Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specif ic marker
FU Lu-ping, XIAO Yong-gui, YAN Jun , LIU Jin-dong , WEN Wei-e, ZHANG Yong, XIA Xian-chun , HE Zhong-hu,
1 National Wheat Improvement Center/Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, P.R.China
2 Institute of Cotton Research, Chinese Academy of Agricultural Sciences, Anyang 455000, P.R.China
3 International Maize and Wheat Improvement Center (CIMMYT) China Offce, Beijing 100081, P.R.China
Abstract Stem lignin content (SLC) in common wheat (Triticum aestivum L.) contributes to lodging resistance. Caffeic acid 3-O-methyltransferase (COMT) is a key enzyme involved in lignin biosynthesis. Characterization of TaCOMT genes and development of gene-specif ic markers could enable marker-assisted selection in wheat breeding. In the present study, the full-length genomic DNA (gDNA) sequences of TaCOMT genes located on chromosomes 3A, 3B, and 3D were cloned by homologous cloning. Two allelic variants, TaCOMT-3Ba and TaCOMT-3Bb, were identif ied and differed by a 222-bp insertion/deletion (InDel) in the 3´-untranslated region (3´-UTR). A co-dominant gene-specif ci marker based on this InDel was developed and designated as TaCOMT-3BM. A total of 157 wheat cultivars and advanced lines grown in four environments were used to validate the associations between allelic patterns and SLC. The SLC of cultivars with TaCOMT-3Ba was signif icantly (P<0.01) higher than that of those with TaCOMT-3Bb, and the marker TaCOMT-3BM could be effectively used in wheat breeding.
Keywords: Triticum aestivum L., caffeic acid 3-O-methyltransferase, stem lignin content, gene-specif ic marker, lodging resistance
1. Introduction
Lodging is an important constraint to wheat production (Fischer and Stapper 1987; Berry et al. 2003), and losses to lodging are reported in the range of 31-80% (Weibel and Pendleton 1964; Easson et al. 1993). Although breeders have reduced lodging risk by introgression of dwarf ing genes to wheat cultivars (Peng et al. 1999; Yang et al. 2012), several studies indicated that yields were also reduced when plants were shortened too much (Allan 1986; Miralles and Slafer 1995; Flintham et al. 1997; Acreche and Slafer 2011). Furthermore, many modern cultivars already have minimum plant heights of 0.7 to 1.0 m for achievement of optimum grain yield (Berry 2013). Therefore, enhancement of the mechanical aspects of stems is another means to improve lodging resistance.
Lignin is a major structural component of secondary cell walls in vascular plants. It contributes to plant growth and development, and provides the plant with mechanical support (Ma 2009; Peng et al. 2014). Various studies indicated that stem lignin content (SLC) is an important factor in mechanical strength and lodging resistance of crop plants. In maize, stems of brown-midrib (bm or bmr) mutants, that were more susceptible to lodging, contained signif icantly lower lignin contents than normal inbred lines (Muller et al. 1971). Lignin accumulation in wheat was negatively correlated with lodging percentage, and positively correlated with stem strength or lodging resistance index (Peng et al. 2014; Zhang et al. 2016; Zheng et al. 2017). Positive and signif icant correlations were also found between SLC and the mechanical strength or lodging resistance in other plant species, such as Arabidopsis (Jones et al. 2001), soybean (Zou et al. 2015), buckwheat (Wang et al. 2015), and rice (Fan et al. 2017). Quantitative trait locus (QTL) analysis of stem strength in maize populations revealed that candidate genes within QTL conf idence intervals of stem strength included those involved in lignin biosynthesis (Flint-Garcia et al. 2003). Therefore, increased SLC is a promising way to strengthen the stem, and thus improve lodging resistance.
Caffeic acid 3-O-methyltransferase (COMT) catalyzes multi-step methylation reactions of hydroxylated monomeric lignin precursors (Poovaiah et al. 2014), and plays a pivotal role in lignin biosynthesis and has potential for genetic engineering to modify lignin content (Ma and Xu 2008). Down-regulation of COMT in transgenic maize resulted in a signif icant decrease in SLC (Piquemal et al. 2002). The spontaneous brown-midrib bm3 maize mutant line exhibits reduced SLC (Barriere and Argillier 1993), and the corresponding gene was verif ied to be COMT (Vignols et al. 1995; Morrow et al. 1997). Four EMS-mutagenized missense mutants of bmr12, which encode COMT in sorghum, showed a signif icant reduction in SLC (Sattler et al. 2012). The expression level of the COMT gene in the bmr-12 mutant of sudangrass was signif icantly reduced, and sequence analysis of the COMT gene revealed two point mutations, of which the second mutation led to a premature termination of translation (Li et al. 2015). Signif icant decreases in SLC were also observed in transgenic tobacco (Ni et al. 1994) and tall fescue (Chen et al. 2004) when COMTs were down-regulated.
