobrevibacter sp. strain AbM4, acetate and butyrate possess different relationships with Methanobrevibacter sp. strain AbM4. Therefore, the positive relationship between butyrate and Methanobrevibacter sp. strain AbM4 observed in the present study might indicate a microbial interaction, in which H2results from the production of butyrate and the growth of protozoa and the PAM Methanobrevibacter sp. strain AbM4. In a complete genomic sequence analysis of Methanobrevibacter sp. strain AbM4 isolated from the abomasal contents of a sheep, Leahy et al. (2013) determined that the composition of Methanobrevibacter sp. strain AbM4 was similar to that of M. ruminantium M1, suggesting a similar methanogenesis pathway and central metabolism for these two strains. They also indicated that the similar structure and function raise the possibility that small molecule inhibitors and vaccine-based CH4mitigation technologies can be developed to reduce CH4emission.
We also found a negative correlation between M. stadtmanae and acetate concentration, although this relationship was not statistically signif icant (P>0.05). M. stadtmanae has been detected in various studies on beef cattle and dairy cows, either by culture-based methods or PCR-based assays targeting the 16S rRNA and mcr A genes (Miller et al. 1986; Fricke et al. 2006; Zhou et al. 2010). M. stadtmanae is known to sustain its growth by using H2to reduce methanol to CH4(Miller and Wolin 1985; Zhou et al. 2009). Previous reports (Hobson and Stewart 1997; McDonald et al. 2002) have shown that some constituents of concentrate feeds are abundant in dietary pectin, which can be converted into methanol by protozoa and the esterase activity of bacteria in the rumen. Carberry et al. (2012) reported increases in protozoa when the concentrate portion of the diet was increased. Thus, a greater potential for increased availability of methanol due to enhanced feed intake in the present study would explain the relatively high abundance of M. stadtmanae. The total feed intake was signif icantly higher for calves in the EWS treatment (2 043.5 g d-1) than in the CWS treatment (1 798.8 g d-1), but similar to that of calves in the CWL treatment (1 959.0 g d-1), which agrees with the higher abundance of M. stadtmanae observed in the CWL (14.73%) and EWS (15.29%) treatments than in the CWS (10.15%) treatment. Therefore, M. stadtmanae appears to be present in the rumen of calves, and its abundance varies with different feed intakes. However, to our knowledge, little information is available regarding M. stadtmanae abundance in calves versus adult cattle.
The comp osition and diversity of methanogens varied in the rumens of Holstein calves under different weaning methods. The Methanobacteriaceae was the most predominant family across the three treatments, which is consistent with the results found in the mature bovine rumen (Zhou et al. 2009). Novel species of Ca. Methanomethylophilus alvus and T. archaeon BRNA1 were detected in some samples. Signif icant correlations were found between ruminal methanogenic archaea and several fermentation variables. This study is the f irst to identify a positive relationship between butyrate and Methanobrevibacter sp. strain AbM4, which may facilitate the elucidation of the ecology and interactions between metabolic variables and the methanogenesis function. Methanobrevibacter sp. strain AbM4 was suggested as a PAM that has a similar function of attracting hydrogen for methanogenesis. Further studies should be conducted to explore the relationships between fermentation prof iles and PAMs.
This study on the methanogenic archaea composition and diversity in Holstein calves after 3 weaning treatments may shed light on approaches to select appropriate nutrition and management practices to develop more specif ic CH4mitigation technologies in intensif ied production systems. However, results in the present study were obtained from animals under the age of 2 mon, and the long-term effect of weaning and diet on the characteristics of methanogenic archaeal community needs further investigation.
5. Conclusion
In summary, the composition and diversity of methanogens in the rumen of Holstein calves varied under different weaning treatments, and a signif icant relationship was observed between ruminal butyrate and Methanobrevibacter sp. strain AbM4, potentially ref lecting correlation between metabolic variables and methanogenesis. These results contribute to further understanding of the effects of weaning treatments on Holstein calves in intensif ied production systems. Knowledge of the methanogen community composition and its correlation with ruminal fermentation characteristics may facilitate the development of more specif ic CH4mitigation technologies.
