Preparation and characterization of atrazine-loaded biodegradable PLGA nanospheres
CHEN Xiao-ting , Tongxin Wang,
1 College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, P.R.China
2 State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops/Key Laboratory of Biopesticide and Chemical Biology, Ministry of Education, Fuzhou 350002, P.R.China
3 CREST Center for Nanomaterials, College of Engineering, Howard University, Washington, D.C. 20059, USA
4 College of Dentistry, Howard University, Washington, D.C. 20059, USA
Abstract Atrazine is the second mostly used herbicide in USA, but low utilization ratio causes severe environmental problem, so controlled release system is highly needed in order to minimize the negative impact on environment. In this paper, a herbicide delivery system, atrazine-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) were prepared by forming an oilin-water emulsion using the emulsion-solvent evaporation method. By varying the preparation conditions of PLGA-NPs, such as sonication time, surfactant content, solvent fraction, and polymer content, the particle sizes of the PLGA-NPs were well controlled from 204 to 520 nm. The morphology and size distribution of PLGA-NPs were evaluated using dynamic light scattering (DLS) and scanning electron microscopy (SEM). Both the encapsulation eff iciency and release prof ile of the herbicide from the PLGA-NPs were typically evaluated by using 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine (atrazine, ATZ) as the model. ATZ encapsulation eff iciency within the PLGA-NPs was ranged from 31.6 to 50.5%. The release prof iles of ATZ-loaded PLGA-NPs exhibited a much slower release rate in comparison with that of pure herbicide. The results demonstrated that the prepared PLGA-NPs had a high encapsulation eff iciency and slow release rate, which could be used as a promising herbicide release system in agriculture to diminish the impact on the environment and minimize the potential harm to the farmers.
Keywords: atrazine, PLGA, nanoparticle, controlled release, drug delivery
1. lntroduction
Herbicides are widely used in modern agriculture for the purpose of inhibiting weeds or reducing tillage. One typical herbicide, 2-chloro-4-ethylamino-6-isopropylamino-1,3,5-triazine (atrazine, ATZ), can inhibit the photosynthesis of the target plants and kill a wide spectrum of weeds under a range of conditions (Moore and Kroger 2010; Zhang et al. 2014). ATZ, as the second mostly used herbicide in USA, had been annually applied with a usage of 35 million kilograms in US agriculture between 1999 and 2004 (Pathak and Dikshit 2012; Zhang et al. 2014). However, both the extremely high dosage of those conventional herbicides and the low utilization ratio leads to the severe environmental problems such as pollution of groundwater, soil, sediments, plants and animals (Anjali et al. 2010). Therefore, controlled release of herbicides or pesticides is highly desirable because it may increase the utilization of agrochemicals and minimize the negative impact on the environment.
Polymer-based microparticles and nanoparticles were explored as agrochemical delivery systems for their advantages including: i) the reduced dosage for weed control due to their larger surface area, easy attachment and fast mass diffusion of nanoparticles; ii) less contamination to environment; and iii) safer application for farmers (Jyothi et al. 2010; Ghormade et al. 2011; Grillo et al. 2011). Since the 1990s, poly(lactic-co-glycolic acid) (PLGA) has been extensively used in drug delivery systems due to its excellent biocompatibility and biodegradability (Soppimath et al. 2001; Musumeci et al. 2006; Joshi et al. 2014). Poly(hydroxybutyrate) (PHB) or poly(hydroxybutyrate-cohydroxyvalerate) (PHBV) microspheres were investigated as delivery system to modify release behavior for ATZ and ametryn (Grillo et al. 2009, 2011; Lobo et al. 2011). The reported encapsulation eff iciency of ametryn was about 40% and the release prof ile was modif ied. Recently, another polymer, poly(epsilon-caprolactone) (PCL), was used to encapsulate herbicides, in which PCL nanoparticles could increase herbicide activity and decrease toxicity (Grillo et al. 2012; Pereira et al. 2014).
