Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats
Nurul Hazirah Che Hamzah, Arifullah Mohammed✉, KNS Sirajudeen, Mohd Asnizam Asari, Zulhazman Hamzah, Ibrahim Khalivulla Shaik
1Faculty of Agro-Based Industry, Universiti Malaysia Kelantan, 17600 Jeli, Kelantan, Malaysia
2Department of Chemical Pathology, School of Medical Sciences, Universiti Sains Malaysia, 16150 Kubang Kerian, Kelantan, Malaysia
3Department of Anatomy, School of Medical Sciences, Universiti Sains Malaysia, Health Campus 16150 Kubang Kerian, Kelantan, Malaysia
4Faculty of Sains Bumi, Universiti Malaysia Kelantan, 17600 Jeli, Kelantan, Malaysia
5Faculty of Pharmaceutical Sciences, UCSI University, 56000 Cheras, Kuala Lumpur, Malaysia
Keywords:Full thickness excision wound Rat model Sprague-dawley rat Alocasia longiloba petiole extract
ABSTRACT Objective: To investigate the wound-healing effect of Alocasia longiloba (A. longiloba) petiole extract on wounds in rats.Methods: Twenty-two male Sprague-dawley rats were randomly assigned to receive 10%solcoseryl gel, phosphate buffer saline, 50% ethanol, 95% ethanol and hexane extracts of A. longiloba at 1.5%, 3% and 6% doses, respectively. A full thicknesses wound (6 mm) was created on the dorsal of the rat; and all rats were applied with the extract solutions, 10%solcoseryl gel and phosphate buffer saline once a day topically until day 12. The wound was photographed on day 1, 6 and 12, and the percentage of wound contraction was calculated. On day 12, rats were sacrificed and histological examination of granulation tissue was carried out using haematoxylin & eosin and Masson’s Trichrome stain to determine the wound healing effect.Results: In this study, 6% of 50% and 95% ethanol extracts of A. longiloba showed 82.50%and 82.32% wound contraction, respectively, and were comparable with 10% solcoseryl gel (82.30%). Meanwhile, phosphate buffer saline treated group showed the lowest wound contraction (69.86%). Histological assessment of wound treated with 6% of 95% ethanol extract of A. longiloba showed distinct epidermal and dermal layer, higher proliferation of fibroblast and more angiogenesis with collagen compared to other wound treated groups.Conclusions: A. longiloba petiole extracts have a wound healing potential and 6% of 95%ethanol extract of A. longiloba is more effective. Further studies are required to understand the wound healing mechanism of action of the extract.
1. Introduction
Wounds can be described as physical injuries to the skin, and cause damage of cells and anatomical function of normal tissues[1]. It can be classified into two types which are acute and chronic. Acute wounds such as cuts or minor burns occur as a result of surgery or trauma, and progress through the normal stages of healing withinthe predicted time frame of usually four weeks. Chronic wounds happen when acute wounds do not progress normally through the stages of healing due to bacterial infection and failure to heal within four weeks[2,3]. The common form of chronic wounds includes diabetic foot ulcers, venous leg ulcers and pressure ulcers[4]. Wound healing is a complex process for restoring the structure and function of injured tissue in order to approach pre-wound characteristics[5,6].
Healing process can be categorized into three overlapping,continuous and precise phases namely inflammation, proliferation and remodelling[7]. The first phase of wound healing is inflammation phase which involves blood coagulation with the aim to stop bleeding by forming a fibrin clot as a temporary filling in the wound and leucocytes into the injured area to prevent bacterial invasion[8].Proliferation phase initiated by macrophages actively develops by fibroblast cells under control of growth factors. Finally, in the remodelling phase, collagen is associated with the existing collagen and protein molecules, thus contributes to scar tissue strength[9].
According to Dorai[10], modern medication has been used to treat wound; however, several challenges have been faced by wound care professional because of the emergence of multi-resistant organism and decrease in newer antibiotics. Therefore, to overcome this problem, they need to develop new formulations via different methods such as ancient healing methods that used traditional medicine. Nowadays, a large number of modern drugs have been developed from natural sources, especially plants used in traditional folk medicine. Traditional medicine has been practiced in India,China, Japan, and other Asian and African countries and now has received much attention by the scientific community for their various uses[11-13].