Since the f irst COMT c DNA was isolated from aspen (Bugos et al. 1991), COMT genes have been cloned and characterized in many other plant species, including maize (Collazo et al. 1992), tobacco (Pellegrini et al. 1993), perennial ryegrass (Heath et al. 1998; Mc Alister et al. 1998), cotton (Ni et al. 2010), sorghum (Gorthy et al. 2013), and kenaf (Kim et al. 2013). The genomic DNA (gDNA) sequences of COMT genes from different species showed varied exon-intron characteristics (Besse et al. 2009). For example, Arabidopsis and barley have three introns, whereas maize, rice, and sorghum have only one. A study on sequence homology of COMT among maize, rice, and sorghum indicated strong selection pressure on the coding sequence, with sequence homology being much lower in the introns, 5´- and 3´-untranslated region (UTR) than in the exons (Bout and Vermerris 2003). A study of genomic COMT sequences in 34 maize inbred lines also revealed insertion/deletion (InDel) variations in introns at the intraspecif ic level (Guillet-Claude et al. 2004).
In common wheat, a stem strength-associated QTL (QSs-3B) was reported in a 7.7-c M interval f lanked by Xgwm108 and Xwmc291 on chromosome 3B (Hai et al. 2005). In addition, an SLC associated COMT cDNA (TaCM, GenBank accession: EF413031) was isolated and mapped between Xtam63b and Xmwg11a in the distal region of chromosome 3B (Ma and Xu 2008; Ma 2009). Based on the IWGSC Reference Sequence v1.0 from International Wheat Genome Sequencing Consortium (IWGSC, http://www.wheatgenome.org/), the physical positions of SSR loci Xgwm108 and Xwmc291 were 738.82 and 790.33 Mb, respectively, while TaCM was closely linked with QSs-3B at the position of 829.39 Mb, indicating that COMT could be the candidate gene for QSs-3B. However, the TaCOMT genes located in the A and D genomes were not characterized, no gene-specif ic marker for TaCOMT was reported, and the associations between these genes and stem lignin accumulation were not explored. Therefore, cloning these genes, exploring their allelic variations, and developing corresponding gene-specif ic markers are of vital importance for further deciphering the function of COMT genes, and for use of these genes in wheat breeding. Thus, the objectives of this study were to: (1) clone TaCOMT genes located on chromosomes 3A, 3B, and 3D; (2) identify their allelic variants among cultivars; and (3) develop corresponding gene-specif ic markers and evaluate their associations with SLC.
2. Materials and methods
2.1. Plant materials
Three cultivars with higher SLC (Yannong 19, Jimai 20, and Ji 035037) and two with lower SLC (Tainong 18 and Taishan 23) were used for TaCOMT genes cloning based on our preliminary study on lodging resistance (data not shown). A set of Chinese Spring (CS) nulli-tetrasomic lines was used to verify the chromosomal locations of target genes and corresponding gene-specif ic markers. A total of 157 cultivars and advanced lines from Yellow and Huai River Valley Facultative Wheat Region of China were used to validate the association between allelic variations of TaCOMT genes and SLC.
2.2. Field trials and sampling
All 157 wheat cultivars and advanced lines were sown at Anyang in Henan Province, China and Suixi in Anhui Province, China during the 2013-2014 cropping season, and sown at Anyang and Shijiazhuang in Hebei Province, China during the 2014-2015 cropping season, providing SLC data from four environments. The experiments were carried out in randomized complete blocks with three replicates in each environment. Each plot comprised six 2-m rows spaced 20 cm apart and 30 cm between adjacent plots. Samples for SLC assays were taken 20 days after heading. Twenty stems with the same heading date in each plot were cut at the soil surface to about 20 cm above the ground. All fresh stems were exposed to 105°C air for 30 min for f ixation and then dried at 80°C for 24 h for preparation of the f inal SLC assay.
