The autophagy gene ATG8 affects morphogenesis and oxidative stress tolerance in Sporisorium scitamineum
ZHANG Bin, CUl Guo-bing, CHANG Chang-qing, WANG Yi-xu, ZHANG Hao-yang, CHEN Bao-shan, DENG Yi-zhen, , JlANG Zi-de
1 Department of Plant Pathology/State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou 510642, P.R.China
2 Integrative Microbiology Research Centre/Guangdong Province Key Laboratory of Microbial Signals and Disease Control, South China Agricultural University, Guangzhou 510642, P.R.China
3 College of Life Science and Technology/State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangxi University, Nanning 530004, P.R.China
Abstract The basidiomycetous fungus Sporisorium scitamineum causes sugarcane smut that leads to severe economic losses in the major sugarcane growing areas in China, India and Brazil, etc. Autophagy is a conserved pathway in eukaryotes for bulk degradation and cellular recycling, and was shown to be important for fungal cell growth, development, and pathogenicity. However, physiological function of autophagy has not been studied in S. scitamineum. In this study, we identif ied a conserved Atg8 protein, named as SsAtg8 and characterized its function. Our results showed that autophagy was blocked in the ssatg8Δ mutant, in nitrogen starvation. The ssatg8Δ mutant formed pseudohypha frequently and was hypersensitive to oxidative stress. However, mating or f ilamenation was unaffected in the ssatg8Δ mutant in vitro. Overall we demonstrate that autophagy is dispensable for S. scitamineum mating/f ilamentation, while critical for oxidative stress tolerance and p roper morphology in sporidial stage.
Keywords: Atg8, autophagy, fungus, Sporisorium scitamineum, morphogenesis, oxidative stress tolerance
1. lntroduction
The sugarcane smut fungus Sporisorium scitamineum experiences three distinct stages of its pathogenic life cycle, based on cell morphology and lifestyle. The haploid sporidium is yeast-like and non-pathogenic, of two mating types, MAT-1 and MAT-2. Sporidia of opposite mating type recognize and fuse with each other and initiate dikaryotic hypha growth, in the host plant canes. Eventually diploid teliospores form in planta by nuclear fusion, and followed by a round of meiosis shortly, giving rise to four haploid sporidia (Sundar et al. 2012). The molecular mechanism of S. scitamineum pathogenicity remains largely unknown although it has been extensively investigated in its close relative smut fungus, the model organism Ustilago maydis.
Autophagy is a highly conserved catabolic process in eukaryotes responsible for bulk degradation of cytosolic contents in vacuole (lysosome) (Ashford and Porter 1962). Autophagy is induced in response to environmental stress or physiological cues, and facilitates cellular remodeling during development and differentiation (Levine and Klionsky 2004; Mizushima 2018). Thus far 41 ATG (AuTophaGy) genes have been identif ied and characterized in yeasts and mammalian cells, therefore elucidating the molecular basis of autophagy (Klionsky et al. 2003; Klionsky 2007; Yao et al. 2015). ATG8 has been established as the most reliable marker for autophagy induction and autophagyassociated vesicular compartments (Ichimura et al. 2000; Kabeya et al. 2000). The biological function of autophagy has been investigated in several pathogenic fungi including Magnaporthe oryzae (Veneault-Fourrey et al. 2006; Deng et al. 2009; He et al. 2012), U. maydis (Nadal and Gold 2010), Cryptococcus neoformans (Hu et al. 2008), Fusarium graminearum (Nguyen et al. 2011; Josefsen et al. 2012), Aspergillus fumugatus (Richie and Askew 2008), Colletotrichum lindemuthianum (Dufresne et al. 1998), C. orbiculare (Asakura et al. 2009), Candida glabrata (Roetzer et al. 2010) and C. albicans (Palmer 2008; Yu Q et al. 2015). However, autophagy function has not been studied in S. scitamineum.
In this study, we identif ied a conserved ATG8 gene in S. scitamineum, named SsATG8. The ssatg8Δ mutants were generated in MAT-1 and MAT-2 background respectively, and assessed in sporidial morphogenesis, growth, mating/f ilamentiation, and resistance to stress conditions, especially host reactive oxygen species (ROS) response during in planta growth that is critical for this fungal pathogenicity. Our results showed that SsATG8 gene is required for autophagy in S. scitamineum, and for morphogenesis and oxidative stress tolerance, as well as for full pathogenicity likely due to survival of host ROS response.
