Phenotypic characterization and genetic mapping of the dwarf mutant m34 in maize
Ll Jie-ping , Soomro Ayaz Ali, XlAO Gui, CHEN Fan-jun , YUAN Li-xing, GU Ri-liang,
1 Lab of Plant-Soil Interaction, Ministry of Agriculture/Department of Plant Nutrition, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193, P.R.China
2 Center for Seed Science and Technology, College of Agronomy and Biotechnology, China Agricultural University, Beijing 100193, P.R.China
Abstract Plant height is one of the most important agronomic traits associated with yield in maize. In this study, a gibberellins (GA)-insensitive dwarf mutant, m34, was screened from inbred line Ye478 by treatment with the chemical mutagen ethylmethanesulfonate (EMS). Compared to Ye478, m34 showed a dwarf phenotype with shorter internodes, and smaller leaf length and width, but with similar leaf number. Furthermore, m34 exhibited smaller guard cells in internodes than Ye478, suggesting that smaller cells might contribute to its dwarf phenotype. Genetic analysis indicated that the m34 dwarf phenotype was controlled by a recessive nuclear gene. An F2 population derived from a cross between m34 and B73 was used for mutational gene cloning and this gene was mapped to a chromosome region between umc2189 and umc1553 in chromosome 1 bin1.10, which harbored a previously identif ied dwarf gene ZmVP8. Sequencing analysis showed a nucleotide substitution (G1606 to A1606) in the sixth exon of ZmVP8, which resulted in an amino acid change (E531 to K531) from Ye478 to m34. This amino acid change resulted in an α-helix changing to a β-sheet in the secondary protein structure and the ‘SPEC' domain changed to a ‘BOT1NT' domain in the tertiary protein structure. Taken together, these results suggested that m34 is a novel allelic mutant originally derived from Ye478 that is useful for further ZmVP8 functional analysis in maize.
Keywords: dwarf, plant height, GA application, ZmVP8, maize
1. lntroduction
Plant height is a vital trait for crop yield. Previous studies have shown that introducing some semi-dominant dwarf ing mutations into several crop plants reduced their height and increased their yield (Hedden 2003; Ravindranath et al. 2011). The dwarf plants also had increased lodging resistance, and improved maturity and harvest index (Weng et al. 2011). In addition, newly released cultivars always had shorter plant height, were more resistant to storm damage, and had higher grain yield than the older varieties (Worland et al. 1998). Therefore, developing dwarf crops is an important breeding target for improving crop yield.
Many genes regulating the dwarf and semi-dwarf phenotypes have been cloned, such as sd-1 in rice (Sasaki et al. 2002) and rht1 in wheat (Peng et al. 1999). Maize (Zea mays L.) is one of the most important cereal crops. Plant height is controlled by several quantitative trait loci in maize (Beavis et al. 1991), and parts of these qualitatively inherited genes have been cloned, such as Dwarf3 (Winkler and Helentjaris 1995), Dwarf8 (Thornsberry et al. 2001), and Br2 (Multani et al. 2003; Xing et al. 2015).
Previous studies indicated that plant hormones, such as gibberellins (GAs), indole-3-acetic acid (IAA), and brassinosteroid (BR), play important roles in determining plant height. For example, four dwarf genes, An1 (Bensen et al. 1995), Dwarf8 (Fujioka et al. 1988), Dwarf1 (Spray et al. 1996), and Dwarf3 (Winkler and Helentjaris 1995) have been reported to encode enzymes that are involved in GA biosynthesis in maize. Dwarf mutant brachytic2 (br2) and semi-dwarf mutant vanishing tassel 2 (vt2) were encoded by IAA metabolic genes (Multani et al. 2003; Knoller et al. 2010). Dwarf phenotype could be achieved by down-regulation of BR biosynthesis gene ZmDWF1 (Tao et al. 2004). In addition to plant hormones, other factors associated with cell division and cell elongation also affect plant height. For example, Viviparous8 (ZmVP8) encodes a putative glutamate carboxy-peptidase protein in maize, and the ZmVP8 mutant had a dwarf phenotype (Suzuki et al. 2008; Lv et al. 2014).
Although some dwarf genes were cloned in previous studies, the molecular mechanisms of plant height regulation in maize are still not well understood. Additional alleles of dwarf genes should be identif ied to uncover the mechanisms of plant height regulation so that these alleles can be used to improve grain yield by regulating plant height in maize breeding.