2.3. Stem lignin content assay
The SLC was determined following Suzuki et al. (2009) with minor modif ications. The dried stems were crushed to pass a 100-mesh sieve and then pulverized with a highthroughput tissue grinder (SPEX SamplePrep 2010 Geno/Grinder, USA; http://www.spexsampleprep.com) for 5 min at 1 750 r min-1. The powdered samples were dried at 60°C for 1 h, then 20 mg each was transferred to a 2-mL microcentrifuge tube with a screw cap (Sangon Biotech, Shanghai; http://www.sangon.com). The sample was extracted once with 2 mL water and centrifuged at 12 000×g for 10 min. The pellet was extracted twice with 1.8 mL methanol (60°C, 20 min), dried in vacuo, and 1 mL of 3 mol L-1HCl and 0.1 mL of thioglycolic acid were added before incubating at 80°C for 10 min and cooling in an ice-water bath. After centrifugation at 12 000×g for 10 min, the pellet was washed with 1 mL water and resuspended in 1 mL of 1 mol L-1NaOH. After gentle shaking at room temperature for 16 h, the solution was centrifuged at 12 000×g for 10 min. A total of 1 mL of supernatant was transferred to a fresh 1.5-mL tube and 0.2 mL of concentrated HCl was added. The tube was chilled at 4°C for 4 h and centrifuged at 12 000×g for 10 min. The pellet was dissolved in 1 mL of 1 mol L-1NaOH and the solution was diluted 50-fold with 1 mol L-1NaOH. Absorbance of the diluted solution at 280 nm was measured against a 1 mol L-1NaOH blank in a UV-Star 96-well plastic microplate (Greiner Bio-One, Germany; http://www.greinerbioone.com) containing 200 µL of the solution in each well on an Absorbance Microplate Reader (SpectraMax Plus 384, Molecular Devices, USA; http://www.moleculardevices.com). The lignin content was reported as the absorbance at 280 nm on a dry weight (DW) basis (Cai et al. 2006; Shan et al. 2008). Each sample was assayed twice and the means were used for subsequent statistical analysis.
2.4. Gene cloning and molecular marker development
The c DNA sequence of a common wheat COMT gene TaCM (GenBank accession: EF413031) was used for BLASTN searching against the wheat genome sequences database in the European Nucleotide Archive (ENA) (http://www.ebi.ac.uk/ena). A stand-alone blast strategy using Triticum urartu (progenitor of the wheat A genome) genomic data (http://gigadb.org/dataset/100050) and Aegilops tauschii (progenitor of the wheat D genome) genomic data (http://gigadb.org/dataset/100054) was also employed for candidate gDNA sequences searching. Based on the candidate sequences, primer sets were designed using Primer Premier 5.0 (Premier Biosoft; http://www.premierbiosoft.com) and synthesized by Sangon Biotech Co., Ltd. (Shanghai; http://www.sangon.com). The chromosome specif icity of each primer set was tested using CS nulli-tetrasomic lines.
Genomic DNA was extracted from seedlings using the CTAB method (Murray and Thompson 1980). PCR was performed in an MJ Research PTC-200 Thermal Cycler (MJ Research, Inc., Waltham, MA) using volumes of 20 µL including 2 µL of 10×buffer, 100 µmol L-1of each d NTP, 2 pmol of each primer, 1 U of rTaq polymerase, and 50 ng of template DNA. All reagents were obtained from TaKaRa Biotechnology Co., Ltd. (Dalian; http://www.takara.com.cn). PCR conditions were 94°C for 5 min followed by 35 cycles of 94°C for 30 s, 53-57°C (depending on primer sets) for 30 s, and 72°C for 2 min, with a f inal extension of 72°C for 10 min. PCR products were separated by electrophoresis in 1.5% (w/v) agarose gels stained with ethidium bromide and visualized using UV light. Target fragments with the expected lengths were recovered and cloned into the p EASY-T5 simple cloning vector (Beijing TransGen Biotech Co., Ltd., Beijing; http://www.transgen.com.cn) and sequenced by Sangon Biotech Co., Ltd. (Shanghai; http://www.sangon.com) with at least three repeats for each clone. PCR and DNA sequencing were repeated at least f ive times for each primer set to ensure the accuracy of the nucleotide sequences. gDNA sequences were aligned using the software DNAMAN (Lynnon Biosoft; http://www.geneious.com). Intron positions were determined by alignment of the gDNA sequences with the TaCM c DNA sequence.