Because ATZ is the second mostly used herbicide in the USA, this work focused on encapsulating ATZ into PLGA nanoparticles. The strategy was to form an oil-in-water emulsion combined with solvent evaporation method. The aim was to improve eff iciency and enhance safety of herbicide application, thus minimizing environmental impacts. The particle size of ATZ-loaded PLGA-NPs was well controlled from 200 to 520 nm by optimizing the preparation conditions. The release rate of PLGA-NPs was effectively decreased compared with that of pure herbicide.
2. Materials and methods
2.1. Materials
Atrazine (analytic al grad e, CAS no. 1912-24-9, formula C8H14ClN5), is white and odorless powder with melting point 173-175°C. It is insoluble in water (0.0347 mg mL-1), slightly soluble in organic solvent (183 mg mL-1in DMSO, 52 mg mL-1in chloroform). Atrazine, dichloromethane (DCM, analytical grade), ethanol (anhydrous, ≥99.5%), polyvinyl alcohol (PVA, Mowiol®10-98) with a molecular weight of 61 kDa and a hydrolysis degree of 98% and PLGA (lactide/glycolide=65/35) with a molecular weight of 40-75 kDa were purchased from Sigma-Aldrich (USA). All water used is MilliQ water f iltered through a 0.2-µm cellulose nitrate membrane.
2.2. Preparation of the ATZ-loaded PLGA-NPs
ATZ-loaded PLGA-NPs were prepared by forming an oil-inwater emulsion method combined with sonication-solvent evaporation method (Budhian et al. 2007). Typically, organic phase of PLGA/ATZ was prepared by dissolving 50 mg of PLGA and 5 mg of ATZ in 5 mL of dichloromethane at room temperature. The aqueous phase (50 mL) was prepared at 75°C using 1.5% (w/v) of PVA. After the organic phase (5 mL) was dropwise added into 50 mL of aqueous phase at 25°C under magnetic stirring, the mixture was sonicated using a probe sonicator S-4000 (Misonix, USA) with an input energy of 40 W for varied time to generate the oil-inwater emulsion. The resulted emulsion was further stirred overnight to completely evaporate DCM. The PLGA NPs were purif ied by using centrifuge (20 000 r min-1, 15 min, Beckman JZ-21M, Japan) at 4°C followed by washing with water three times in order to remove the unloaded ATZ and loosely bonded surfactant. The purif ied nanoparticles were resuspended in 1 mL of water and freeze-dried using a Labconco Lyophilizer (Freezone 4.5, Labconco, USA).
2.3. Size distribution and morphology of PLGA-NPs
The nanoparticle size and size distribution were measured by laser dynamic light scattering (DLS) using a Nano-ZS Zetasizer (Malvern, UK). Size measurements were performed in triplicate following a 1/10 dilution of the nanoparticles suspension in distilled water at 25°C, then, sonicated for 5 min. The polydispersity index ranges from 0 to 1.
The morphology and particle size of the dry powder were observed on a Zeiss Auriga FIB (focused ion beam)-SEM Crossbeam Workstation using an InLens detector at an accelerated voltage of 2 kV. For SEM study, a small amount of the freeze-dried powder of PLGA-NPs was put on a double-sided tape attached on an aluminum stub.
2.4. Evaluation of the encapsulation effciency of ATZ-loaded PLGA-NPs
The herbicide content within the nanoparticles was determined by UV-Vis Spectrophotometry (Agilent, USA). A weighted freeze-dried powder of ATZ-loaded PLGA NPs (5-10 mg) was suspended in 5 mL of ethanol, the suspension was maintained under slight agitation for 30 min (60 r min-1, 30°C) to completely collapse the PLGA nanospheres. After centrifugation at 20 000 r min-1for 10 min, the supernatant was collected and analyzed using a UV-Vis-NIR Spectrophotometer (Agilent, USA). The absorption at the wavelength of 223 nm was used to evaluate the amount of atrazine encapsulated in the nanoparticles. A calibration curve was established by using the standard solutions of pure atrazine in ethanol. Measurements were performed in triplicate for each batch. The encapsulation eff iciency (EE, %) could be calculated by following equation:
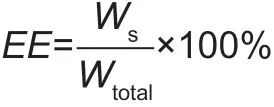
Where, Wsis the weight of loaded ATZ in nanoparticles and Wtotalis the theoretical weight of ATZ.