2. Materials and methods
2.1. Growth conditions and fungal strains used in this study
Teliospores of sugarcane smut collected from the f ields in Guangdong Province of China (21°12´36´´N; 101°10´12´´E) by Yan et al. (2016) was maintained in Prof. Jiang Zide's lab. South China Agricultural University, and the WT17 (MAT-1 mating-type) or WT18 (MAT-2 mating-type) haploid sporidia isolated from such teliospores were used in this study. The culture medium used in this study include YePSA medium (yeast extract 1%, peptone 2%, sugar 2%, agar 2%), YePS liquid medium (yeast extraction 1%, peptone 2%, sugar 2%, p H 7.0), YePS soft medium (yeast extract 1%, peptone 2%, sugar 2%, agar 0.65%), YePSS medium (yeast extract 1%, peptone 2%, sugar 2%, D-sorbitol 18.17%, agar 2%), PDA (Beijing Dingguo, HB0233-12) medium (2% agar, p H 7.5), and MM-N medium (minimum medium lacking nitrogen resource, glucose 1%, KH2PO40.15%, MgSO40.05%, KCl 0.05%, 1 000× dilution of TES (Trace element solution: ZnSO42.2%, H3BO31.1%, MnCl·4H2O 0.5%, FeSO4·7H2O 0.5%, Co Cl2·6H2O 0.17%, Cu SO4·5H2O 0.16%, NaMoO4·2H2O 0.13%, Na2EDTA·2H2O 5%, p H 6.5)). For mating/f ilamentation assay, the equal volume of haploid sporidia of MAT-1 or MAT-2 were mixed and plated on the solid medium, and kept in dark at 28°C incubator for 2-3 d before photographing. For assessment of stress tolerance, wild-type (WT) or ssatg8Δ mutant sporidia of serial diluted concentration from 107to 103mL-1were inoculated on YePSA, PDA or MM-N medium, and allowed to grow in dark at 28°C incubator for 3 d before photographing. For growth assay, sporidia of S. scitamineum wild-type (MAT-1) and ssatg8Δ mutant were cultured in 50 mL of YePS liquid medium at 28°C, with shaking at 200 r min-1for 24 h. An aliquot of such cultured sporidia were then diluted to fresh YePS liquid medium, adjusting to cell density of 105cells mL-1, and cultured for another 28 h under the same condition. Measurement of OD600with spectrophotometer (Thermo, NanoDrop 2000C) was performed every 4 h to monitor the yeast-like (budding) growth of wild-type or mutant strain.
2.2. Nucleic acid-related manipulation
Fungal genomic DNA was extracted using a Fungal DNA Kit (Omega, America, D3390-04). PCR amplif ication was performed using PrimeSTAR®HS DNA Polymerase (TaKaRa, Japan, R010A). PCR product purif ication was performed using Cycle Pure Kit (Omega, D2500-02) or Gel Extraction Kit (Omega, D6492-02). In Southern blot assay, NEB restriction enzymes PstI (ER0611) and EcoR I (ER0271) were used for digestion of genomic DNA. DIG-High Prime DNA Labeling and Detection Starter Kit I (Roche, America, Catalog number: 11745832910) was used for probe labeling. Amersham Hybond TM-N+(GE Healthcare, America, RFN303B) membrane was used for blotting. NBT/BCIP Stock Solution (Roche, 11681451001) was used for probed band detection. For total RNA extraction, Fungal RNA Kit (Omega, R6840-01) was used. PrimeScriptTMRT Master Mix (TaKaRa, RR036A) was used for c DNA systhesis. SYBR®Premix Ex TaqTMII (TaKaRa, RR820A) for real-time qPCR, and the reaction was run on qTOWER3G (Analytikjena, Germany).
2.3. Generation of deletion constructs
Construction of two fragments for the replacement of SsATG8 gene by the HPT (recombinant hygromycin, Hygr) gene follows the strategy described (Chung et al. 2002; Yang and Chung 2012). The f lanking DNA (1-kb 5´ and 3´) of the SsATG8 gene was PCR amplif ied using WT S. scitamineum genomic DNA as template, and the HPT gene in plasmid p EX2 (Yan et al. 2016) as template. The primers were as listed in Table 1. The 5´- or 3´-f lanking sequences respectively fused with two half partialoverlapping HPT sequences, were generated by fusion PCR and verif ied by sequencing.
2.4. PEG-mediated protoplast transformation
Polyethylene glycol (PEG)-mediated protoplast transformation follows the established protocol (Yu et al. 2015b) with modif ication as follows. Enzyme digestion of the wild-type MAT-1 or MAT-2 sporidia was performed using the lysing enzyme (Sigma-Aldrich, Germany, L1412) dissolved in SCS solution (20 mmol L-1trisodium citrate and 1 mol L-1D-sorbitol, p H 5.8) to reach the f inal concentration of 4 mg mL-1, incubated at 28°C for 30 min. 40% PEG (Sigma-Aldrich, 202444) solution was prepared with STC solution (10 mmol L-1Tris-HCl, pH 7.5; 1 mol L-1D-sorbitol and 100 mmol L-1CaCl2) as solvent. A total of 1-5 µg PCR amplif ied fragments and 1 µL heparin solution (15 mg mL-1; Beijing Dingguo, China, DH157) were mixed with the protoplasts and incubated with 40% PEG solution on ice for 10 min. For regeneration, the protoplasts were streaked on the 3-layer regeneration medium: one layer of YePS soft medium on top of two lays of YePSS medium, with only the bottom YePSS layer containing 400 µg mL-1hygromycin B (Calbiochem, CAS: 53-84-9), and incubated at 28°C until transformants appeared.