Development of gene-specif ic markers was based on polymorphisms of target genes cloned from different cultivars. For cleaved amplif ied polymorphic sequence (CAPS) marker development, PCR products were digested using restriction enzyme Bmg BI. The reaction mixtures contained 5 µL PCR product, 0.5 µL Bmg BI (10 000 U mL-1), 2 µL 10× NEBuffer in total volumes of 20 µL and incubated at 37°C for 2 h. Digested PCR products were separated by electrophoresis in 2.0% (w/v) agarose gels.
2.5. Statistical analyses
Analysis of variance (ANOVA) and correlation analysis were conducted using the PROC GLM and PROC CORR in SAS v9.0 (SAS Institute; http://www.sas.com), respectively. The broad-sense heritability (h2) for SLC was calculated using the formulain which,andwere variance estimates of cultivar, cultivar×environment interaction, and residual error, respectively, and r and e were replicates per environment and the number of environments, respectively. The best linear unbiased estimations (BLUE) of SLC across four environments were calculated using the ANOVA function in IciMapping v4.0 software (Li et al. 2007; http://www.isbreeding.net). Differences in SLC among genotypes were tested using Fischer's protected LSD.
3. Results
3.1. Phenotypic evaluation
SLC across the four environments ranged from 6.84×103to 19.95 × 103OD280kg-1DW (Appendix A), and correlation coeff icients among four environments ranged from 0.67 to 0.77 (P<0.0001) (Table 1). SLC was signif icantly (P<0.0001) affected by cultivars, environments, and cultivar×environment interaction (Table 2). The broad-sense heritability was 0.90, indicating that SLC phenotype was mainly controlled by genetic factors, allowing selection for SLC in early generations of a breeding program.
3.2. Allelic variants of TaCOMT genes
The wheat genomic scaffold, IWGSC_CSS_3B_scaff_10655791, was obtained through a BLASTN search against the ENA using the TaCM c DNA (GenBank accession: EF413031) sequence as a query. The T. urartu genomic scaffold 2957 and A. tauschii genomic scaffold 50108, which shared high sequence similarity with the TaCM c DNA in the aligned regions, were also obtained by stand-alone BLASTN using the same query. Based on these sequences, three primer sets COMT_A, COMT_B, and COMT_D (Table 3) were designed to clone the corresponding full-length gDNA sequences of TaCOMT genes. Each primer set was tested with a set of CS nulli-tetrasomic lines and the target genes were located on chromosomes 3A, 3B, and 3D (Fig. 1-A), designated as TaCOMT-3A, TaCOMT-3B, and TaCOMT-3D, respectively.
Two allelic variants with full length of 1 721 bp (cloned from cultivars Tainong 18, Jimai 20, and Taishan 23) and 1 499 bp (from Yannong 19 and Ji 035037) were detected at the TaCOMT-3B locus, designated as TaCOMT-3Ba and TaCOMT-3Bb, respectively (Appendix B). Alignment of these two gDNA and TaCM c DNA sequences indicated two exons (398 and 673 bp) and one intron (140 bp) for both variants. The intron sequences were bordered with typical GT-AG structures. No p olymorphism was detected at 5´-UTR, whereas a 222-bp InDel was identif ied at the 3´-UTR between two allelic variants. Both alleles contained a 1 071-bp open reading frame (ORF) with a single nucleotide polymorphism (SNP) in exon 2. The deduced 356 amino acid polypeptide sequences were conserved and had a predicted molecular weight of 38.6 kDa.
