Antioxidant compounds and capacities of Gac (Momordica cochinchinensis Spreng) fruits
Ali Abdulqader, Faisal Ali,2, Amin Ismail,3✉, Norhaizan Mohd Esa,4
1Department of Nutrition and Dietetics, Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
2Biochemistry & Molecular Biology Department, University Hospital, Faculty of Medicine and Health Sciences, Sana’a University, Yemen
3SReredseaanrgc,h S Celeanntegro ro,f M Eaxlcaeyllseiant, Non-Communicable Diseases (NNCD) Faculty of Medicine and Health Sciences, Universiti Putra Malaysia, 43400
4Laboratory of Molecular Biomedicine, Institute of Bioscience, Universiti Putra Malaysia, 43400, Serdang, Malaysia
Keywords:Momordica cochinchinensis Phytochemicals Carotenoids Antioxidants Polyphenols High-performance liquid chromatography
ABSTRACT Objective: To identify and determine the composition of antioxidant compounds, and to evaluate the antioxidant abilities of Gac fruit parts (peel, pulp, seed and aril) grown in Malaysia.Methods: LC-MS/MS was used for identification of antioxidant compounds and UV-Vis for estimation of the contents of phenolics, flavonoids, and carotenoids. Lycopene and β-carotene were quantified using high-performance liquid chromatography. DPPH (2, 2-diphenyl-1-picrylhydrazyl) and ferric reducing antioxidant power assays were employed to evaluate antioxidant capacities.Results: Phytochemicals were found amongst all the fruit parts. Notably, significant amounts of carotenoids [(107.4 ± 4.5), (85.7 ± 4.4), (110.6 ± 2.1) mg/100 g dry weight (DW)], and relatively high levels of both phenolics [(27.3 ± 1.7), (28.9 ± 2.4), (30.8 ± 2.7) mg/100 g DW]and flavonoids [(38.1 ± 2.2), (8.8 ± 1.3), (24.5 ± 3.3) mg/100 g DW] were found in the fruit’s peel, pulp and aril, respectively. Seed part also showed a relatively high level of flavonoids [(18.1± 2.3) mg/100 g DW]. Lycopene and β-carotene were found to be significantly high (P < 0.05)in aril [(579.3 ± 22.7) and (621.0 ± 35.0) µg/g DW], followed by peel [(51.0 ± 7.5) and (210.0± 12.5) µg/g DW] and pulp [(37.6 ± 10.9) and (205.6 ± 22.1) µg/g DW)]. Antioxidant assays revealed that aril possessed the highest scavenging activity (IC50 = 865 µg/mL), while the peel possessed the highest ferric reducing power of 140 µmol FeSO4/µg.Conclusions: The current results demonstrate that Gac fruit grown in Malaysia is a rich source of phytochemicals, especially carotenoids, and possesses antioxidant activities. Thus, such findings suggest Gac fruit as a source of an antioxidant plant.
1. Introduction
Phytochemicals have attracted significant attention because of their pharmacological and therapeutic properties against different chronic diseases[1]. Tropical fruits have been reported to contain health-promoting components for human benefits. Mostly,these health benefits are attributed to the nutritional value and bioactive compounds in these resources[2,3]. Given the warm tropical environment in Southeast Asia, this provides the ideal conditions for the growth and abundance of fruits and vegetables,including Gac fruit which is reputed to be an exceptional source of phytochemicals[4,5]. Accordingly, Gac (Momordica cochinchinensis Spreng) is a seasonal tropical fruit that is widely grown throughout Southeast Asian countries such as Vietnam, Thailand, Laos, and Malaysia. Traditionally, the fruit has been used for the treatment of certain diseases such as diabetes and eye disorders. In Vietnam, the rice is commonly mixed with aril part of the fruit as a traditional meal called “xoi gac”, giving the rice a red, shiny colour[6,7]. Furthermore,some studies have suggested that Gac fruit is an extraordinary source of bioactive compounds such as carotenoids, polyphenols and essential fatty acids[8-10]. Also, Gac fruit extracts exhibited anticancer,anti-inflammatory and antioxidant activities[11-14]. However, the active compounds underlying Gac fruit health benefits potential have not been well studied. Notably, the contents of bioactive compound composition might be affected or varied due to agronomic factors,climatic and environmental conditions[15]. But, limited studies, if any, have investigated the phytochemical composition in Gac fruit grown in Malaysia. Furthermore, the majority of previous studies have mainly focused on the aril part of the fruit. Wasteful disposal of the other parts of the fruit (i.e. seed, peel, and pulp) contributes to environmental disposal problems. Therefore, this study aims to identify and quantify the phytochemical composition and evaluate the antioxidant activities of different parts (peel, pulp, seed, and aril)of Gac fruit grown in Malaysia.