2.5. Microscopy
Microscopy and imaging was performed using an Axio Observer Z1 Microscope (Zeiss, Jena, Germany) equipped with an sCMOS Camera (PCO Edge, Kelheim, Germany).
2.6. Transmission electron microscopy (TEM)
Wild-type or the ssatg8Δ sporidia were starved for 6 h in MM-N liquid medium containing 2 mmol L-1PMSF (phenylmethanesulfonyl f luoride, Sigma-Aldrich, USA, 10837091001), and processed following the established protocol (Deng et al. 2009) for TEM observation and imaging. TEM was performed on a FEI Tecnai 12 Spirit TEM (Thermo-Fisher, Holland).
2.7. Pathogenicity assay
The susceptible sugarcane variety ROC22 was inoculated with mixed fungal sporidia from WT or mutant combination (of opposite mating-type) through injection at 4-5-leaves seedling stage, and the symptoms were photographed at 90-180 d post inoculation (dpi).
2.8. ROS staining
The susceptible sugarcane variety ROC22 seedlings were inoculated with mixed fungal sporidia from WT or mutant combination (of opposite mating-type) through injection at 4-5-leaves stage. At 40-48 h post inoculation (hpi) the inoculated sites were cut from the seedling and immersed in 1 mg mL-1DAB (3,3´-diaminobenzidine, p H 3.8; Sigma, D8001) solution for 8 h, followed by de-stain with ethanol:acetic acid solution (94:4, v/v) and section for microscopic observation and imaging.

Table 1 Primers used in this study
3. Results
3.1. ldentif ication of a conserved autophagy gene Ss ATG8
We identif ied the S. scitamineum ATG8 by using the S. cerevisiae Atg8 protein sequence (accession number: KZV12988.1) to search against the S. scitamineum genome available on NCBI website (taxid: 49012), using the tBlastn homology search algorithm (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=tblastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome). A single highly related (2e-57) S. scitamineum gene (SPSC_01070), here named SsATG8 encoding a 118-aa protein, was identif ied. Alignment of the SsAtg8 protein with other fungal, plant and human (LC3) Atg8 proteins showed a high degree of amino acid conservation, including the conserved C-terminal glycine residue (Fig. 1-A), subject to post-translational modif ication, which is essential for protein function (Kabeya et al. 2000). Phylogenetic analysis showed that SsAtg8 is highly conserved, especially within smut fungi (Fig. 1-B).

Fig. 1 SsATG8 encodes a conserved autophagy-related ubiquitin-like protein. A, amino acid sequences arrangement of SsAtg8 protein (accession number: CDU22440.1) and its orthologs in Sporisorium reilianum SRZ2 (Sr, CBQ69980.1), Ustilago maydis (Um, XP_011391873.1), Ustilago hordei (Uh, CCF50371.1), Saccharomyces cerevisiae (Sc, KZV12988.1), Magnaporthe oryzae (Mo, ACJ06588.1), Arabidopsis thaliana (At, AAM64870.1), and Rattus norvegicus (Rn, AAQ94605.1). Amino acid sequences alignment was conducted using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) and BoxShade (https://embnet.vital-it.ch/software/BOX_form.html). The boxed, triangle-labeled residue is the conserved C-terminal glycine essential for post-translational modif ication and Atg8 protein function in autophagy induction. B, phylogenetic analysis conducted in MEGA7 (Kumar et al. 2016), with the aforementioned 8 sequences. The evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987). The optimal tree with the sum of branch length=1.59263886 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1 000 replicates) are shown next to the branches (Felsenstein 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the JTT matrix-based method (Jones et al. 1992) and are in the units of the number of amino acid substitutions per site. All positions with less than 50% site coverage were eliminated.
The ssatg8Δ mutants were generated in wild-type MAT-1 and MAT-2 background respectively, using a modif ied PEGmediated protoplast transformation methodology (Yu J et al. 2015) by complete replacement of the SsATG8 open reading frame (ORF) with HPT-resistant selection marker, derived from two PCR amplif ied truncated but partially overlapped HPT fragments f lanked by 5´- and 3´-untranslated region (UTR) of the SsATG8 ORF (Appendix A-a). Transformants 1716 and 1825 were conf irmed as ssatg8Δ mutants in two mating-type background respectively, by PCR or Southern blot (Appendix A-b-c) and qPCR (Appendix A-d), and were used in the following assessment.