2.5. Release prof ile of ATZ-loaded PLGA-NPs
The dual-compartment system was employed to investigate the release prof ile of ATZ from the PLGA-NPs or ATZ alone (Grillo et al. 2011). A cellulose membrane (Spectrum, USA) with molecular pore-exclusion size of 8-10 kDa was employed to separate the donor compartment (suspension of PLGA-NPs or pure ATZ) from the acceptor compartment containing 150 mL of deionized water. The pore size of the membrane does not allow the passage of nanoparticles or other polymers such as PLGA and PVA except the free herbicide. The system was maintained under slight agitation (60 r min-1, 30°C). Aliquots of 3 mL were periodically taken from the receptor compartment until the absorbance became stabilized. The solutions for UV-Vis analysis were put back into the acceptor compartment in order to maintain the solution at a constant volume (150 mL). The experiments were carried out in triplicate.
3. Results and discussion
3.1. Effect of preparation variables on nanoparticles' morphology and size
Oil-in-water emulsion method combined with sonicationsolvent evaporation was used to prepare the ATZ-loaded PLGA NPs. Because the particle size and morphology of the NPs are critical parameters that affect the kinetics of drug release and uptake of the targeted plants, a number of experimental variables including the sonication time, surfactant content, PLGA content and the volume ratio of aqueous to organic phase were fully evaluated and optimized for the preparation of PLGA-NPs.
Sonication timeSonication energy played a key role in formation of the emulsion, and thus signif icantly affected the particle size. The sonication energy was adjusted by varying sonication time (0.5 to 10 min) with a constant power (Table 1). With increasing sonication time, the particle size of PLGA-NPs was gradually decreased from 364 nm for 0.5 min to 234 nm for 10 min. In the meantime, the polydispersity index (PDI) was reduced from 0.28 for 0.5 min to less than 0.1 for 10 min. It revealed that the increase of the sonication time led to a reduction in the particle size as well as the size distribution. Longer sonication time released greater sonication energy, which led to a rapid and even dispersion of polymeric organic phase as nanodroplets, and thus smaller polydispersity, smaller size and monomodal distribution prof ile of nanoparticles. This conclusion is consistent with previous report on praziquantel-loaded PLGA NPs (Mainardes and Evangelista 2005). Moreover, size reduction was faster at the beginning of sonication time than later, which was similar with the f inding of Feczkó et al. (2011).
Based on the above results, 5 min of sonication time was chosen for the subsequent preparation, in order to get relatively small size and narrow distribution of NPs, and also avoid side effects due to the input of excessive energy.
Surfactant contentThe surfactant, PVA was important in the preparation of PLGA NPs, because it could reduce the surface energy, facilitate emulsion formation, and stabilize the nanoparticles. The inf luence of PVA concentration was evaluated from 0.25 to 2.0% (w/v) and the corresponding results were shown in Table 2. The particle size dramatically decreased from 520 to 283 nm, when the PVA content increased from 0.25 to 1.5% (w/v). Meanwhile, the PDI of the PLGA-NPs showed signif icant decrease with the increase of the PVA concentration from 0.25 to 1.5%. However, when the PVA content was higher than 1.5%, the particle size exhibited little change and the size distribution tended to be broad. This observation was consistent with previous report on the praziquantel- or haloperidol-loaded PLGA NPs (Mainardes and Evangelista 2005; Budhian et al. 2007) or PLGA-BSA nano- and microparticles (Feczkó et al. 2008), in which the decrease in particle size with increasing PVA concentration was also observed.
The emulsif ication and stabilization of the nanoparticles are determinant factors for emulsion-solvent evaporation method. An insuff icient amount of emulsif ier would fail in stabilizing the nanoparticles, and thus the nanoparticles would tend to aggregate, leading to larger size of nanoparticles or precipitation (Feng and Huang 2001). The stabilizing effect of PVA and the high viscosity of the PVA solution prevented the emulsif ied droplets from interf lowing (Feczkó et al. 2008). In the following preparation, 1.5% of PVA was chosen in order to get relatively smaller nanoparticles and stable emulsions.