2. Materials and methods
2.1. Chemicals and reagents
The 2, 2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma-Aldrich,Steinheim, Germany), 2,4,6-tripyridyl-s-triazine (TPTZ) (Sigma-Aldrich, Buchs, Switzerland), Folin–Ciocalteu’s reagent,butylated hydroxytoluene, ascorbic acid, gallic acid, quercetin,β-carotene, and lycopene standards were purchased from Sigma-Aldrich (St. Louis, USA). Fisher Scientific supplied the acetonitrile, dichloromethane, and methanol for the HPLC analysis (Loughborough, UK). All other chemicals used in the extraction, including the chemicals used in phenolic and flavonoids determinations were of analytical grade, otherwise stated.
2.2. Fruits sampling and preparation
Five fruits were obtained from the International Tropical Fruits Network, Selangor, Malaysia. The fruits were selected randomly at ripe stage during the harvesting season in September of 2017 and kept at -80 ℃ until they were ready to use. The fruits were then taken and thawed at room temperature prior to sample preparation.Then, the fruits were separated carefully to avoid mix between materials into four parts: peel, pulp, seed, and aril, before cutting into small pieces. Each part was then lyophilised using a freeze dryer at -45 ℃ for 3 d (BT2K, VirTis, Warminster, USA) and was ground using a blinder in order to obtain powder form and kept at -40 ℃ for later experiments.
2.3. Determination of nutritional composition
Considering that only the aril part of the fruit is processed as food additives and cooked with rice while other fruit parts are not eatable, only the aril part was chosen for determination of nutritional composition. To determine the ash, moisture, fat, protein, and carbohydrate contents of fresh Gac fruit aril, proximate analysis was employed in accordance with the official analysis methods adopted from the Association of Official Analytical Chemists[16].All determinations were performed in triplicate of three independent experiments.
2.4. Extraction for determination of total phenolics and flavonoids
One gram of dried fruit sample (peel, pulp, seed, and aril) was weighed and shielded from light. Then, each sample was mixed with 20 mL of 70% ethanol and vigorously shaken using an orbital shaker (SHO-2D, Daihan Scientific, Seoul, Korea) at 180 rpm for 2 h. Lastly, the mixture was then filtrated before analysis for total phenolics and flavonoid contents[8].
2.5. Estimation of total phenolics
Five hundred microliters of each fruit sample were mixed with 2.5 mL of Folin-Ciocalteu solution (10 times diluted with distilled water) before stood at room temperature for 5 min. Then, 2 mL of sodium carbonate (7.5 % v/w) solution was added and mixed well.After 90 min, the absorbance was measured at 765 nm using a UV/Vis spectrophotometer (UV 1800, Shimadzu, Kyoto, Japan). The total phenolics content was then calculated based on the standard calibration curve of the different concentrations of gallic acid (50-250 µg/mL). The result was given as the gallic acid equivalent per 100 g of dry weight (mg GAE/100 g DW) of the mean value of the three measurements[17].
2.6. Estimation of total flavonoids
Briefly, 500 µL of each sample was mixed with 2.25 mL of distilled water and 150 µL of 5% NaNO2solution for 5-7 min. Then, 300 µL of 10% AlCl3·6H2O solution was added and allowed to react for 5 min. Finally, 1 mL of 1 mol/L NaOH was then added and well mixed using a vortex mixer. The absorbance was read at 510 nm using a UV/Vis spectrophotometer. Various concentrations of quercetin in ethanol were used to draw the standard curve (100-500 µg/mL). The result was explained based on the standard curve of quercetin as mg quercetin equivalents to 100 g dry weight (mg QE/100 g DW) of the mean value of three measurements[8].