First we characterized for autophagy activity. Autophagy is normally induced by nitrogen depletion (Tsukada and Ohsumi 1993). Consistent with their fungal orthologs, SsATG8 is required for autophagy in response to nitrogen starvation, as visualized by transmission electron microscopy (TEM) analysis (Fig. 2). Numerous vesicles were apparent in the wild-type vacuole lumen under nitrogen starvation, but not seen in starved ssatg8Δ sporidia (Fig. 2). Therefore, we concluded that we identif ied and verif ied a conserved autophagy gene SsATG8 in S. scitamineum, essential for autophagy induction in response to nitrogen starvation.
3.2. Function of Ss ATG8 in S. scitamineum sporidial growth, morphogenesis, and mating/f ilamentation
Next, we characterized the ssatg8Δ mutants for various aspects of yeast-like sporidial growth, mating/f ilamentation and pathogenesis. When grown on solid medium, the ssatg8Δ (1716 and 1825) sporidial colony appeared wrinkle, less smooth compared to the wild-type (MAT-1 and MAT-2), especially under extended culture (>1 wk; Fig. 3-A). Under microscope, we frequently noticed elongated pseudohyphae in the ssatg8Δ sporidial culture, which was rarely seen in the WT (Fig. 3-B). We propose that it may be resulted from failure of f ission of the newly formed sporidia budding from the mother cell. When cultured in liquid YePS medium, the ssatg8Δ sporidia grew more slowly than the WT in the initial 16 h, and reached the comparable level with WT at the stationary phase (16 h onwards; Fig. 3-C). Therefore we propose that SsAtg8 (autophagy) may be required for proper sporidial growth in S. scitamineum.
We also examined the mating/f ilamentation of the ssatg8Δ mutant, by mixing its sporidia with the WT sporidia of opposite mating type. Furthermore, we mixed ssatg8Δ sporidia of two mating types and assessed their mating/f ilamentation. We observed that mating between ssatg8Δ and the opposite wild-type mating-type strain was slightly reduced than that between the two wild-type strains (Fig. 3-D). Mating between the two ssatg8Δ mutants was further reduced (Fig. 3-D). Under microscope we observed long and smooth dikaryotic hyphae formed by mating of the two wild-type sporidia, as well as of ssatg8Δ mutants (Fig. 3-E). In the ssatg8Δ mutants, we also saw pseudohypha formed by a chain of sporidia with failure in f ission (Fig. 3-E, arrows).
Overall, we found that SsAtg8 (autophagy) plays an important role in sporidial growth and morphogenesis, while may be dispensable for mating/f ilamentation in S. scitamineum.
3.3. Ss ATG8 is involved in oxidative stress tolerance and cell wall integrity
Next we tested the hypersensitivity towards osmotic stress, oxidative stress, and cell wall integrity stress, in ssatg8Δ mutants compared to the WT. The ssatg8Δ mutants displayed comparable tolerance as WT towards high osmotic pressure caused by NaCl, and the cell wall stress caused by SDS (Fig. 4). In contrast, ssatg8Δ mutants 1716 and 1825 were hypersensitive to 1 mmol L-1H2O2, and the cell wall stress caused by Congo red, compared to the WT (Fig. 4, boxed region). Addition of c AMP was unable to enhance resistance towards H2O2, in either WT or mutants (Fig. 5-A). This result indicates that autophagy-mediated tolerance to oxidative stress is independent on c AMP signaling pathway.

Fig. 2 SsATG8 is essential for autophagy in Sporisorium scitamineum. The images show the transmission electron microscopy (TEM) analysis of starved sporidia. Wild-type and ssatg8Δ sporidia were grown in YePS medium for 16 h and subject to nitrogen starvation for 8 h, and processed for thin-section transmission electron microscopy. Boxed regioned in the wild-type (WT) image was enlarged to show numerous structures (arrows) inside the vacuolar lumen, indicating autophagy induction. Vacuoles in the ssatg8Δ sporidia were empty (arrowheads). Scale bar for image was labeled individually.