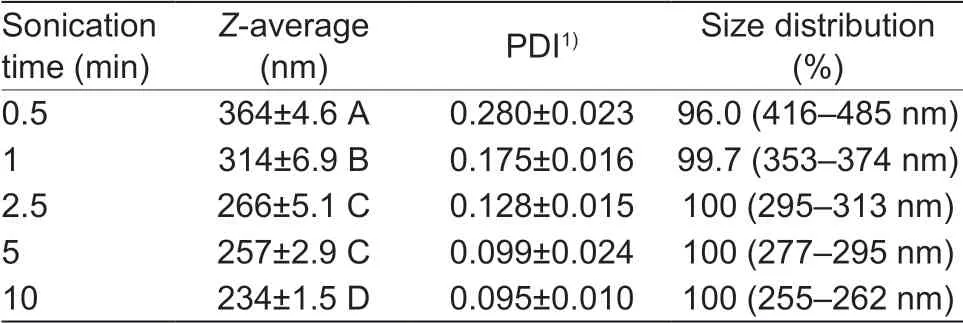
Table 1 Effect of sonication time on the particle size and size distribution
The morphology and size distribution of NPs were further conf irmed by the SEM observation. Fig. 1 shows that PLGANPs are spherical with smooth surface. Similar as the DLS result described above, SEM observations conf irmed that the particle size decreased with the increase of the PVA surfactant content from 0.25 to 1.5%.

Table 2 Effect of PVA content on the particle size and distribution of PLGA-NPs1)
In addition to the particle size change, an interesting f inding from SEM analysis was that it became more diff icult to obtain the satisfactory SEM images, particularly when the particles became smaller. Fig. 1 also shows that the morphological changed and it seems that the spherical particle became less with the increase of PVA content or decrease of particle size. When the imaging parameters such as aperture size and acceleration voltage were kept the same, the smaller particles from Fig. 1-D became agglomerated or meted together. Even though the voltage was reduced to 0.5 kV from 2 kV, the particles melting still could be observed. On the contrary, the larger particle from Fig. 1-A seldom became agglomerated during SEM observation. This might be due to that the smaller particles had relatively thinner PLGA shell, therefore, they might be easily destroyed or melted by the electron current during SEM imaging.
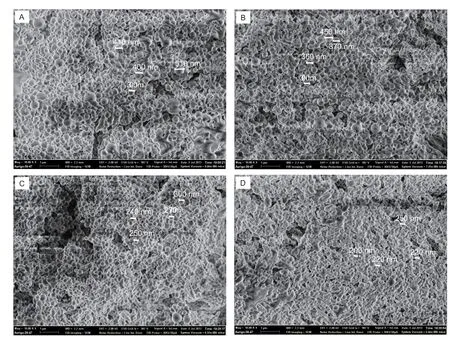
Fig. 1 Representative scanning electron microscopy (SEM) images of poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) with different content of polyvinyl alcohol (PVA). A-D, 0.25, 0.50, 1.0, and 1.5% PVA, respectively.
DCM content (aqueous to organic phase (W/O) volume ratio)In addition to the surfactant content, the ratio of W/O played a key role to stabilize and control the size and size distribution of the emulsion. The effect of the W/O ratio was evaluated by varying the volume of DCM from 2 to 15 mL while keeping the volume of aqueous phase as a constant of 50 mL. It is shown that an increase in organic solvent volume led to the increase of particle size and wider size distribution (Table 3). One possible reason for the increased particle size might be resulted from the reduced net shear stress. Because the preparation was using a constant external energy input, increase of the whole volume reduced the shear stress, thus increasing particle size (Song et al. 2008b). On the other hand, with increasing the DCM volume from 2 to 15 mL, the PVA concentration in the f inal emulsion was decreased from 1.44 to 1.15%, which also led to larger particle size. In order to get a better solubility of ATZ and PLGA and avoid aggregates of nanoparticles, 5 mL of DCM (W/O ratio of 10/1) was chosen for preparation of PLGA-NPs.