2.7. Extraction and estimation of total carotenoids
One gram of dried fruit sample (peel, pulp, seed and aril) was mixed with 20 mL of hexane, acetone and 70% ethanol at the ratio of 2:1:1 using a conical flask[8]. The mixture was then covered by foil and magnetically stirred at 40 ℃, and 300 rpm for 1 h (MR, Hei-tec,Schwabach, Germany). All samples were then filtered and allocated in another conical flask. To stimulate liquid separation for polar and non-polar compounds, 5 mL of distilled water was next added and allowed to separate for 10 min. Finally, the upper layer containing the carotenoids was allocated and measured for total carotenoids content. The total carotenoids were determined based on the standard curve of β-carotene, the absorbance at 450 nm for the fruit samples and standard concentrations was measured using a UV/Vis. The total carotenoids content of each extract was expressed as mg β-carotene equivalents to 100 g dry weight[18].
2.8. Extraction and quantification of individual carotenoids (β-carotene and lycopene)
In this procedure, 1 g of dried fruit powder was weighed and mixed with 100 mL of hexane, acetone, and 70% ethanol at the ratio 2:1:1 respectively and shielded from light. Following 1 h of magnetic stirring, all samples were then filtered and deposited in a conical flask. Next, 10 mL of distilled water was added in order to trigger the separation of polar and non-polar liquid compounds for 10 min. Following separation, the upper layer containing the carotenoids was allocated and evaporated using a rotary evaporator until completely dry (R-210, Buchi, Flawil, Switzerland). The final dryness of the carotenoids pigments was reconstituted using 10 mL of dichloromethane and filtered using 0.22 µm syringe membrane before depositing in a number of small vials for high-performance liquid chromatography (HPLC) analysis.
The compositions of β-carotene and lycopene in the fruit parts were measured employing HPLC (Agilent 1100, Palo Alto, USA).The HPLC system comprises a quaternary pump, auto-injector,degasser, and diode-array detection (DAD). For the separation of the individual carotenoids (β-carotene and lycopene), a reversedphase C18 column (Alltech, Licosphere, United States) (250 mm× 4 mm, 5 µm I.D) was used. The analysis was performed with the mobile phase system, consisting of three solvents (acetonitrile,dichloromethane, and methanol) at the ratio of (5:4:1 v/v/v). The injection volume was 20 µL, the flow rate was 0.9 mL/min, the column temperature 30 ℃, and the time was set to 30 min. Also,the UV spectra of eluted compounds were recorded at 450 nm.Accordingly, the β-carotene and lycopene were assessed based on the retention times and standard curves of standards, and all the results given as µg/g of dry weight[19].
2.9. Identification of chemical compounds using LC-MS/MS
All samples (peel, pulp, seed, and aril) were approximately weighed (0.1 g) in a microcentrifuge tube. Next, 2 mL of methanol was added, and the contents vigorously mixed before centrifugation at 10 000 rpm for 3 min. The mixture for each sample was then filtered through a 0.45 µm nylon membrane filter before injection into LC-MS/MS. The identification was carried out using a Perkin Elmer Flexar FX15 ultra HPLC system. The system incorporated a degasser, binary pump, autosampler, and column heater, connected to a Sciex 3200 hybrid trap triple quad tandem mass spectrometer(UHPLC-MSMS) equipped with an ESI ion source (Toronto,Canada). For chromatographic separation of possible bioactive compounds, a reverse phase C18 column (Phenomenex Synergy,Torrance, USA) (100A, 100 mm × 3 µM × 2.0 mm) was used. The analysis was undertaken with mobile phases consisting of solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid). The gradient elution was then performed;5%-95% B: 0.01-10.0 min, holding for 2 min and then reverting back to 10% B in 0.1 min and re-equilibration for 3 min. The overall flow rate was 250 µL/min, the injection volume was 20 µL, and the total time of injection was 15 min. The single ion monitoring (SIM) modality was performed to quantify the molecular ions of the polyphenol compounds.Importantly, the SIM analysis, in this experiment, was set to scan all ions greater than 100 m/z and all ions smaller than 1 200 m/z. The turbo interface was configured to operate in a negative mode and the drying temperature set at 500 ℃. Accordingly, phenolic acids and flavonoids were identified using a combination of liquid chromatography with mass spectrometry (ESI-LC-MS/MS) based on their ultraviolet (UV)spectra, retention time, mass spectra and through comparison with the gathered standard library information that includes 500 established compounds.
2.10. Extraction for antioxidant assays
In this procedure, 20 g of weighed each dried samples were extracted with 70% ethanol at the ratio of 1:20 (w/v). The samples remained on the orbital shaker for 1 h and then were filtered. The resulting filtrate was evaporated using a rotary evaporator. The extract after evaporation (sticky and dark colour liquid extract) was then dried using a freeze dryer. Finally, the dried extract was used to prepare various concentrations of each sample in ethanol for antioxidant evaluation.