In this study, the maize dwarf mutant m34 was isolated with a map-based cloning strategy. Mutant m34 had a new allele of ZmVP8 with a single nucleotide alteration at position 1 606 (G to A) in the sixth exon. This nucleotide alteration resulted in an amino acid change at residue 536 from Glu to Lys, which eliminated a ‘SPEC' domain in natural VP8 protein. These results suggested that locus Glu536 might be critical for ZmVP8 function in regulating plant height in maize, providing a new mutant allele for uncovering the function of ZmVP8.
2. Materials and methods
2.1. Plant materials and growth conditions
The maize dwarf mutant m34 was derived from inbred line Ye478 through treatment with the chemical mutagen ethylmethanesulfonate (EMS). Ye478 and m34 were grown in China Agricultural University experiment f ield in Shangzhuang, Beijing. Morphological traits including plant height, distance between internodes, leaf number, and length and width of leaves were measured at 21, 48, 78, and 90 days after sowing (DAS). The mean of each trait was estimated from at least 10 plants. All the statistical analyses, signif icance tests, and analyses of variation were performed in IBM SPSS Statistics 19 (Statistical Program for Social Sciences).
2.2. Histological analysis
A total of 10-mm transection specimens of internodes from Ye478 and m34 plants at 21 DAS were put in a buffer solution for 30 min. Under a scanning electron microscope, specimens were picked out and put into a 2.5% glutaraldehyde f ixative (p H 7.2, osmotic pressure 300 to 400) for 2 h, followed by 3-5 washes using phosphate buffer solution (p H 7.2). Specimens were then f ixed in 1% osmium tetraoxide f ixative for 2 h and washed 3-5 times with phosphate buffer solution. The specimens were dehydrated in an ethanol series of 30, 50, 70, 80, 90, and 100% and were dried using a LEICA EM CPD (critical point drier; Leica, Germany). Dried samples were mounted on a suitable working stage and coated with platinum (Pt) in a high vacuum (Eiko IB.3, ION COATER). A scanning electron microscope (HITACHI S-3000N) was used for imaging.
2.3. GA response assay
Ye478 and m34 were grown in the experimental f ield in 2014. At 19 DAS, 1×10-5mol L-1GA3(Sigma, St. Louis, MO) was applied to Ye478 and m34 seedlings; meanwhile sterilized water was applied as a control. At 27 DAS, the heights of both plants were measured (Winkler et al. 1994; Souza et al. 2001; Lv et al. 2014).
2.4. Mapping of dwarf mutant m34
An F2segregation population was derived from a cross between m34 and B73. Plant leaves were sampled for DNA extraction using the CTAB method (Doyle 1987) and genotyped with a SNP chip developed by the National Maize Improvement Center, China (GoldenGate assay, Illumina; Fan et al. 2003). Four simple sequence repeat (SSR) markers around the targeted locus were detected by SNP chip analysis, selected from the Maize Genome Database (MaizeGDB, http://www.maizegdb.org/ss.php), and used to genotype the individuals from the segregation population. Polymerase chain reaction (PCR) and gel electrophoresis were performed as described in the SSR method manual by MaizeGDB to narrow the genetic window (Dellaporta et al. 1983).
2.5. ldentif ication of the mutation site in m34
Total RNA from leaves of Ye478 and m34 at 21 DAS was isolated using Trizol reagent (TaKaRa, Dalian, China) and was converted into c DNA using a PrimeScript™ RT Reagent Kit with gDNA Eraser kits (TaKaRa, China). According to the B73 reference genome sequence, ZmVP8 open reading frames (ORFs) were amplif ied by KOD enzyme (TOYOBO, Japan) using the cDNA as templates. The PCR products were sequenced and compared between Ye478 and m34. The primers used for PCR amplif ication were vp8cdsF: 5´-ATGCCGCACTCCGTCCTGG-3´ (forward) and vp8cdsR: 5´-TCATGGGGTCACCAAAGAACTAAAA-3´ (reverse).
2.6. Linkage analysis between the nucleotide alteration and the dwarf phenotype
The m34 M3population was generated by self-pollination of m34 heterogeneous plants. Individual leaves were collected from each plant, and genomic DNA was extracted using the CTAB method. The genotypes of all individuals were identif ied by PCR using mutant and non-mutant specif ic primers. The forward primers were vp8-Ye478F (5´-TCTGCTTCATCAGTCACAG-3´) and vp8-m34F (5´-TCTGCTTCATCAGTCACAA-3´) for wild type and mutant, respectively. The underlined letter in forward primers indicated different SNPs between the two primers responding for wild type and mutant. The reverse primer was vp8-R (5´-TCTTCCTTGGAGCCCTTCAG-3´). The phenotype of each plant was investigated at the mature stage.