Alocasia longiloba (A. longiloba) is a genus of rhizomatous or bulbous perennials plants with large heart-shaped leaves belonging to the family of Araceae[14]. It is one of the most familiar medicinal plants which grow abundantly throughout the year in all Southeast Asian countries. It can be found in rainforest and regrowth understory, in swampy areas, well drained slopes, and occasionally on rocks[15]. This species is known as ‘Ray tai la dai’ by Vietnamese and has been used to treat hives, itch, and pimples[16]. It is also known as ‘keladi candik’ by local Malays and has been used to treat external wound. Malay traditional healers use juices from petiole and apply it onto the wound to stop the bleeding and improve wound healing[8]. Inspired by its ability to treat wound, this study was conducted to investigate the efficacy of topical application of A.longiloba macroscopically and the histological methods pertaining to wound healing in rats.
2. Materials and methods
2.1. Plant collection and extraction
A. longiloba plants were collected from Pasir Mas, Kelantan,Malaysia. The petiole parts from the plants were washed thoroughly under running tap water and dried at 40 ℃ in an oven. Then the dried petioles were ground into powder using electrical blender.Thirty grams of dry powdered petioles were extracted using 300 mL of 50% ethanol (50E), 95% ethanol (95E) and hexane solvent respectively with Soxhlet extractor. Rotary evaporator was then used to concentrate the extracts by evaporating the solvents. Finally, the concentrated extracts were stored in dry airtight container at 4 ℃until further use. A. longiloba extracts at 15 mg, 30 mg and 60 mg were dissolved in 1 mL of phosphate buffer saline (PBS) solution to obtained 1.5%, 3.0% and 6.0% of extracts respectively. The mixture was stirred to become homogeneous and then applied topically to the wound area once a day.
2.2. Experimental animals
Male Sprague-dawley rats weighing 180-200 g of 8-12 weeks old were purchased from Laboratory Animal Research Unit, Animal Research and Service Centre Universiti Sains Malaysia Health Campus (ARASC USM), Malaysia and were used in this study.Ethical approval for this wound healing study was obtained from Animal Ethic Committee, USM Kubang Kerian, Malaysia [approval project code: USM/Animal Ethic Approval/2016/(104)(800) on 27th November 2017]. The rats were acclimatized for a week at the ARASC USM Laboratory and housed in separate cages at a temperature of (22 ± 20) ℃ with light-dark cycle of 12 h. The rats were fed with conventional rodent chow diet (Altromin, Germany)and water ad libitum. The rats were divided into eleven groups (n =2) and were topically applied with the extracts as follows:
Group A: PBS (negative control)
Group B: 10% solcoseryl gel (10SG) (positive control)Group C: 1.5% of 50E extract Group D: 3% of 50E extract Group E: 6% of 50E extract Group F: 1.5% of 95E extract Group G: 3% of 95E extract Group H: 6% of 95E extract Group I: 1.5% of hexane extract Group J: 3% of hexane extract Group K: 6% of hexane extract
All rats were anesthetized using pentobarbital (60 mg/kg) and their dorsal experimental sites were shaved and wiped with 70%alcohol swab followed by povidone-iodine solution. A fullthickness integument excisional wound (6 mm) was excised by using standardized sterile biopsy punch with diameter size of 6 mm and depth biopsy thickness of 3 mm. For post-operative care and monitoring, each rat was placed in individual cage with the base of its floor which was covered by wood bedding. Daily treatment of 20 µL of PBS and respective plant extracts were applied topically to each rat starting from Day 0 to Day 12 for group A and C to K. Meanwhile,group B was topically applied with 20 mg of 10SG daily.
2.3. Macroscopic evaluation of wound
To evaluate the wound healing features macroscopically, the excision wound and its contracting diameter (mm) were observed.The diameter of wounds was photographed and measured using Vernier calliper on day 1, day 6 and day 12 for each treatment as well as control group of rats.
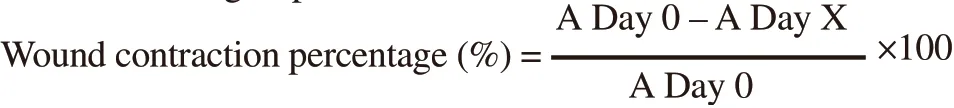
Where, Day X = Day 1: Day 6: Day 12 and A = diameter of wound
On the day 12, after macroscopic evaluation, the rats were euthanized with 100% carbon dioxide (CO2) inhalation. Then the granulation tissues were removed using a sterile biopsy punch, 6 mm in diameter and fixed in 10% buffered formalin for the microscopic studies.