2.11. DPPH free radical scavenging assay
The DPPH working solution was prepared with a concentration of 0.1 mM in 80% methanol[20]. Then, 1 mL of various concentrations of Gac fruit parts (150, 300, 600, 1 200 µg/mL) respectively, were mixed with an equal volume of DPPH, and 250 µg/mL of vitamin C was used as a positive control. Following 30 min of incubation at room temperature in dark condition, the absorbance was measured using a UV/Vis at 517 nm. The antioxidant percentage activity was calculated using the following formula:
% DPPH radical scavenging capacity = [(A1- A2) / A1] × 100
Where, A1= absorbance of the DPPH solution without fruit extract,A2= absorbance of the DPPH solution with fruit extract.
2.12. Ferric reducing antioxidant power (FRAP) assay
FRAP working solution was freshly prepared consisting of acetate buffer (300 mM) at pH 3.6, TPTZ reagent (10 mM) in 10 mL of 40 mM HCL solution, and 20 mM ferric chloride in distilled water, at the ratio of 10:1:1. Next, 100 µL of various concentrations (150, 300,600, and 1 200 µg/mL) respectively of Gac samples, and butylated hydroxytoluene (BHT) as a positive control were allowed to react for 4 min with 3 mL of the FRAP working solution. The absorbance was measured at 593 nm using UV/Vis, and the results were calculated based on the plotted standard curve of FeSO4ranging from 40 to 250µM[21].

Figure 1. HPLC chromatograms of carotenoid standards (β-carotene and lycopene) and Gac fruit parts (aril, peel, and pulp).
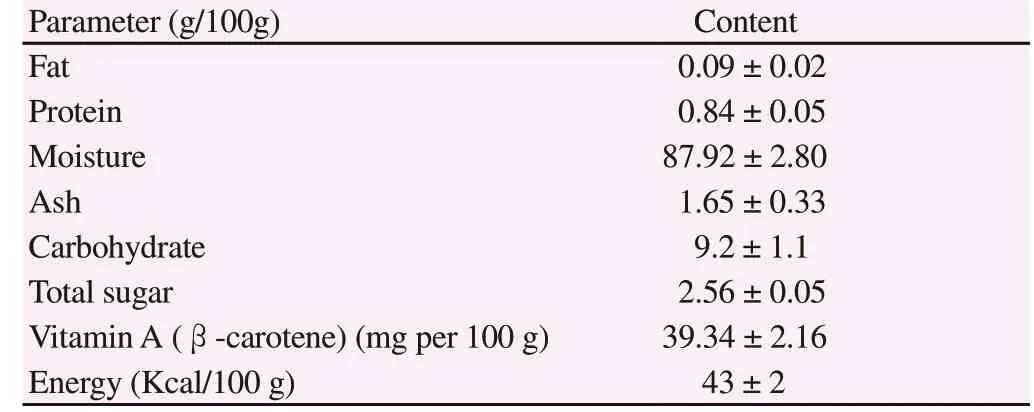
Table 1. Proximate composition of Gac fruit aril.
2.13. Statistical analysis
The values were presented as mean ± standard deviation (SD) of the three replicates. Also, the analysis of the significance and differences among the means was undertaken through One-way ANOVA and Tukey’s post hoc multiple comparison test. Notably, all experimental data values were statistically subjected using the GraphPad PRISM program version 6.01.
3. Results
3.1. Proximate composition
Nutritional values (fat, protein, moisture, ash, carbohydrate,total sugar and vitamin A activity) of aril part of Momordica cochinchinensis Spreng are tabulated in Table 1. The results showed that provitamin A activity was high, suggesting that Gac fruit is extremely rich in carotenoids. Furthermore, fat and protein levels were found in minimal amounts, while the moisture value content was very high. In addition, the peroxide was 5.63 mEq/kg oil and pH was 5.63.
3.2. Total carotenoids, phenolics, and flavonoids content
The total carotenoids, phenolics, and flavonoids contents in the Gac fruit parts (peel, pulp, seed, and aril) are represented in Table 2.The results indicated a noticeable variation between the carotenoids,phenolics and flavonoids. Furthermore, the carotenoids were predominant in the whole fruit parts except in the seed. The aril was found to have the highest carotenoids content followed by peel,pulp and seed. The level of phenolics was found to be quite similar in the peel, pulp and aril. The aril recorded the highest phenolics level, followed by the pulp, peel and seed. The flavonoids content varied among the four parts, with the peel showing the highest level followed by the aril, seed and pulp.