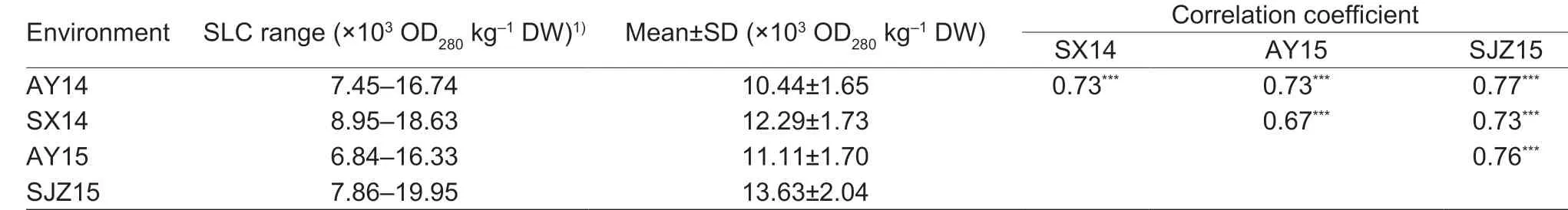
Table 1 Statistical analysis of stem lignin content (SLC) evaluated on 157 wheat accessions across four environments
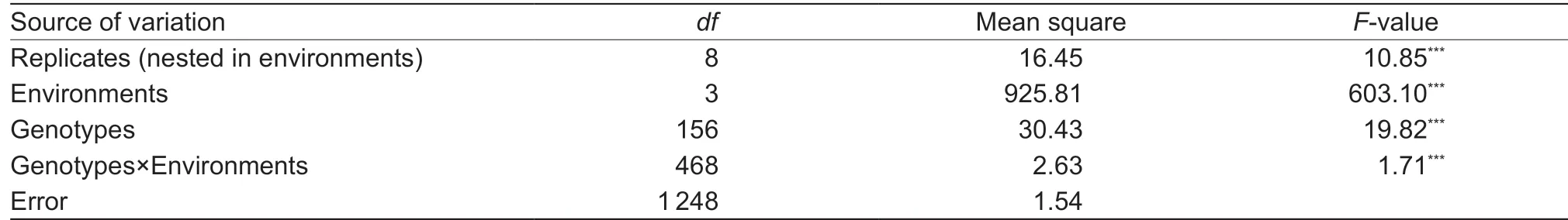
Table 2 Analysis of variance of stem lignin content (SLC) in 157 wheat accessions
For TaCOMT-3A, two allelic variants with full length of 1 622 bp (cloned from cultivars Tainong 18, Jimai 20, and Ji 035037) and 1 621 bp (from Taishan 23) were also identif ied, designated as TaCOMT-3Aa and TaCOMT-3Ab, respectively (Appendix C). And for TaCOMT-3D, a 1 735-bp gDNA sequence (Appendix D) was cloned from Taishan 23. These two TaCOMT genes shared the same exon-intron structure as alleles at the TaCOMT-3B locus (Fig. 2). A 1-bp InDel in exon 2, leading to a premature stop codon to the CDS of TaCOMT-3Ab, was detected among two alleles at locus TaCOMT-3A. Sequence alignment among the f ive TaCOMT gDNA sequences indicated that the exons were highly conserved with a sequence similarity of 97.9%, and the introns were more variable with 81.7% similarity.
3.3. Development of gene-specif ic markers
A co-dominant marker TaCOMT-3BM based on the 222-bp InDel in TaCOMT-3B (Table 3) was developed, yielding 638 and 416 bp fragments in genotypes TaCOMT-3Ba and TaCOMT-3Bb, respectively (Fig. 1-B). The location of the marker in chromosome 3B was verif ied using CS nullitetrasomic lines (Fig. 1-A). Among the 157 cultivars and advanced lines screened by TaCOMT-3BM, TaCOMT-3Ba, and TaCOMT-3Bb were present in 105 and 52 accessions, respectively (Appendix A). Statistical analysis indicated the SLC of cultivars with TaCOMT-3Ba was signif icantly (P<0.01) higher than those with TaCOMT-3Bb (Table 4), conf irming the association between allelic patterns and SLC.

Table 3 Primer sets for cloning TaCOMT genes and gene-specif ic marker TaCOMT-3BM

Fig. 1 Chromosome locations and allelic variations of TaCOMT genes. A, c hromosome specif icity of primer sets COMT_A, COMT_B, COMT_D, and marker TaCOMT-3BM. M indicates DNA ladder DL2000; 1 to 23 indicate the Chinese Spring (CS) nulli-tetrasomic lines N1A-T1D, N1B-T1D, N1D-T1B, N2A-T2D, N2B-T2A, N2D-T2A, N3A-T3D, N3B-T3D, N3D-T3B, N4A-T4D, M4B-T4D, N4DT4B, N5A-T5D, N5B-T5A, N5D-T5A, N6A-T6B, N6B-T6A, N6D-T6B, N7A-T7D, N7B-T7D, N7D-T7A, CS, and H2O, respectively. B, allelic variations of TaCOMT genes tested with marker TaCOMT-3BM and TaCOMT-3AM. 1 to 13 indicate cultivars Aikang 58, Bainong 64, Jimai 22, Liangxing 99, Shijiazhuang 8, Xiaoyan 6, Zheng 9023, Yumai 18, Zhoumai 16, Jimai 21, Linmai 2, Huaimai 18, and H2O corresponding to marker TaCOMT-3BM; and Zhongmai 871, Zhou 8425B, Jimai 22, Linmai 2, Han 6172, Wanmai 38, Yanzhan 4110, Yumai 50, Neixiang 5, Shi 4185, Huaimai 21, Wanmai 53, and H2O corresponding to TaCOMT-3AM, respectively.