The authors wish to thank colleagues at the Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing, for assistance in recording and preparing the data sets. This study was supported by the Key Program for International S&T Cooperation Projects of China (2016YFE0109000), the National Key R&D Program of China (2017YFF0211702), the National Natural Science Foundation of China (41475126 and 31802085), and the Young Scientist Lifting Project, China (2017-2019).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
References
Abecia L, Ramos-Morales E, Martínez-Fernandez G, Arco A, Martín-García A I, Newbold C J, Yáñez-Ruiz D R. 2014. Feeding management in early life inf luences microbial colonisation and fermentation in the rumen of newborn goat kids. Animal Production Science, 54, 1449-1454.
Amato K R, Yeoman C J, Kent A, Righini N, Carbonero F, Estrada A, Gaskins H R, Stumpf R M, Yildirim S, Torralba M, Gillis M, Wilson B A, Nelson K E, White B A, Leigh S R. 2013. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. The ISME Journal, 7, 1344-1353.
Bach A, Ahedo J, Ferrer A. 2010. Optimizing weaning strategies of dairy replacement calves. Journal of Dairy Science, 93, 413-419.
Baldwin R L, Mc Leod VI K R, Klotz J L, Heitmann R N. 2004. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. Journal of Dairy Science, 87, E55-E65.
Bodas R, Prieto N, García-González R, Andrés S, Giráldez F J, López S. 2012. Manipulation of rumen fermentation and methane production with plants secondary metabolites. Animal Feed Science and Technology, 176, 78-93.
Cabezas-Garicia E H, Krizsan S J, Shingf ield K J, Huhtanen P. 2017. Between-cow variation in digestion and rumen fermentation variables associated with methane production. Journal of Dairy Science, 10, 4409-4424.
Carberry C A, Kenny D A, Han S, Mc Cabe M S, Waters S M. 2012. Effect of phenotypic residual feed intake and dietary forage content on the rumen microbial community of beef cattle. Applied and Environmental Microbiology, 78, 4949-4958.
Chaney A L, Marbach E P. 1962. Modif ied reagents for determination of urea and ammonia. Clinical Chemistry, 8, 130-132.
Diao Q Y, Zhang R, Tu Y. 2017. Current research progresses on calf rearing and nutrition in China. Journal of Integrative Agriculture, 12, 2805-2814.
Dong L F, Ferris C P, McDowell D A, Yan T. 2015. Effects of diet forage proportion on maintenance energy requirement and the eff iciency of metabolizable energy use for lactation by lactating dairy cows. Journal of Dairy Science, 98, 1-10.
Dong L F, Xu X C, Zhang N F, Tu Y, Diao Q Y. 2017a. Effects of different feeding methods and space allowance on the growth performance, individual and social behaviors of Holstein calves. Journal of Integrative Agriculture, 6, 1375-1382.
Dong L F, Zhang W B, Zhang N F, Tu Y, Diao Q Y. 2017b. Feeding different dietary protein to energy ratios to Holstein heifers: effects on growth performance, blood metabolites and rumen fermentation parameters. Journal of Animal Physiology and Animal Nutrition, 1, 30-37.
Eadie J M. 1962. Inter-relationships between certain rumen ciliate protozoa. Journal of General and Applied Microbiology, 4, 579-588.
Eckert E, Brown H E, Leslie K E, DeVries T J, Steele M A. 2015. Weaning age affects growth, feed intake, gastrointestinal development, and behavior in Holstein calves fed an elevated plane of nutrition during the preweaning stage. Journal of Dairy Science, 98, 6315-6326.
EPA (U. S. Environmental Protection Agency ). 2009. Ruminant livestock. [2012-05-01]. https://www.epa.gov/sites/production/f iles/signpost/cc.html
EPA (U.S. Environmental Protection Agency). 2018. Inventory of U.S. Greenhouse Gases Emissions and Sinks 1990-2016. [2018-04-12]. https://www.epa.gov/ghgemissions/inventory-us-greenhouse-emissions-and-sinks
Finlay B J, Fenchel T. 1989. Hydrogenosomes in some anaerobic protozoa resemble mitochondria. FEMS Microbiology Letters, 65, 311-314.