Table 2. Carotenoids, phenolics, and flavonoids contents in Gac fruit parts(peel, pulp, seed, and aril).
The results are expressed as mean ± SD (n = 3). Values not sharing the same letters are significantly different at P < 0.05. GAE, Gallic acid equivalent;QE, Quercetin equivalent; DW, Dry weight.
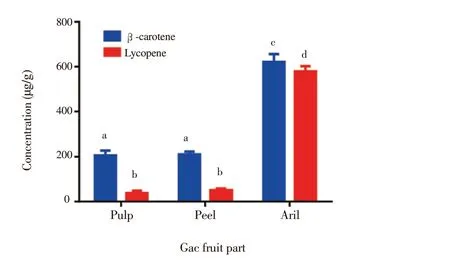
Figure 2. Quantification of β-carotene and lycopene in Gac fruit parts.Results are expressed as mean ± SD (n = 3). Bars not sharing the same letters are significantly different at P < 0.05.
3.3. β-carotene and lycopene contents
Figure 1 illustrates the typical chromatogram of the fruit parts and standards, in which the elution order and retention time for lycopene was 6.1 and for β-carotene 8.1 min. The concentrations of β-carotene and lycopene of the Gac fruit (peel, pulp, and aril)are summarised in Figure 2. As observed, lycopene and β-carotene were found in all parts. Furthermore, Gac aril displayed the highest carotenoids content of both lycopene and β-carotene, followed by peel and pulp. However, no peaks were detected of the both carotenoids in the seed part.
3.4. Identification of phytochemical compounds in Gac fruit parts by LC-MS/MS
Phytochemical compounds obtained from the LC-MS/MS analysis,of each fruit part (peel, pulp, seed, and aril) are displayed in Table 3.Approximately fifteen compounds were identified by comparing and searching in the standard library information based on the retention time and mass spectra acquired in Figure 3. The present screening highlighted a number of organic acid, polyphenols and fatty acids distributed among the fruit parts which included aconitic, malic, and cinnamic acid in the peel part, the p-hydroxycinnamoyl moiety in the pulp part, the cinnamic acid in the aril, and sinapic acid in the seed. Furthermore, rutin was discovered in the peel, and quercetin glucoside and quercetin were found to be present in the seed part.Also, a saponin compound named momordin Ⅱ was found in the peel. Notwithstanding, a number of fatty acids were also identified;decanoic, linolenic, octadecanoic, and oxooctadecanoic acid were distributed in all fruit parts. Other unknown compounds were also discovered in the fruit parts using the LC-MS/MS technique;however, more advanced techniques would be required to identify and define these compounds correctly.
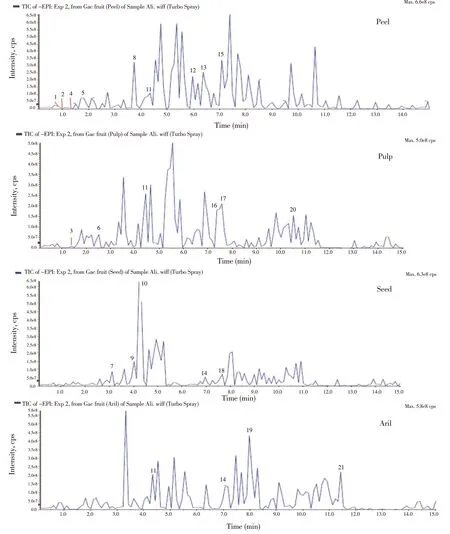
Figure 3. LC-MS/MS chromatograms of Gac fruit parts (peel, pulp, seed and aril).
3.5. DPPH free radical scavenging activities of Gac fruit parts
The percentage inhibition activity of the Gac fruit parts is represented in Figure 4. The results showed differences in the radical scavenging activities among the fruit parts. The aril part exhibited the highest scavenging activity, whereas the pulp part showed the lowest antioxidant activity compared to other parts. The inhibition concentration (IC50) of each part based on this experiment was 1 435(pulp), 1 086 (peel), 1 035 (seed), and 865 (aril) µg/mL, respectively.The results, therefore, indicate that the scavenging activity of each fruit part is accelerated with increasing concentrations for each part of the fruit.