3. Results
3.1. Phenotypic characterization of the new maize dwarf mutant m34 from Ye478 inbred lines
The ethylmethanesulfonate (EMS) mutant m34, derived from Ye478 inbred lines, had a severe dwarf phenotype (Fig. 1). The morphological traits of Ye478 and m34 were estimated at 21, 48, 78, and 90 DAS. Signif icant reductions in plant height were observed in mutant plants as compared to Ye478, with 55, 49, 49, and 38% reductions at 21, 48, 78, and 90 DAS, respectively (Fig. 2-A). Meanwhile, m34 showed signif icantly shorter internodes, about 62, 64, 72, and 55% the size of those in Ye478 at 21, 48, 78, and 90 DAS, respectively (Fig. 2-B); the internodes were all shorter than those in Ye478 at 90 DAS (Fig. 2-C). However, m34 showed a similar number of leaves to Ye478 at all four growth stages (Fig. 2-D), indicating that dwarf ing of m34 was due to shorter internodes but not a smaller number of nodes (Fig. 1-B).
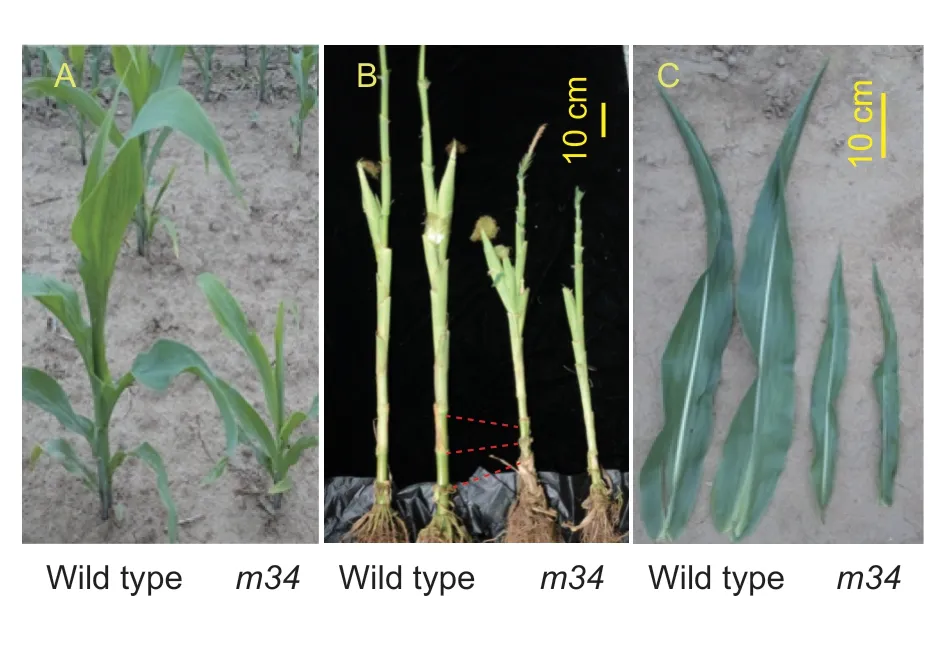
Fig. 1 Phenotypes of the wild type (Ye478) and m34. A, appearance of plants grown in f ield at the V6 stage (six visible leaves). B, images of the stems of plants at the R1 stage (silking stage). C, images of the ear leaf at the R1 stage.
Compared to Ye478, m34 plants had shorter and narrower leaves in addition to shorter plant height (Fig. 1-C). Leaf length in m34 was reduced by about 37, 26, 48, and 49% at 21, 48, 78, and 90 DAS, respectively (Fig. 2-E). Leaf widths were also signif icantly reduced to about half of those in Ye478 at all four growth stages (Fig. 2-F).
3.2. Mutant m34 develops shorter guard cells
To characterize the plant anatomy basis for the dwarf phenotype of m34, we investigated the guard cell morphology from the internode using scanning electron microscopy. The results showed that m34 had signif icantly smaller guard cells than Ye478 (Fig. 3-A). The length of guard cells in Ye478 was around 38 µm, while that in m34 was only around 33 µm (Fig. 3-B). This result suggested that smaller cell size might contribute to the dwarf phenotype of m34.