PLGA contentThe effect of the PLGA content on NPs size was evaluated between 25 and 75 mg of PLGA. Table 4 shows that the mean diameter of PLGA NPs increased with the increase of PLGA amount, especially, in the treatment of 75 mg of PLGA. When the W/O ratio and PVA amount was kept the same, increasing PLGA content led to the increase of the viscosity of the organic phase which would reduce the net shear stress, thus promoting the formation of droplets with larger size. Also, the increase of the viscosity could hinder rapid dispersion of PLGA solution into the aqueous phase, resulting in larger nanoparticles after evaporating the organic solvent (Mainardes and Evangelista 2005; Song et al. 2008a). In comparison to the sonication time, PVA concentration and DCM volume, PLGA content showed less effect on the particle size control.

Table 3 Effect of DCM volume on the particle size and size distribution1)
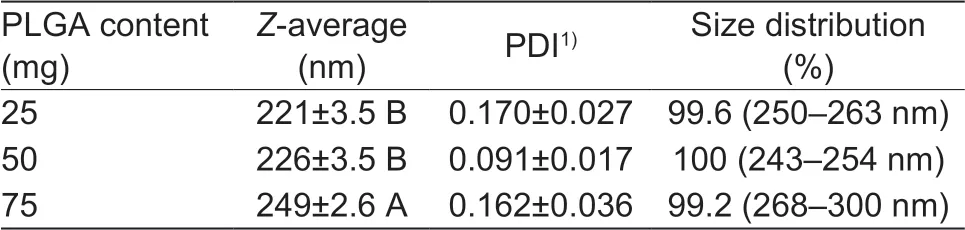
Table 4 Effect of PLGA content on the particle size and size distribution1)
3.2. Encapsulation effciency of ATZ-loaded PLGA- NPs
The encapsulation eff iciencies (EE, %) of the ATZ-loaded PLGA-NPs were determined by complete collapsing the nanoparticles using ethanol to completely release the encapsulated ATZ, which was determined by UV-Vis spectroscopy based on calibration curve using the standard ATZ with known concentration. The encapsulation eff iciencies for the nanoparticles prepared from 0.25 to 1.5% of PVA were ranged from 31.6 to 50.5%. In comparison to those previous reports, the EEs for our nanoparticles are similar to that of other herbicide-loaded microparticles prepared using similar method. As an example, ATZ-loaded PHBV microspheres had a mean size ranged from 5.1-24.0 µm and the EE values ranged between 3.3 and 24.7% (Lobo et al. 2011). Similarly, ametryn (AMT)-loaded PHB/PHBV microspheres had a mean size of 6 µm and the EE values of AMT-PHB and AMT-PHBV microparticles were 34.3 and 38.2%, respectively (Grillo et al. 2011). However, the EE of the prepared ATZ-loaded PLGA NPs is much lower than that of the ATZ-loaded poly(epsiloncaprolactone) nanoparticles (400-500 nm), which possessed an astonished EE (93%) (Pereira et al. 2014).
3.3. Release prof ile of ATZ-loaded PLGA-NPs
The release prof ile of ATZ-loaded PLGA-NPs as well as free ATZ were depicted in Fig. 2. In the dual-compartment, only the dissolved ATZ can pass through the membrane. Analysis of the release kinetics curves indicated that the encapsulated ATZ exhibited a much slower rate in comparison with that of un-encapsulated one. The time for half cumulative release (50%) for pure ATZ was around 3.5 h. While the larger nanoparticle (500 nm) showed a 9.0 h of the half cumulative release time, the smaller nanoparticle (250 nm) was 24 h. To reach a cumulative release level of 75%, the time for pure ATZ, PLGA-NPs of 500 nm and 250 nm was around 8, 48 and 96 h, respectively. The above results indicated that PLGA nanoparticles had a slower release rate than that of pure ATZ and smaller particle size led to even slower release rate than that of the larger particle.