Fricke W F, Seedorf H, Henne A, Kruer M, Liesegang H, Hedderich R, Gottschalk G, Thauer R K. 2006. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2for methane formation and ATP synthesis. Journal of Bacteriology, 188, 642-658.
Friedrich M W. 2005. Methyl-coenzyme M reductase genes: Unique functional markers for methanogenic and anaerobic methane-oxidizing archaea. Methods in Enzymology, 397, 428-442.
Geishauser T. 1993. An instrument for the collection and transfer of ruminal f luid and for the administration of water soluble drugs in adult cattle. Bovine Practice, 27, 38-42.
Greenwood R H, Morrill J L, Titgemeyer E C, Kennedy G A. 1997. A new method of measuring diet abrasion and its effect on the development of the forestomach. Journal of Dairy Science, 80, 2534-2541.
Gerber P, Vellinga T, Opio C, Steinfeld H. 2011. Productivity gains and greenhouse gas emissions intensity in dairy systems. Livestock Science, 139, 100-108.
Guzman C E, Bereza-Malcolm L T, de Groef B, Franks A E. 2015. Uptake of milk with and without solid feed during the monogastric phase: Effect on f ibrolytic and methanogenic microorganisms in the gastrointestinal tract of calves. Animal Science Journal, 87, 378-388.
Hill T M, Bateman II H G, Aldrich J M, Schlotterbeck R L. 2012. Methods of reducing milk replacer to prepare dairy calves for weaning when large amounts of milk replacer have been fed. Professional Animal Science, 28, 332-337.
Hobson P N, Stewart C S. 1997. The Rumen Microbial Ecosystem. Blackie Academic and Professional, London, England.
Johnson K A, Johnson D E. 1995. Methane emissions from cattle. Journal of Animal Science, 73, 2483-2492.
Kay M, Fell B F, Boyne R. 1969. The relationship between the acidity of the rumen contents and rumenitis in calves fed barley. Research in Veterinary Science, 10, 181-187.
Khan M A, Lee H J, Lee W S, Kim H S, Kim S B, Ki K S, Ha J K, Lee H G, Choi Y J. 2007. Pre- and postweaning performance of Holstein female calves fed milk through step-down and conventional methods. Journal of Dairy Science, 90, 876-885.
Khan M A, Weary D M, von Keyerslingk M A G. 2011. Effects of milk ration on solid feed intake, weaning, and performance in dairy heifers. Journal of Dairy Science, 94, 1071-1081.
Leahy S C, Kelly W J, Li D, Li Y, Altermann E, Lambie S C, Cox F, Attwood G T. 2013. The complete genome sequence of Methanobrevibacter sp. AbM4. Standards in Genomic Science, 8, 215-227.
Li Z P, Wright A D G, Liu H L, Fan Z Y, Yang F H, Zhang Z G, Li G Y. 2015. Response of the rumen microbiota of Sika deer (Cervus nippon) fed different concentrations of tannin rich plants. PLoS ONE, 10, 1371.
Luton P E, Wayne J M, Sharp R J, Riley P W. 2002. The mcr A gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landf ill. Microbiology, 148, 3521-3530.
Martin C, Morgavi D P, Doreau M. 2010. Methane mitigation in ruminants: from microbe to the farm scale. Anim a l, 4, 351-365.
Mc Donald P, Edwards R A, Greenhalgh J F D, Morgan C A. 2002. Animal Nutrition. 6th ed. Longman, Harlow, United Kingdom.
Michalowski T, Belzecki G, Kwiatkowska E, Pajak J J. 2003. The effect of selected rumen fauna on f ibrolytic enzyme activities, bacterial mass, f ibre disappearance and fermentation pattern in sheep. Journal of Animal Feed Sciences, 1, 45-64.
Miller T L, Wolin M J. 1985. Methanosphaera stadtmaniae gen. nov., sp. nov.: A species that forms methane by reducing methanol with hydrogen. Archives of Microbiology, 141, 116-122.
Miller T L, Wolin M J, Zhao H X, Bryant M P. 1986. Characteristics of methanogens isolated from bovine rumen. Applied and Environmental Microbiology, 51, 201-202.