3.3. The m34 mutant is a GA-insensitive dwarf mutant
To evaluate the effects of GAs for growth control of m34 mutant, GA3and water were applied to Ye478 and m34 at 19 DAS in f ield. After 8 days (at 27 DAS), the heights of Ye478 and m34 without GAs treatment increased by 73.8 and 66.7%, respectively. Meanwhile, the ratios increased slightly to 84.2 and 72.8% of Ye478 and m34 after GA treatment, respectively. The analysis of variance showed that the genotype×treatment effect was not signif icant (P=0.248; Table 1). This result indicated that GA3treatment had a similar effect on Ye478 and m34.
3.4. Fine mapping of dwarf mutant m34
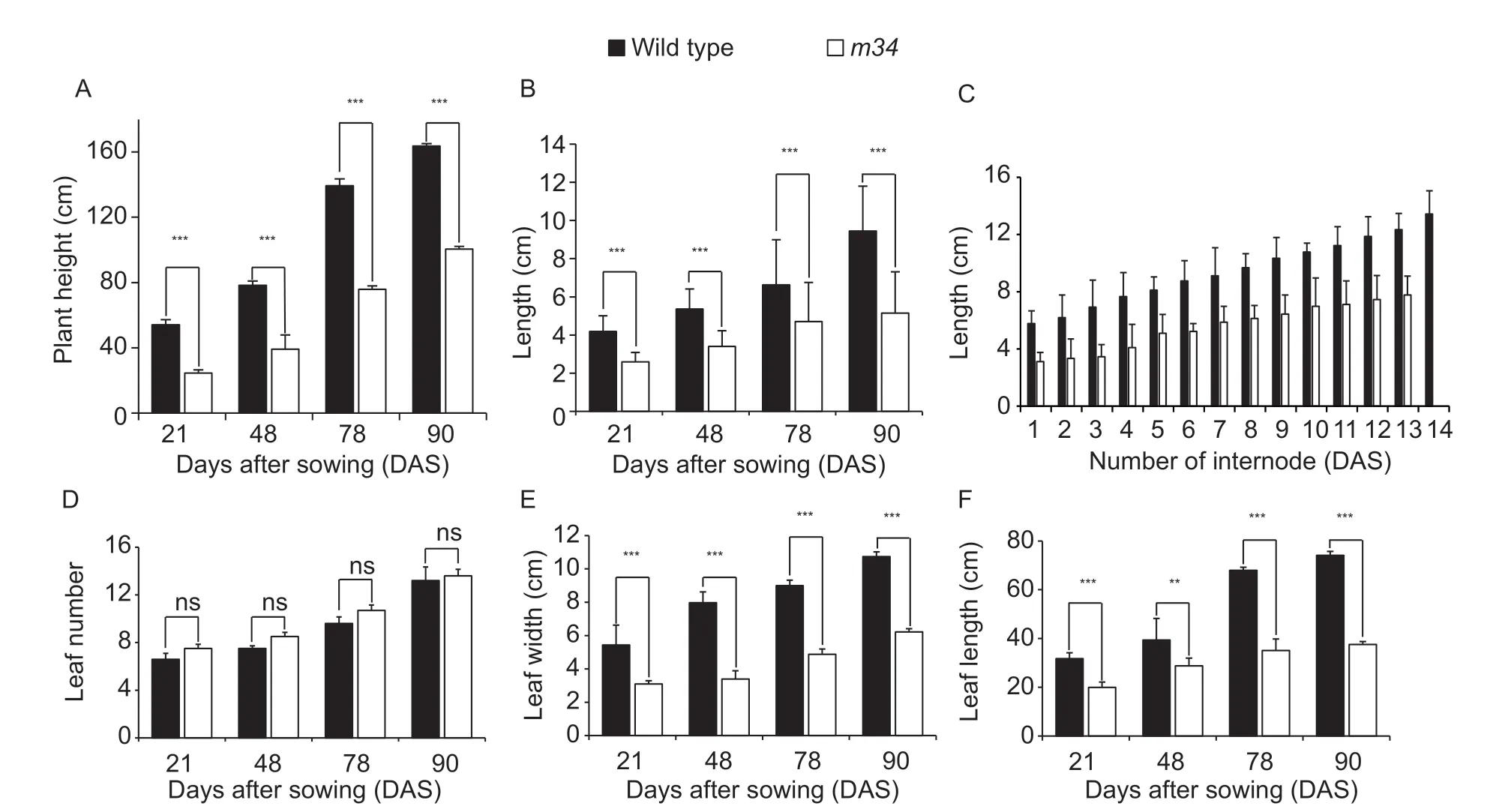
Fig. 2 Phenotypic performance of wild type (Ye478) and m34 at four growth stages at 21, 48, 78, and 90 days after sowing (DAS). A, plant height. B, average internode distance. C, length of every internode at 90 DAS. D, leaf number. E, leaf width. F, leaf length. ** and *** indicate signif icant differences at P<0.01 and P<0.001 levels, respectively. ns indicates not signif icant. Data are expressed as mean±SD.