The 1-bp InDel in exon 2 of TaCOMT-3A resulted in polymorphism of Bmg BI restriction sites, i.e., three Bmg BI cleavage sites were found for TaCOMT-3Aa, whereas two were in TaCOMT-3Ab (Appendix C). Based on this characteristic, a CAPS marker, designated as TaCOMT-3AM, was developed to discriminate the two alleles. The PCR products amplif ied by primer set COMT_A were used for digestion with Bmg BI. Four fragments of 932, 84, 39, and 567 bp were generated for TaCOMT-3Aa, whereas three of 932, 84, and 605 bp were produced for TaCOMT-3Ab; the specif ic 567 and 605 bp bands were used to distinguish between TaCOMT-3Aa and TaCOMT-3Ab (Fig. 1-B). Among 157 cultivars and lines screened by the marker TaCOMT-3AM, 126 and 23 accessions had alleles TaCOMT-3Aa and TaCOMT-3Ab, respectively (Appendix A); however, there was no signif icant difference between the corresponding SLC of contrasting genotypes (Table 4). Analysis of SLC among four TaCOMT combinations indicated that the TaCOMT-3Aa/3Ba genotype had signif icantly (P<0.05) higher SLC than that of the others (Table 4).
4. Discussion
4.1. Molecular characterization of TaCOMT genes
The common wheat genome comprises three closely related subgenomes, and it is necessary to ensure that primer sets used for gene cloning are chromosome-specif ic. Achievements in genome sequencing of T. urartu (Ling et al. 2013) and A. tauschii (Luo et al. 2013) are extremely helpful for wheat gene isolation. In the present study, primer sets COMT_A, COMT_B, and COMT_D, were conf irmed to be chromosome 3A-, 3B-, and 3D-specif ic, respectively. Of the three primer sets, COMT_B was designed based on the common wheat genome scaffold, whereas COMT_A and COMT_D were designed directly based on the T. urartu and A. tauschii scaffolds. In addition, the full-length gDNA sequence of TaCOMT-3Aa shared a 99.3% similarity with the corresponding region of the T. urartu genomic scaffold 2957; similarly, the TaCOMT-3D gDNA shared 99.9% similarity with the corresponding region of the A. tauschii genomic scaffold 50108. These results indicated that TaCOMT genes from different progenitors were highly conserved during wheat evolution.
The TaCOMT genes shared exon-intron structures with those from monocotyledons like maize (Collazo et al. 1992) and sorghum (Bout and Vermerris 2003), i.e., two exons interspaced by one intron, whereas those from dicotyledons such as tobacco (Toquin et al. 2003) and Arabidopsis (Besse et al. 2009) exhibited two or three introns, indicating there had been a divergence in COMT genes in evolution of monocotyledons and dicotyledons. Exons of COMT genes from wheat chromosomes 3A, 3B, and 3D showed the same lengths with high sequence similarity, whereas the introns were more variable, implying an absolute necessity for correct exon structures of COMT genes for normal function. The deduced polypeptide sequence of wheat COMT gene comprise 356 amino acids and showed a high degree of similarity with those from maize (86.1%) and sorghum (85.4%), further conf irm ing the closer relationship of COMT genes from wheat and other monocotyledons.
4.2. Sequence divergence of allelic variants at the TaCOMT-3B and TaCOMT-3A loci
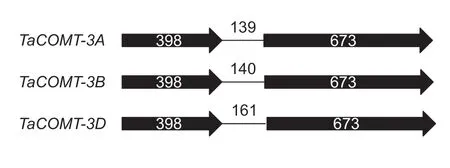
Fig. 2 Schematic representation of the exon-intron structures of TaCOMT genes. The black arrows indicate exons; lines represent introns; and numbers denote sequence lengths (bp).