Mirzaei M, Dahkhah N, Baghbanzadeh-Nobari B, Agha-Tehrani A, Eshraghi M, Imani M, Shiasi-Sardoabi R, Ghaffari M H. 2018. Effects of preweaning total plane of milk intake and weaning age on intake, growth performance, and blood metabolites of dairy calves. Journal of Dairy Science, 101, 4212-4220.
Morvan B, Dore J, Rieu-Lesme F, Foucat L, Fonty G, Gouet P. 1994. Establishment of hydrogen-utilizing bacteria in the rumen of the newborn lamb. FEMS Microbiology Letters, 117, 249-256.
Moss A R, Jouany J P, Newbold J. 2000. Methane production by ruminants: Its contribution to global warming. Annales De Zootechnie, 49, 231-253.
Nemati M, Amanlou H, Khorvash M, Moshiri B, Mirzaei M, Khan M A, Ghaffari M H. 2015. Rumen fermentation, blood metabolites, and growth performance of calves during transition from liquid to solid feed: Effects of dietary level and particle size of alfalfa hay. Journal of Dairy Science, 98, 7131-7141.
Roth B A, Keil N M, Gygax L, Hillmann E. 2009. Inf luence of weaning method on health status and rumen development in dairy calves. Journal of Dairy Science, 92, 645-656.
Seedorf H, Kittelmann S, Janssen P H. 2015. Few highly abundant operational taxonomic units dominate within rumen methanogenic archaeal species in New Zealand sheep and cattle. Applied and Environmental Microbiology, 81, 986-995.
Terré M, Bach A, Devant M. 2006. Performance and behaviour of calves reared in groups or individually following and enhanced growth feeding program. Journal of Dairy Research, 73, 480-486.
Tomkins N W, Denman S E, Pilajun R, Wanapat M, McSweeney C S, Elliott R. 2015. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Animal Feed Science and Technology, 200, 25-34.
Tóthová T, Piknova M, Kisidayova S, Javorsky P, Pristas P. 2008. Distinctive archaebacterial species associated with anaerobic rumen protozoan Entodinium caudatum. Folia Microbioogica, 53, 259-262.
Yáñez-Ruiz D R, Macías B, Pinloche E, Newbold C J. 2010. The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiology Ecology, 72, 272-278.
Yáñez-Ruiz D R, Abecia L, Newbold C J. 2015. Manipulating rumen microbiome and fermentation through interventions during early life: A review. Frontiers in Microbiology, 6, 1133.
Yu Z T, García-González R, Schanbacher F L, Morrison M. 2008. Evaluations of different hypervariable regions of archaeal 16S rRNA genes in prof iling of methanogens by archaea-specif ic pcr and denaturing gradient gel electrophoresis. Applied Environmental Microbiology, 74, 889-893.
Zhou M, Hernandez-Sanabria E, Guan L L. 2009. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed eff iciencies. Applied Environmental Microbiology, 75, 6524-6533.
Zhou M, Hernandez-Sanabria E, Guan L L. 2010. Characterization of variation in rumen methanogenic communities under different dietary and host feed eff iciency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Applied Environmental Microbiology, 76, 3776-3786.
Zhou M, Chen Y H, Griebel P J, Guan L L. 2014. Methanogen prevalence throughout the gastrointestinal tract of preweaned dairy calves. Gut Microbes, 5, 628-638.
Zhou M, Hünerberg M, Beauchemin K A, Mc Allister T A, Okine E K, Guan L L. 2013. Individuality of ruminal methanogen/protozoa populations in beef cattle fed diets containing dried distillers' grain with solubles. Acta Agriculture Scandinavica (Section A - Animal Science), 62, 273-288.
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specif ic marker
- Phenotypic characterization and genetic mapping of the dwarf mutant m34 in maize
- Morphological diversity and correlation analysis of phenotypes and quality traits of proso millet (Panicum miliaceum L.) core collections
- Field identif ication of morphological and physiological traits in two special mutants with strong tolerance and high sensitivity to drought stress in upland rice (Oryza sativa L.)
- Crosstalk of cold and gibberellin effects on bolting and f lowering in f lowering Chinese cabbage
- Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.)