Fig. 3 Scanning electron microscopy examination of guard cells in internodes of wild type (Ye478) and m34 mutants. A, microscopic observation of guard cell. B, length of guard cell in wild type and m34. * indicates signif icant difference at P<0.05 level. Data are expressed as mean±SD.
The isolated m34 mutants were crossed to Wu312 and B73. About 75 and 120 plants from f ive m34×B73 and 10 m34×Wu312 F1populations, respectively, were selfpollinated to develop the F2segregation populations. Among the 11 198 plants of the F2generation of m34×B73, a segregation ratio of 3.27:1 (8 573 tall and 2 625 dwarf) was observed for wild type and dwarf mutant. Similarly, in 18 812 m34×Wu312 F2plants, a 3.77:1 ratio (14 886 tall and 3 926 dwarf) was observed. These results indicated that the m34 dwarf mutant was genetically regulated by a single recessive gene.
Bulk segregation analysis (BSA) was used for preliminary mapping of the dwarf mutant m34. DNA from 50 short and tall plants from the F2population of m34×B73 were bulked to construct separate dwarf and tall pools. By screening 3 072 SNPs assays on parental (Ye478 and B73) pools, 1 153 SNP markers were revealed to be polymorphic between the two parents and were then used to screen the dwarf and tall pools. In the dwarf pool, most of the polymorphic SNPs had heterozygous alleles derived from both parents, which was same as that in the tall pool. However, SNPs between PZE-101181259 and PZE-101256289 (in chromosome 1 bin 1.10) in the dwarf pool only contained homozygous alleles from Ye478, indicating that these SNPs were linked to the dwarf phenotype of m34 (Fig. 4-A).
For f ine mapping, 290 F2plants that had the dwarf phenotype were selected from the F2population of m34×B73 and genotyped with SSR markers bnlg1025, umc2029, umc2189, and umc1553. Then, the m34 locus was narrowed to the genetic window between umc2189 and umc1553 (Fig. 4-B). In the candidate genetic window, the gene ZmVP8 was identif ied; this gene has been reported to show a dwarf phenotype (Evans and Poethig 1997; Lv et al. 2014). Thus, we presumed that ZmVP8 was the candidate gene controlling the m34 dwarf phenotype.
3.5. ldentifying the mutation sites of ZmVP8 in mutant m34
According to the B73 reference genome sequence, the ORF of ZmVP8 was amplif ied from Ye478 and m34. Sequence analysis revealed a polymorphic SNP in the sixth exon at position 1 606 between Ye478 (G) and m34 (A) (Fig. 4-C). Meanwhile, information on all SNPs in the exon of ZmVP8 among 513 maize lines was acquired from the webside (http://www.maizego.org/Resources.html) (Yang et al. 2011). The data showed that there were only f ive SNPs in the exon of ZmVP8 among all lines; detailed information about the SNPs is shown in Table 2. These SNPs did not include the SNP at position 1 606 (G/A). The results indicated that the A to G substitution in m34 may not exist in the natural population and is produced by artif icial EMS treatment.
To further conf irm the associations between the nucleotid e alteration of 1 606 (G/A) and the dwarf phenotype in m34, 129 M3plants were generated with 17 dwarfs and 112 normal plants. Primer pair vp8-m34F and vp8-R could generate specif ic PCR products from m34, while primer pair vp8-Ye478F and vp8-R was specif ic to wild-type plant (Fig. 5). For the M3plant, all 17 dwarfs could generate PCR products when using primer pair vp8-m34F and vp8-R, but failed to produce any product with primer pair vp8-Ye478 and vp8-R. All normal plants could generate PCR products when using primer pair vp8-Ye478 and vp8-R, and some produced additional PCR products with primer pair vp8-m34F and vp8-R (Fig. 5). These results suggested that the nucleotide alteration at codon 1 606 (G/A) was tightly linked with the dwarf phenotype, supporting the hypothesis that ZmVP8 was the corresponding gene for m34 mutant.