2.4. Microscopic evaluation of wound
The fixed tissues were processed, embedded in paraffin wax and sectioned (3 µm-5 µm). These sections were strained with hematoxylin and eosin (H&E) as well as Masson’s Trichrome staining. Histological analysis of the tissue was carried out using light microscopy to visualize and semi-quantitate the histological features of wound healing (epithelization, fibroblast proliferation,new vessel formation and collagen organization).
3. Results
3.1. Wound contraction rate
Healing pattern of wound closure was observed in rats starting from day 0 to day 12. Figure 1 shows that the scab started to form on day 1 and started to drop on day 6, then replaced with pinkish color of granulation tissue until day 12. Wound treated with 6% of 50E and 95E extract of A. longiloba showed completely closed just like in 10SG (positive control) on Day 12. Whereas the negative control that was treated with PBS showed wide granulation tissue area as compared to other treated groups and this revealed that the wound was not completely recovered. The percentage of wound contraction is shown in Figure 2 and a significant contraction was observed in the treated group as compared to the negative group. On the twelfth day, the highest percentage of wound contraction was found in 6% of 50E extract with 82.50% of contraction, followed by 6% of 95E with 82.32%, positive group with 82.30% and 6% of hexane extract with 79.37%. Meanwhile, wound treated with PBS showed the lowest wound contraction percentage (69.86%).
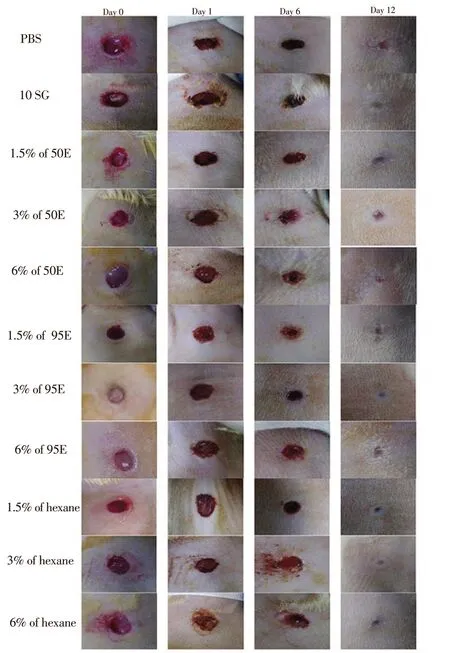
Figure 1. Macroscopic images of excision wound healing on day 0, 1, 6 and 12 in mice treated with 1.5%, 3% and 6% of A. longiloba extracts and the control groups on Sprague-Dawley rats. Scab starts to form on day 1 and starts to drop on day 6, then replaces with pinkish color of granulation tissue until day 12. Wound treated with 6% of 50% and 95% ethanol (50E and 95E)extract of A. longiloba shows completely closed as in 10% solcoseryl gel(10SG) (positive control) on Day 12. The negative control that is treated with PBS shows wide granulation tissue area, demonstrating that the wound is not completely recovered.
3.2. Microscopic analysis
The epithelization findings for all groups were shown in Figure 3. The results showed that granulation tissue of positive control(10SG) revealed a well demonstrated and distinct epidermal and dermal layer. Meanwhile in negative control group (PBS), it showed the epidermal layer was not as definite as in the positive control.Granulation tissues from 50E and 95E of A. longiloba treatments also revealed a distinct epidermal and dermal layer. The epidermal layer was thicker with higher concentration of the extracts from 1.5% to 6%. The skin tissue from the group treated with 6% of 95E extract demonstrated a thicker and well demonstrated epidermal layer as well as distinct dermal layer compared to 6% of 50E extract treatment. However, the skin tissue of the group with treatment of hexane extract of A. longiloba demonstrated thinner epidermal layer compared to the other extract treatments.