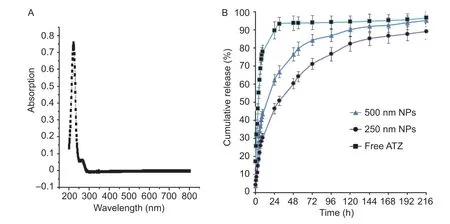
Fig. 2 UV-Vis spectrum of atrazine (ATZ, A) and release prof ile of ATZ-loaded PLGA-NPs in water (B, 60 r min-1, p H 7.0, 30°C), PLGA, poly(lactic-co-glycolic acid); NPs, nanoparticles. Data are mean±SD (n=3).
Normally, smaller particles led to faster releasing rate due to the greater surface area. In our research, two types of nanoparticles with 500 and 250 nm prepared from varied PVA amount (from 0.25 or 1.5% of PVA content, respectively) were selected to evaluate the releasing rate of ATZ from nanoparticles. The results indicated that PLGA nanoparticles with larger size (500 nm) had a slower release rate than that of pure ATZ and smaller particle size (250 nm) led to even slower release rate than that of the larger particle (500 nm). The possible reason could be attributed to the PVA amount within the nanoparticles. In this study, the PVA amount for 250 nm particles is 1.5%, which is about 6 times that of 500 nm particles (0.25%). Because PVA is non-degradable emulsif ier, higher amount of PVA within the 250 nm particles could lead to greater stability than that of 500 nm particles, thus slower drug releasing rate than that of 500 nm particles. In addition, another reason might be due to the greater encapsulation eff iciency of ATZ within the smaller nanoparticles. Increasing PVA content from 0.25 to 1.5% of PVA led to smaller particles (from 500 to 250 nm), as well as greater encapsulation eff iciency of ATZ within nanoparticles (from 31.6 to 50.5%, respectively). Consequently, both greater PVA content and greater encapsulation eff iciency of ATZ from smaller nanoparticles led to slower releasing rate from the smaller nanoparticles with 250 nm than those from 500 nm.
Therefore, our above formulation technology to control the particle size could provide an effective strategy to control the release prof ile of the ATZ.
4. Conclusion
ATZ-loaded PLGA-NPs was successfully prepared by an emulsion method. The effects of 4 experimental variables including sonication time, PVA amount, DCM/water ratio and PLGA content on the particle size and size distribution were fully evaluated. While PLGA content did not show obvious effect, variation of other variables signif icantly affected the particle size and size distribution. While increase sonication time or PVA content led to smaller size and narrower distribution, increase DCM amount (less water/DCM ratio) led to larger particle size. By optimizing the variables, the particle size was well controlled in the range between 204 and 520 nm. Both SEM and DLS measurements conf irmed the success of the particle size control. The encapsulation eff iciencies of the ATZ-loaded PLGA-NPs were 31.6-50.5%. In comparison to pure ATZ, the ATZloaded PLGA-NPs exhibited a much slower released rate. The sustained release behavior of the nanoparticle due to the well-controlled particle size suggested that the ATZ-loaded PLGA-NPs could be developed into an environmentally friendly herbicide delivery system for agricultural applications which may diminish the impact on the environment and minimize the potential harm to farmers.
Acknowledgements
The authors thank the f inancial supports of the National Natural Science Foundation of China (30671347), the Commonweal Specialized Research Fund of China Agriculture (201103016), and the Fujian Provincial Science Foundation, China (2012J01079).
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specif ic marker
- Phenotypic characterization and genetic mapping of the dwarf mutant m34 in maize
- Morphological diversity and correlation analysis of phenotypes and quality traits of proso millet (Panicum miliaceum L.) core collections
- Field identif ication of morphological and physiological traits in two special mutants with strong tolerance and high sensitivity to drought stress in upland rice (Oryza sativa L.)
- Crosstalk of cold and gibberellin effects on bolting and f lowering in f lowering Chinese cabbage
- Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.)