Table 1 Analysis of plant height of Ye478 (wild type) and m34 after gibberellin (GA) treatment
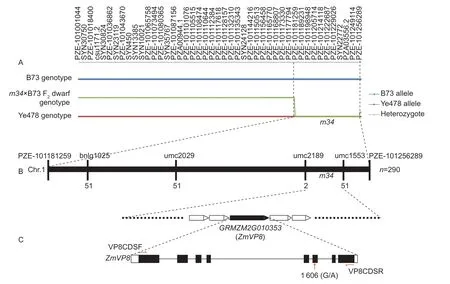
Fig. 4 Fine mapping of the m34 gene. A, primary mapping of m34 gene by bulk segregation analysis (BSA). B, f ine mapping of the m34 gene, the numbers 51 and 2 represent recombinants that were detected from 290 dwarf progeny plants in F2 population. C, structure of the candidate gene ZmVP8.
The ORF of three VP8 homologs were extracted from Oryza sativa (Os03g57660), Sorghum bicolor (Sobic.001G062900), and Brachypodium distachyon (Bradi1g06230) and used for alignment of ZmVP8 genes from cereals. The results showed that the nucleotide 1 606 (G) position in ZmVP8 was a conservative locus among the four homologs (Fig. 6-A).
After translating the ORF into protein sequence, the G/A (1 606) alteration resulted in an amino acid change at residue 536 from glutamic acid (Glu) to lysine (Lys). The protein sequence of Os03g57660, Sobic.001G062900, and Bradi1g06230 were also conserved to have a Glu at this position (Fig. 6-B).
The ZmVP8 protein domain of Ye478 and m34 was queried in the simple modular architecture research tool (SMART) database. It was found that natural ZmVP8 protein had a α-helix structure around position 536 in its secondary structure, while this structure was not present in the mutant protein (Fig. 7-B). In the tertiary protein structure, natural ZmVP8 had a ‘SPEC' domain, which was conserved in all cereal VP8 proteins, but this domain changed to a ‘BOT1NT' domain when Glu (513) changed to Lys (Fig. 7-A).
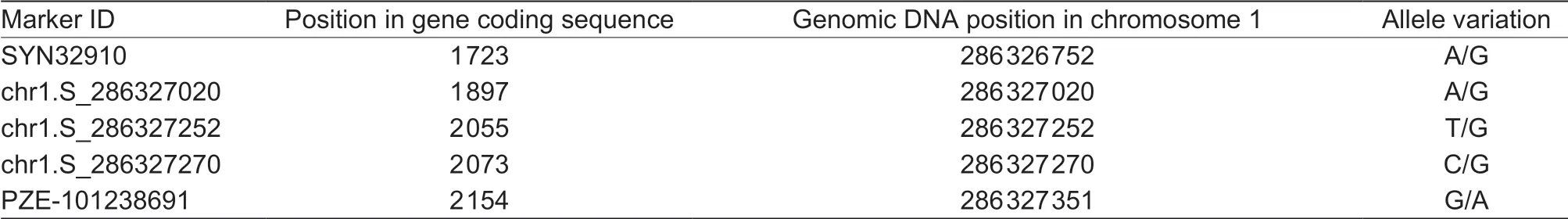
Table 2 Information of f ive SNPs in the exon of ZmVP8 among 513 maize lines1)
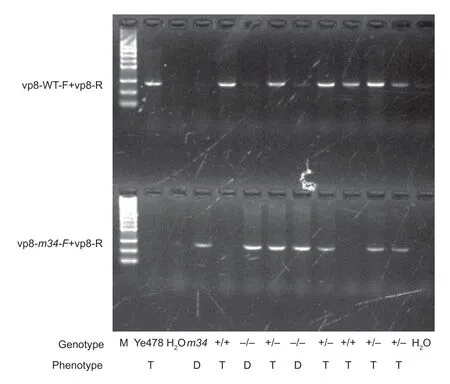
Fig. 5 Linkage analysis of ZmVP8 and the dwarf phenotype in M3 population. The gel shows some of the PCR amplif ication results from the M3 individuals using primer pair vp8-WT and vp8-R, and primer pair vp8-m34 and vp8-R. M, DNA marker. +/+, homozygous wild type (Ye478); +/-, heterozygote; -/-, homozygous mutant. T, tall plant; D, dwarf plant.