Table 3. Phytochemical compounds identified by LC-MS/MS in Gac fruit parts.
3.6. Ferric reducing antioxidant activities of Gac fruit parts
Figure 5 illustrates the power reducing activity of the Gac fruit samples (aril, seed, pulp, and peel). Different concentrations were used and compared with synthesised BHT as a potent antioxidant agent, thereby demonstrating the ability of the fruit samples to reduce the brown colour of the ferric chloride (Fe3+) solutions to a blue colour (Fe2+). Accordingly, Gac peel displayed the highest reducing power (140 µmol FeSO4/µg) while Gac aril exhibited the lowest reducing activity (91 µmol FeSO4/µg) at the concentration of 1 200 µg/mL compared with BHT (207 µmol FeSO4/µg).Furthermore, the FRAP reducing power capacity of the fruit parts showed an accelerating trend with increased concentrations. Notably,all the results were equivalent to the FeSO4standard curve.
4. Discussion
Gac fruit has been declared to be a rich source of phytochemicals and to possess antioxidant activities. In this study, phytochemical compositions and antioxidant potential activities of Gac fruit parts(peel, pulp, seed and aril) grown in Malaysia were investigated.The results showed that carotenoids, phenolics and flavonoids were found in all parts. β-carotene and lycopene were found in high concentrations in the aril and moderated concentrations in the peel and pulp parts of the fruit. By testing the scavenging activity and ferric reducing power activities, Gac fruit parts appeared to possess antioxidant activities.
Carbohydrate, sugar, protein, fat and other metabolic substrates are major sources of energy found in foods. Therefore, recognition of the nutritional value is important in the energy metabolic system.Importantly, the moisture and protein levels identified in this study were found to be in agreement with the previous study that reported 92.47% moisture and 0.96 g/100 g protein[22]; while, both the energy and fat values were found to be much lower than another study that reported 125 Kcal for energy and 7.9 g/100g fat[23].
In general, the existence of bioactive compounds including carotenoids, phenolics and flavonoids in certain plants affords more prominence to their potentiality, superiority and utilisation in the therapeutic and pharmaceutical aspect. These active compounds have been well-known as natural antioxidants and for their significant roles in the prevention of a large number of diseases[24]. Obviously, the aforementioned bioactive compounds were found among all the fruit parts. Clearly, the carotenoids in the peel, pulp and aril were about three times higher than the phenolics or flavonoids, which indicate that Gac fruit parts are a significant source of carotenoids. Indeed, Gac aril is the only part that is usually processed and considered, while other fruit parts (peel, pulp and seed) are discarded and wasted[6,7]. So that,this study might draw more attention toward the utilization of all Gac fruit parts as valuable sources of phytochemicals. In comparison with other underutilized tropical fruits that are also cultivated in Malaysia such as Bacang, Durian Cerapu, and Jambu, total carotenoids found in this study were found to be several times higher[25]. In addition,total phenolics values reported in this study were similar and much lower while flavonoids contents were much higher than some other tropical fruits such as pineapple, papaya, mango, and banana[26].The differences of the bioactive compounds content might be due to genetics and geo-agriculture reasons.
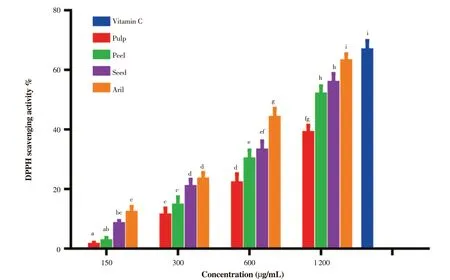
Figure 4. Scavenging activity of Gac fruit parts at different concentrations (150, 300, 600, and 1 200 µg/mL) compared with 250 µg/mL vitamin C. Results are expressed as mean ± SD (n = 3). Bars not sharing the same letters are significantly different at P < 0.05.

Figure 5. Reducing power capacities of Gac fruit parts and BHT as a positive control at different concentrations (150, 300, 600, and 1 200 µg/mL). Results expressed as mean ± SD (n = 3) as (µmol FeSO4/µg) based on the FeSO4 standard curve. Bars not sharing the same letters are significantly different at P < 0.05.