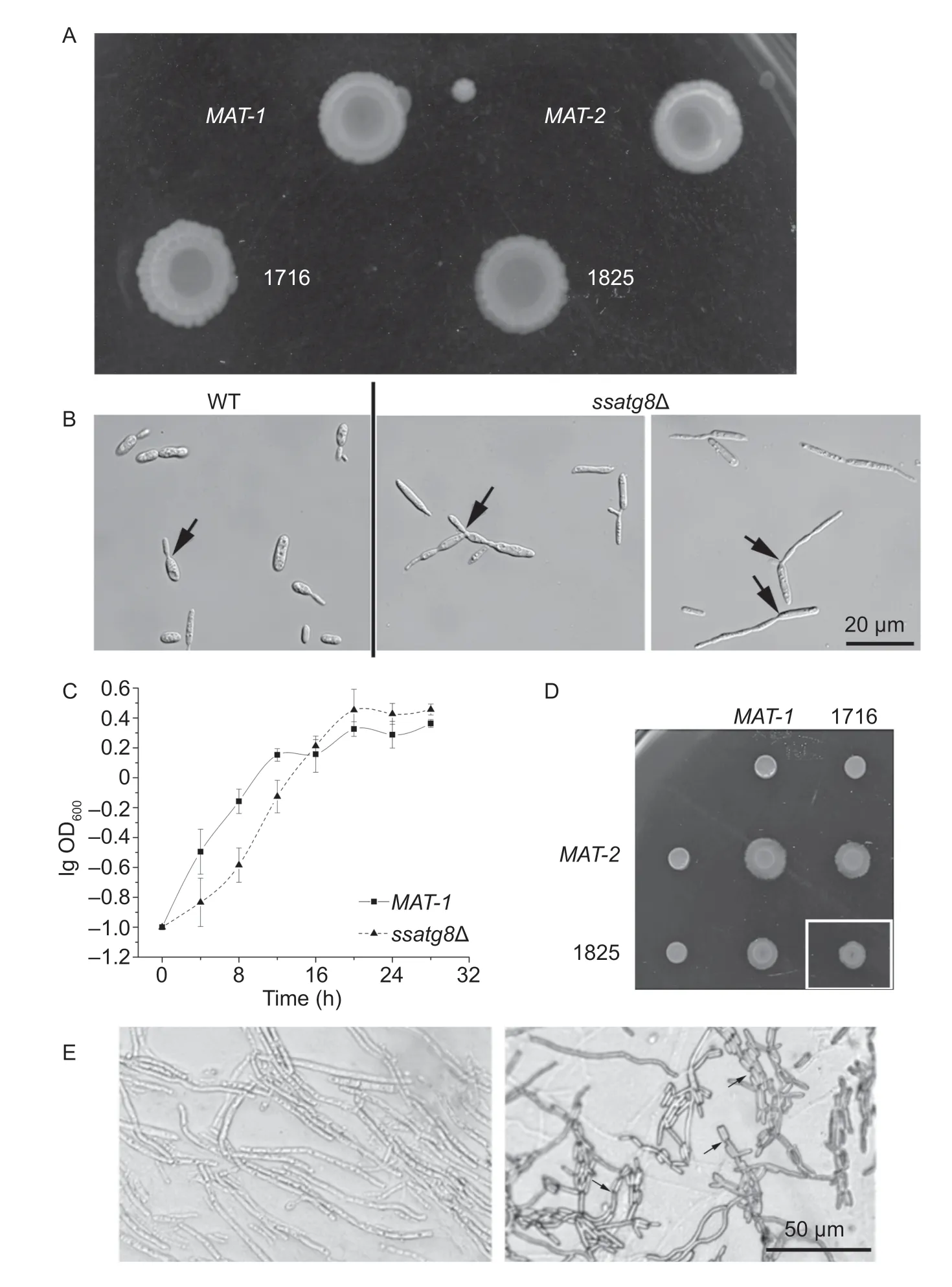
Fig. 3 Functional analysis of SsATG8 in Sporisorium scitamineum morphogenesis, growth and mating/f ilamentation. A, colony morphology of two wild-type strain (MAT-1 and MAT-2) and ssatg8Δ mutants in these two mating-type backgrounds (1716 and 1825). Sporidia of each strain was spotted onto the YePSA medium and allowed to grow for 7 d, before photographed. B, microscopic observation and imaging of wild-type and ssatg8Δ sporidia. Arrows depict the new sporidia connecting to mother cell. Scale bar= 20 µm. C, a 24-h culture of S. scitamineum sporidia was diluted into YePS liquid medium at cells per mL. The number of cells at each specif ic time point was acquired by measuring the OD absorption at 600 nm. The growth curve for wild-type (MAT-1; square) or ssatg8Δ (triangle) was prepared by plotting the logarithmic values of OD600 vs. incubation time. Mean±SE was derived from three independent biological repeats. D, mating/f ilamentation of wild-type (MAT-1 and MAT-2) and ssatg8Δ mutants (1716 and 1825) was assessed on PDA solid medium. Photographs were taken 3 d post inoculation. Boxed region denotes mating between two ssatg8Δ mutants, resulting in further reduced f ilamentation. E, microscopic observation and imaging of dikaryotic hypha formed after sexual mating, in wild-type and ssatg8Δ mating cultures. Arrows depict the pseudohypha formed by connecting sporidia, with failure in f ission. Scale bar=50 µm.