4. Discussion
4.1. The mechanism underlying ZmVP8-controlled organ development in maize
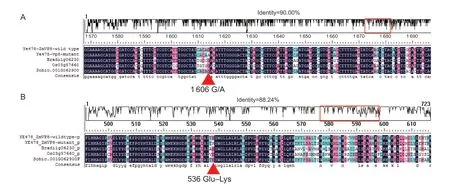
Fig. 6 Comparison of natural ZmVP8 and mutated ZmVP8. Alignment of DNA (A) and protein (B) sequences of natural ZmVP8 from Ye478 (wild type), mutated ZmVP8 from m34, and other three homologous genes from Oryza sativa (Os03g57660), Sorghum bicolor (Sobic.001G062900), and Brachypodium distachyon (Bradi1g06230). The nucleotide G at position 1 606 in the sixth exon, and the corresponding amino acid glutamic acid 536 (Glu536) in ZmVP8 were conserved among the four monocotyledon species. The G (1 606) in nucleotide sequence and Glu536 in protein sequence were marked by a triangle.

Fig. 7 The protein structure prediction for natural VP8 (Ye478, WT) and mutated VP8 (m34) (A) and the different structures between the ‘SPEC' domain and the ‘BON1NT' domain (B). TMR, transmembrane region; PUA, pseudouridine synthase and archaeosine transglycosylase domain; PA, protease associated domain; P_M28, peptidase_M28 domain; XPGN, xeroderma pigmentosum G N-region; SPEC, spectrin repeats; BOP1NT, this N terminal domain is found in BOP1-like WD40 proteins; TFR, the dimerization domain found in the transferrin receptor.
In this study, we reported a single nucleotide alteration in the ZmVP8 gene. ZmVP8 has been cloned to encode a putative glutamate carboxy-peptidase protein, which is homologous to Arabidopsis ALTERED MERISTEM PROGRAM1 (AMP1) (Suzuki et al. 2008; Lv et al. 2014). AMP1 is a member of the M28 family (also called aminopeptidase Y family) of carboxypeptidases with a pivotal role in plant development and stress adaptation (Helliwell et al. 2001). Previous studies concluded that both ZmVP8 and AMP1 had effects on the development of plant height, leaf formation, seed production, and lateral root growth (Chaudhury et al. 1993; Evans and Poethig 1997; Vidauree et al. 2007; Lv et al. 2014). However, the functional mechanism underlying VP8/AMP1 regulation was still unclear.
In Arabidopsis, mutant amp1 has a higher cytokinin biosynthesis rate and elevated total cytokinin levels (Nogué et al. 2000). Seven cytokinin synthase genes encoding ISOPENTENYL TRANSFERASE (IPT) were signif icantly upregulated in amp1 seedlings (Huang et al. 2015), indicating that amp1 may regulate plant height by accelerating cytokinin synthase and elevating cytokinin level. Meanwhile, in maize, the expression of Abphyl1, a negative regulator of cytokinin signaling, decreased signif icantly in vp8 mutants (Giulini et al. 2004; Kurakawa et al. 2007). Together with the fact that ZmVP8 was a GA-insensitive dwarf mutant (Table 1), it could be suggested that ZmVP8 controlled maize organ development through cytokinin hormone pathway.
Previous studies have shown that ZmVP8 represses ZmL1Lb gene expression, and ZmL1Lb worked as an upstream activator to activate the ZmFUS3 and ZmABI3 B3 domain to control the viviparous phenotype and regulate ABA accumulation in maize (Suzuki et al. 2008). This result suggested that ZmVP8-mediated dwarf phenotype might also be involved in the ABA pathway.
According to previous studies, ZmVP8 gene encodes a putative peptidase. Several classes of plant peptides have been identif ied to be involved in signal transduction (Boller 2005), and VP8 may be involved in plant signaling through its peptide processing function. A subcellular localization study showed that AMP1-GFP fusion protein was localized to endo-membranes in Arabidopsis (Vidauree et al. 2007), indicating that the AMP1/VP8 peptidases may process peptides in intracellular compartments.