Table 4 Statistical analysis of the association between TaCOMT genotypes and stem lignin content (SLC) in 157 wheat accessions
In the present study, two allelic variants of TaCOMT-3B were isolated from common wheat cultivars. One SNP that caused no diversity to the deduced amino acid sequence was detected in exon 2. A 222-bp InDel was detected in the 3´-UTR, and based on this variation, a gene-specif ic marker TaCOMT-3BM was developed. UTRs have crucial roles in post-transcriptional regulation of gene expression, and variations in UTR might affect the translation eff iciency and the stability of mRNA (Bashirullah et al. 2001; Mignone et al. 2002). The InDel mutation in the 3´-UTR of the two allelic variants of TaCOMT-3B might cause a different transcription level and thus result in variable SLC among cultivars.
Two allelic variants of TaCOMT-3A were also isolated, and SNPs were detected in exons, introns, and 5´- and 3´-UTR (Appendix C). One of these involved a 1-bp InDel in exon 2, and resulted in stop codons at the 3´-end of the CDS of TaCOMT-3Ab. Previous research indicated there were f ive consensus regions at the C-terminal ends of O-methyltransferases, and regions I and IV were crucial for their normal enzyme function (Ibrahim et al. 1998). Since the 1-bp deletion in exon 2 of TaCOMT-3Ab caused loss of regions IV and V in the polypeptide sequence (Appendix E), the protein probably performs a deviant function. Furthermore, this mutation brought to the diversif ication of Bmg BI cleavage sites (Appendix C), and based on this characteristic, a gene-specif ic marker TaCOMT-3AM was successfully developed. The association of SLC with different genotypes of TaCOMT-3A was analyzed, but the result showed there was no signif icant difference between the corresponding SLC of the two genotypes (Table 4). A possible explanation for this unexpected result is that the TaCOMT-3A locus plays only a minor role in controlling lignin content; however, further study needs to be conducted to investigate the relative contributions of the TaCOMT homoeologs to lignin biosynthesis.
4.3. Effec tiveness of the gene-sp ec if ic marker TaCOMT-3BM
Gene-specif ic markers that can accurately discriminate allelic variants at a locus are ideal tools for marker-assisted selection in wheat breeding (Bagge et al. 2007; Liu et al. 2012). Lignin is an important factor in the mechanical strength of stems and thus lodging resistance in wheat. A recent research showed that the SLC of Chinese modern cultivars are higher than that of older cultivars (Zhang et al. 2016), thus development of markers for SLC is of high value for wheat breeding. To date, however, no gene-specif ic marker for SLC was reported for wheat. Here, a co-dominant gene-specif ic marker, TaCOMT-3BM, was developed and validated by testing 157 wheat cultivars and advanced lines. This co-dominant gene-specif ic marker can be used in wheat breeding programs aimed at increased lodging resistance.
5. Conclusion
The gDNA sequences of TaCOMT genes located on wheat chromosomes 3A, 3B, and 3D were isolated and verif ied. A co-dominant gene-specif ic marker, TaCOMT-3BM, based on a 222-bp InDel at TaCOMT-3B locus was developed and validated on 157 wheat cultivars and advanced lines. This marker can be effectively used for selection of SLC in wheat breeding programs.
Acknowledgements
We thank Prof. R. A. McIntosh from Plant Breeding Institute, University of Sydney, for reviewing this manuscript. This study was supported by the National Natural Science Foundation of China (31161140346 and 31461143021), the Beijing Municipal Science and Technology Project, China (D151100004415003), the National Key Technology R&D Program of China (2014BAD01B05), and the earmarked fund for China Agriculture Research System (CARS-3-1-3).
Appendicesassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Phenotypic characterization and genetic mapping of the dwarf mutant m34 in maize
- Morphological diversity and correlation analysis of phenotypes and quality traits of proso millet (Panicum miliaceum L.) core collections
- Field identif ication of morphological and physiological traits in two special mutants with strong tolerance and high sensitivity to drought stress in upland rice (Oryza sativa L.)
- Crosstalk of cold and gibberellin effects on bolting and f lowering in f lowering Chinese cabbage
- Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.)
- Seedling and adult plant resistance to leaf rust in 46 Chinese bread wheat landraces and 39 wheat lines with known Lr genes