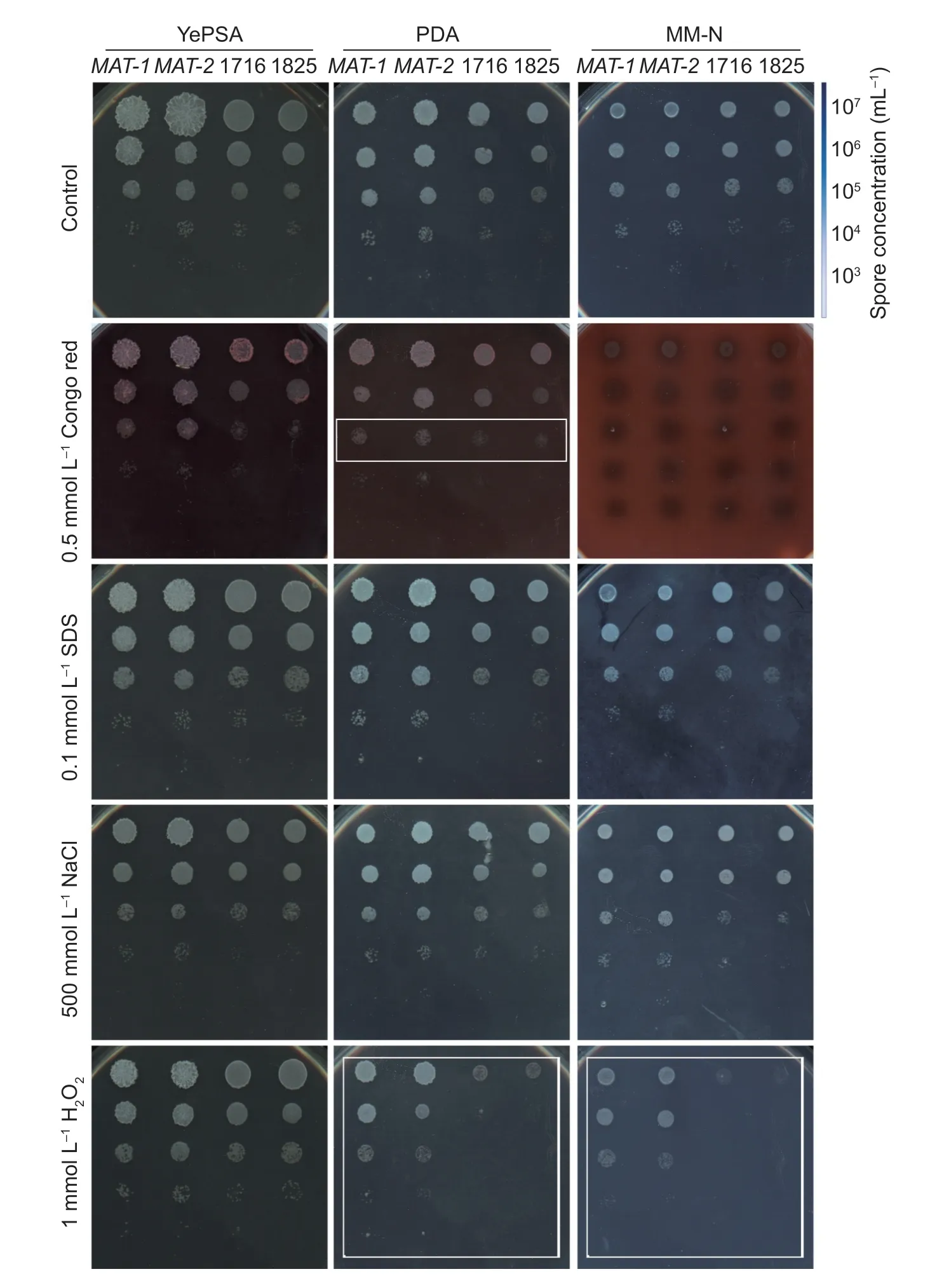
Fig. 4 SsATG8 is involved in stress tolerance. Serially diluted cells of wild-type MAT-1, MAT-2, or ssatg8Δ mutants 1716 and 1825 were spotted onto YePSA, PDA or MM-N medium supplemented with Congo red (0.5 mmol L-1), SDS (0.1 mmol L-1), NaCl (500 mmol L-1), or H2O2 (1 mmol L-1). Images were taken at 72 h post inoculation.