Foods-rich lycopene and β-carotene such as tomato, melon,carrots and spinach are essential for the daily nourishment and consumption due to their health promoting benefits. The aril part of the fruit showed the highest contents of the aforementioned carotenoids, which was in agreement with previous studies.However, the information about the levels of these carotenoids in the aril part was largely varied. Recently, Gac aril was reported to have 1 644 µg/g of lycopene which was much higher than this study and 205 µg/g of β-carotene which was found to be lower compared to the current result[27]. Interestingly, the level of lycopene reported in this study was about four times higher than the lycopene found in fresh tomato (140 µg/g)[28], which was also mentioned by a previous study[5]. Lycopene and β-carotene were also found in moderate concentrations in the peel and pulp parts, which was also confirmed by previous study that reported significant concentrations among these parts[8]. This finding confirmed that peel and pulp parts of Gac fruit are also significant sources of lycopene and β-carotene.However, several factors including agro-environmental, extraction processes and drying methods affect the total contents of carotenoids and other bioactive compounds. Therefore, more optimisation methods are required for good recovery of carotenoids and other bioactive compounds found in the Gac fruit. Carotenoids have been found to possess several therapeutic effects via their antioxidants,cancer chemoprevention activities, and their roles in the prevention of eyes disorders such as cataract and diabetic retinopathy[29-33]. Recently, the extracts obtained from Gac fruits parts showed promising potential against hyperglycaemia-induced proliferative diabetic retinopathy biomarkers in vitro[34]. This finding might be attributed to the presence of lycopene, β-carotene and other bioactive components as reported in this study.
The LC-MS/MS data, as described above, was successfully obtained in order to detect several phytochemicals based on the mass spectrum. The majority of known compounds were observed in the peel part, while little-known compounds were found in other parts. Most importantly, rutin and quercetin were detected in the peel and seed parts. These two flavonoids are well acknowledged for their pharmacological properties including but not limited cataract and radio protective activities via reducing levels of advanced glycation end products, aldose reductase and malondialdehyde[35,36].The current LC-MS/MS data also confirmed the presence of fatty acids[4,37]. In fact, the fatty acids trigger the absorption of carotenoids via the human digestive system thereby increasing its bioavailability[38]. However, more researches on the isolation and purification of the bioactive compounds and their therapeutic potential separately might be highly advantageous.
DPPH and FRAP tests provide a convenient, cost-effective and evident observation regarding the potential antioxidant capacities of plants extracts based on colour changes. The results of DPPH and FRAP conducted in this study exhibited strong antioxidant activity of the aril and peel parts of the fruit when compared to the synthesised controls vitamin C and BHT. Recently, a number of studies have also demonstrated that Gac fruit parts possessed antioxidant capacities which were consistent with these findings[18,11]. In this study, both the aril and peel appeared to possess higher contents of carotenoids,phenolics and flavonoids. Total contents of these phytochemicals are positively linked with the antioxidant capacities[8,39]. Therefore,the variation of the inhibition activities observed amongst the fruit parts might be due to the variation of the levels in the phytochemical contents.
In conclusion, this study provided information about the composition of bioactive compounds and antioxidant activities of Gac (Momordica cochinchinensis Spreng) fruit parts grown in Malaysia. The results of this study have demonstrated that Malaysian Gac fruit contains a significant level of multi-bioactive compounds especially carotenoids (β-carotene and lycopene), and relatively high levels of polyphenols. The bioactive compounds found in the Gac fruit parts might be potentially isolated, purified and utilised as supplements, natural food colourants or for the ethnopharmacological and biomedicine applications. However, serious efforts would be required in order to study the biological activities and the potential health benefits of the bioactive compounds found in Gac fruit.
Conflict of interest statement
The authors have no conflicts of interest to declare related to this study.
Acknowledgments
We would like to appreciate Dr. Mohd Desa and the International Tropical Fruits Network, Malaysia for providing the Gac fruits. We also gratefully thank Mr. Hasbullah for his technical assistance in HPLC.
FundingThis research was funded by the Putra Graduate Initiative under Universiti Putra Malaysia Research Grant (GP-IPS/2017/9527300).
 Asian Pacific Journal of Tropical Biomedicine2019年4期
Asian Pacific Journal of Tropical Biomedicine2019年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats
- Falcaria vulgaris extract attenuates diabetes-induced kidney injury in rats
- GC-MS analysis and anti-mosquito activities of Juniperus virginiana essential oil against Anopheles stephensi (Diptera: Culicidae)
- Antimalarial activities of butanol and ethylacetate fractions of Combretum nigricans leaf
- Epidemiology, clinical features and transmission of re-emerging arboviral infection chikungunya