Arabidopsis AMP1 expression is relatively high in rapidly dividing tissues such as in shoot and root meristems and AMP1 has been proven to suppress re-specif ication of stem cell niches (SCN) in the circular peripheral zone to maintain organ formation through meristem (Huang et al. 2015). Thus, ZmVp8 is likely required for normal cell division and expansion processes in the developing embryo (Suzuki et al. 2008). In this study, dwarf mutant m34 had shorter guard cells in internodes than wild type, further supporting the idea that the AMP1/Vp8 gene functioned in cell division. Taken together, we suggest that ZmVP8 may be involved in plant cell division through the cytokinin and ABA hormone signal pathway, but not through the GA signal pathway, by its peptidase processing function.
In this study, the ‘SPEC' domain (including spectrin alpha and beta subunits, alpha-actinin and dystrophin) in Ye478 was changed to ‘BOT1NT' domain in m34, a domain that is found in the N-terminal region of BOP1-like WD40 proteins. Bop1 is a nucleolar protein involved in rRNA processing, thereby controlling the cell cycle (Yan et al. 1993; Kim et al. 2006). This change in the tertiary protein structure may contribute to the change of ZmVP8 protein function in plant cell division and lead to the dwarf phenotype.
4.2. Potential application of ZmVP8 mutant for maize improvement
After adoption of short cultivars in the last century, known as the Green Revolution, cereal crops, especially rice and wheat, experienced dramatic increases in yield production. However, some famous dwarf genes that regulated the dwarf and semi-dwarf phenotypes were cloned and found to have negative effects on yield production (Winkler and Helentjaris 1995; Thornsberry et al. 2001). Thus, it is necessary to clone more dwarf genes and identify desirable alleles to balance the plant height and yield output.
Maize vp8 mutant has been reported to show a pleiotropic phenotype, including the viviparous (Robertson 1955) and dwarf phenotypes (Evans and Poethig 1997). Expression of ZmVP8 seemed to be abundant in maize organs at every growth stage (https://www.maizegdb.org/gene_center/gene/GRMZM2G010353; Fig. 8), supporting its multiple functions in plant development.
The mutant phenotype of ZmVP8 might be different in different genetic backgrounds, such as development of defective embryos in the W22 background (Suzuki et al. 2008) and dwarf phenotypes in the K36 and Ye478 backgrounds (Lv et al. 2014; Fig. 1). In a previous study, d2003 (another VP8 mutant) generated a premature stop codon to create a truncated protein (Lv et al. 2014). The plant heights of homozygous d2003 were different in different backgrounds (Lv et al. 2014), further suggesting that the function of ZmVP8 might depend on some other factors in different genetic backgrounds. In the future, it will be necessary to identify more alleles in ZmVP8 from natural populations, mutants, and applied genome editing technology to create more breeding materials to balance plant height and yield production.
5. Conclusion
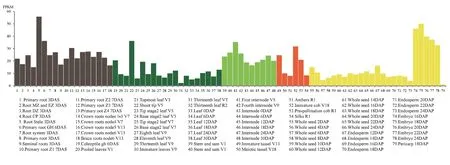
Fig. 8 Expression pattern of ZmVP8 in different organs and developmental stages in maize. Expression data were extracted from https://www.maizegdb.org
In this study, the maize GA-insensitive dwarf mutant m34, which is derived from inbred line Ye478 background, was identif ied and characterized. Compared to WT, m34 developed a severe dwarf phenotype, with shorten internodes, and shorter and narrower leaves. Genetic analysis indicated that the dwarf phenotype in m34 was controlled by a recessive gene. Genetic mapping implied that ZmVP8 was the candidate gene controlling the dwarf phenotype. Sequence analysis showed that a single nucleotide alteration at codon 1 606 (G/A) in the sixth exon of ZmVP8 might be responsible for the dwarf phenotype in m34.
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFD0101803), the earmarked fund for China Agriculture Research System (CARS-02-10), the National Natural Science Foundation of China (31771891), and the Chinese University Scientif ic Fund (2015ZH001).
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specif ic marker
- Morphological diversity and correlation analysis of phenotypes and quality traits of proso millet (Panicum miliaceum L.) core collections
- Field identif ication of morphological and physiological traits in two special mutants with strong tolerance and high sensitivity to drought stress in upland rice (Oryza sativa L.)
- Crosstalk of cold and gibberellin effects on bolting and f lowering in f lowering Chinese cabbage
- Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.)
- Seedling and adult plant resistance to leaf rust in 46 Chinese bread wheat landraces and 39 wheat lines with known Lr genes