We noticed that ssatg8Δ strains were hypersensitive to oxidative stress when grown on PDA or MM-N medium, while they were comparable to the wild-type strains when grown on YePSA medium (Fig. 4). We inferred that such hypersentivity towards oxidative stress may correlate to different carbon sources used in PDA and MM-N medium (glucose) and that in YePSA (sucrose). To test this hypothesis, we assessed oxidative stress tolerance on carbonhydrate-swapping YePSA and MM-N media. On either YePSA (sucrose as carbon source) or YePDA (D-glucose as carbon source), the hypersensitivity towards oxidative stress in the ssatg8Δ mutants (1716 and 1825) were comparable to that of WT strains (MAT-1 and MAT-2, Fig. 5-B). Similarly, swapping of carbon source did not change sensitivity of the ssatg8Δ sporidia towards H2O2when cultured on MM-N medium (Fig. 5-B). This result indicates that autophagy-mediated tolerance to oxidative stress was glucose-independent.
Overall, we found that in S. scitamineum, SsAtg8 plays an important role in resistance to ROS, as well as cell wall integrity stress, both of which may be encountered by the pathogen during invasive growth within the host.

Fig. 5 SsAtg8 is required for tolerance to oxidative stress, independent on c AMP/PKA signaling or carbon source. A, sporidia from wild-type (MAT-1 and MAT-2) and ssatg8Δ mutants (1716 and 1825), of serial dilution, were spotted on solid PDA medium, with or without supplement of 1 mmol L-1 H2O2 and/or 10 mmol L-1 c AMP. Images were taken 72 h post inoculation. B, serially diluted cells of wild-type (MAT-1 and MAT-2) and ssatg8Δ mutants (1716 and 1825) were spotted onto the YePDA (D-glucose as sole carbon source) or MM-N-Sucrose (sucrose as sole carbon source) medium, without (control) or supplemented with 1 mmol L-1 H2O2. Images were taken 72 h post inoculation.
3.4. Ss Atg8 is required for full pathogenicity of S. scitamineum
Next, we performed pathogenicity assay with the wild-type strain and the ssatg8Δ mutant respectively to assess the function of SsAtg8 in S. scitamineum infection. The highly susceptible sugarcane variety ROC22 was inoculated by injection with the mixed fungal cells of different combinations (of opposite mating types) as follows: WT/ssatg8Δ, ssatg8Δ/ssatg8Δ, and with wild-type strains WT/WT as positive control. We found that mixed WT/ssatg8Δ sporidia could cause symptom of black “whip” formation but to a reduced extend compared to the WT/WT inoculation, while inoculation of the ssatg8Δ/ssatg8Δ sporidia of two opposite mating-types produced no symptoms at all (Fig. 6). This result indicates that SsAtg8 (autophagy) plays a critical role in full pathogenicity of S. scitamineum.
To further investigate the possible function of SsAtg8 (autophagy) in S. scitamineum pathogenicity, we performed a staining DAB (3,3´-diaminobenzidine) for evaluating ROS response in S. scitamineum-sugarcane pathosystem, at 40-48 hpi. At this early stage of S. scitamineum infection, we could not detect obvious ROS burst in the sugarcane tissues infected by WT/WT dikaryotic hyphae or by WT/ssatg8Δ (Fig. 7). In contrast, ssatg8Δ/ssatg8Δ inoculation resulted in elevated level of ROS in the infected host cells as stained by DAB (Fig. 7).
Overall we propose that autophagy is essential for full pathogenicity of S. scitamineum, likely by serving a function in survival under the host ROS response triggered by fungal infection.
4. Discussion

Fig. 6 SsAtg8 required for full pathogenicity of Sporisorium scitamineum. The susceptible sugarcane variety ROC22 was inoculated with mixed fungal sporidia from WT or mutant combination (of opposite mating-type) through injection at 4-5-leaves seedling stage, and the symptoms were assessed and documented at ~180 d post inoculation (dpi). Black dash-line boxed region was enlarged for a better view of black “whip” formation. Infection assay was performed with at least 10 plants/inoculation. Quantif ication of infection was illustrated as at the bottom of the image.

Fig. 7 SsAtg8 may be involved in coping with host reactive oxygen species (ROS) response during infection. Microscopic sections of sugarcane cells infected by WT/WT, WT/ssatg8Δ, or ssatg8Δ/ssatg8Δ mixed sporidia, and stained by 1 mg mL-1 DAB for ROS. Injection of YEPS medium serves as the blank control. Arrowheads denote the sugarcane cells with no obvious ROS burst, in WT/WT or WT/ssatg8Δ inoculation; arrows for the host cells with elevated ROS stained by DAB, in infected plant cells by ssatg8Δ/ssatg8Δ. WT, wild-type. Scale bar=100 µm.
Autophagy is viewed as a cellular protection response to stress conditions including starvation, oxidative stress, and/or to physiological clues to differentiation in many organisms (Tsukada and Ohsumi 1993; Levine and Klionsky 2004; Navarro-Yepes et al. 2014). As a marker and an essential regulator in autophagosome formation, Atg8 protein is required for autophagy (including non-selective and selective) in yeasts and numerous pathogenic fungi, and is indispensible for their pathogenicity. Biological function of autophagy in pathogenic fungi includes promoting spores/conidia formation and/or invasive growth within host tissues, likely involving nutrient catabolism and oxidative stress tolerance (Dufresne et al. 1998; Veneault-Fourrey et al. 2006; Hu et al. 2008; Palmer 2008; Richie and Askew 2008; Asakura et al. 2009; Deng et al. 2009; Nadal and Gold 2010; Roetzer et al. 2010; Nguyen et al. 2011; He et al. 2012; Josefsen et al. 2012; Yu Q et al. 2015). In this work, we have explored the process of autophagy in the plant pathogenic fungus S. scitamineum using a reverse genetic approach. Our results show that a tight connection exists among autophagy and several aspects of S. scitamineum biology.
We have demonstrated here that nitrogen depletion is able to trigger autophagy in haploid S. scitamineum sporidia, as shown by the accumulation of autophagic bodies within the vacuoles of wild-type, nitrogen-starved cells. The absence of autophagic bodies from vacuoles of nitrogen-starved ssatg8Δ mutant cells strongly supports the hypothesis that autophagy activity is lost. We noticed that with loss of autophagy, the mutant sporidia altered the budding process, as a higher frequency of lateral buds seen and f ission (detachment) between mother and daughter cells failed, which is similar as reported in U. maydis autophagydef icient mutants (Nadal and Gold 2010). Such motherdaughter cell detachment defect resulted in pseudohypha formation in the S. scitamineum sporidial colony under extended culture as well as in mating condition. Although autophagy is not essential for S. scitamineum mating/f ilamentation, it does play an important role in tolerance to oxidative stress.
It has been shown in several f ilamentous fungi including M. oryzae and U. maydis, that autophagy serves glucose production function (via vacuolar hydrolysis of glycogen) critical for spores (conidia or teliospores) formation (Deng et al. 2009; Nadal and Gold 2010). However, it seems that glucose production is not necessary for f ilamentation, in U. maydis (Nadal and Gold 2010). In contrast, high concentration of glucose suppresses f ilamentation after sexual mating (Yan et al. 2016). This may at least partially explain why autophagy is dispensable for S. scitamineum mating/f ilamentation. SsAtg8 (autophagy) also seems to be essential for full pathogenicity in S. scitamineum, similar as that reported in U. maydis (Nadal and Gold 2010). Given that nutrient availability is generally limited, and ROS is triggered upon perception of pathogen infection (Apostol et al. 1989; Scheel 1998), in the host plant, the ability of the fungus to induce autophagy might be crucial for its overall pathogenic development. Staining of host ROS response at early stage of infection supported our hypothesis, as infection of mixed wild-type sporidia of two opposite mating-types did not produce detectable ROS at the site of inoculation, while in contrast, autophagy-def icient mutants triggered obviously elevated level of host ROS.
5. Conclusion
We identif ied an ortholog of ATG8 gene in the sugarcane smut fungus S. scitamineum and studied its function by characterizing the ATG8-deletion mutant in important aspects. Our results displayed that SsATG8 gene is essential for proper sporidial growth and morphogenesis, while may be dispensible for sexual mating/f ilamentation. SsATG8 is important for S. scitamineum pathogenicity as it may be involved in coping with host ROS response at early stage of infection. Overall, our study for the f irst time revealed that the autophagy gene ATG8 affects morphogenesis and oxidative stress tolerance in the sugarcane smut fungus S. scitamineum.
Acknowledgements
We thank Dr. Huang Jilei (Instumental Analysis and Research Center, South China Agricultural University) for assisting in TEM analysis and imaging. This research was supported by the National 973 Program of China (2015CB150600) and the Natural Science Foundation of Guangdong Province, China (2017A030310144).
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
 Journal of Integrative Agriculture2019年5期
Journal of Integrative Agriculture2019年5期
- Journal of Integrative Agriculture的其它文章
- Characterization of TaCOMT genes associated with stem lignin content in common wheat and development of a gene-specif ic marker
- Phenotypic characterization and genetic mapping of the dwarf mutant m34 in maize
- Morphological diversity and correlation analysis of phenotypes and quality traits of proso millet (Panicum miliaceum L.) core collections
- Field identif ication of morphological and physiological traits in two special mutants with strong tolerance and high sensitivity to drought stress in upland rice (Oryza sativa L.)
- Crosstalk of cold and gibberellin effects on bolting and f lowering in f lowering Chinese cabbage
- Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.)