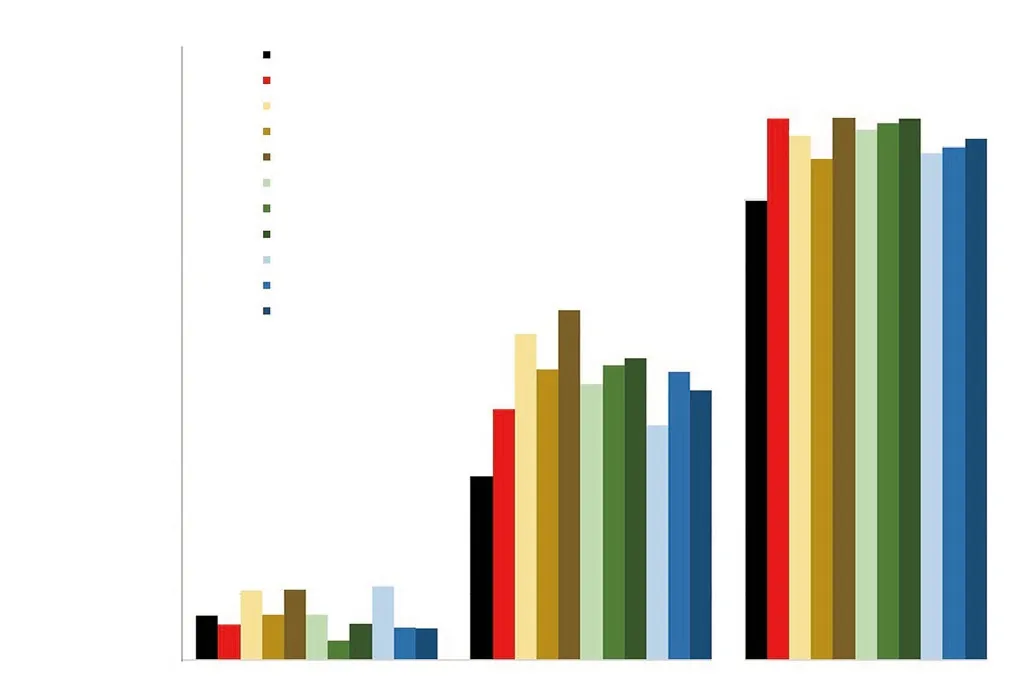
Figure 2. Percentage of wound contraction for wounds treated with PBS (negative control), 10SG (positive control), 1.5%, 3% and 6% each of 50E, 95E and hexane extract of A. longiloba on day 1, 6 and 12.
The fibroblasts appeared spindle-shaped as presented in Figure 4. Tissue of positive control demonstrated high proliferation of fibroblast with intense distribution in the granulation tissue;whereas in negative control, it showed less number of fibroblast with large gap in between each fibroblast within the granulation tissue. The skin tissue with treatment of 6% of 95E A. longiloba extract also demonstrated higher proliferation of fibroblast with intense distribution in the granulation tissue as compared to other A. longiloba extract treatment. In contrast, the hexane treatment demonstrated lower number of fibroblast in the granulation tissue.
The new vessels were identified by the presence of luminar structures lined with flat endothelial cells of bulging nucleus as shown in Figure 5. A large number of new vessels with intense distribution were shown in granulation tissue of positive control.However, the skin tissue in negative control showed less distribution of new vessels. The skin tissue of 6% of 95E A. longiloba extract treatment demonstrated high number of new vessels distribution in the granulation tissue compared to others A. longiloba extracts treatment.
Masson’s Trichrome images shown in Figure 6 revealed that there was dense composition and well-organized arrangement of collagen fibrils in skin tissue of positive control, while the skin tissue of negative control showed the collagen fibrils were loosely arranged in an irregular manners and presented with large gaps between each fibril unit. Compared to other A. longiloba extracts treated groups,the dense composition and well-organized arrangement of collagen fibrils were observed in 6% of 95E extract treated group, which thus was considered to be more effective.
4. Discussion
Wounds are physical injuries of the skin. Wound will undergo healing process which is a complex process initiated in response to an injury to restore function and integrity of the damaged tissues.The aim of wound treatment is to shorten the desired time for healing and to avoid risks of complications[17]. All three phases namely inflammation, proliferation, and remodelling must occur in the proper sequence and time frame for a successful wound healing[18].In this study, topical application of A. longiloba extracts accelerated the rate of wound healing, and also increased the presence of collagen, fibroblasts, and blood capillaries in granulation tissue in comparison to the negative control.
Wound contraction takes part in the proliferation phase which occurs through the centripetal movement of the tissues surrounding the wound. The process of wound contraction depends on the ability of tissues to repair the damage, the damage extent and type, and also the general health of tissue[19]. The rate of wound contraction might be a result of the enhanced activity of fibroblast mediated by specialized myofibroblasts. Therefore, the rate of contraction can determine the period of epithelization and the amount of collagen deposited[20].
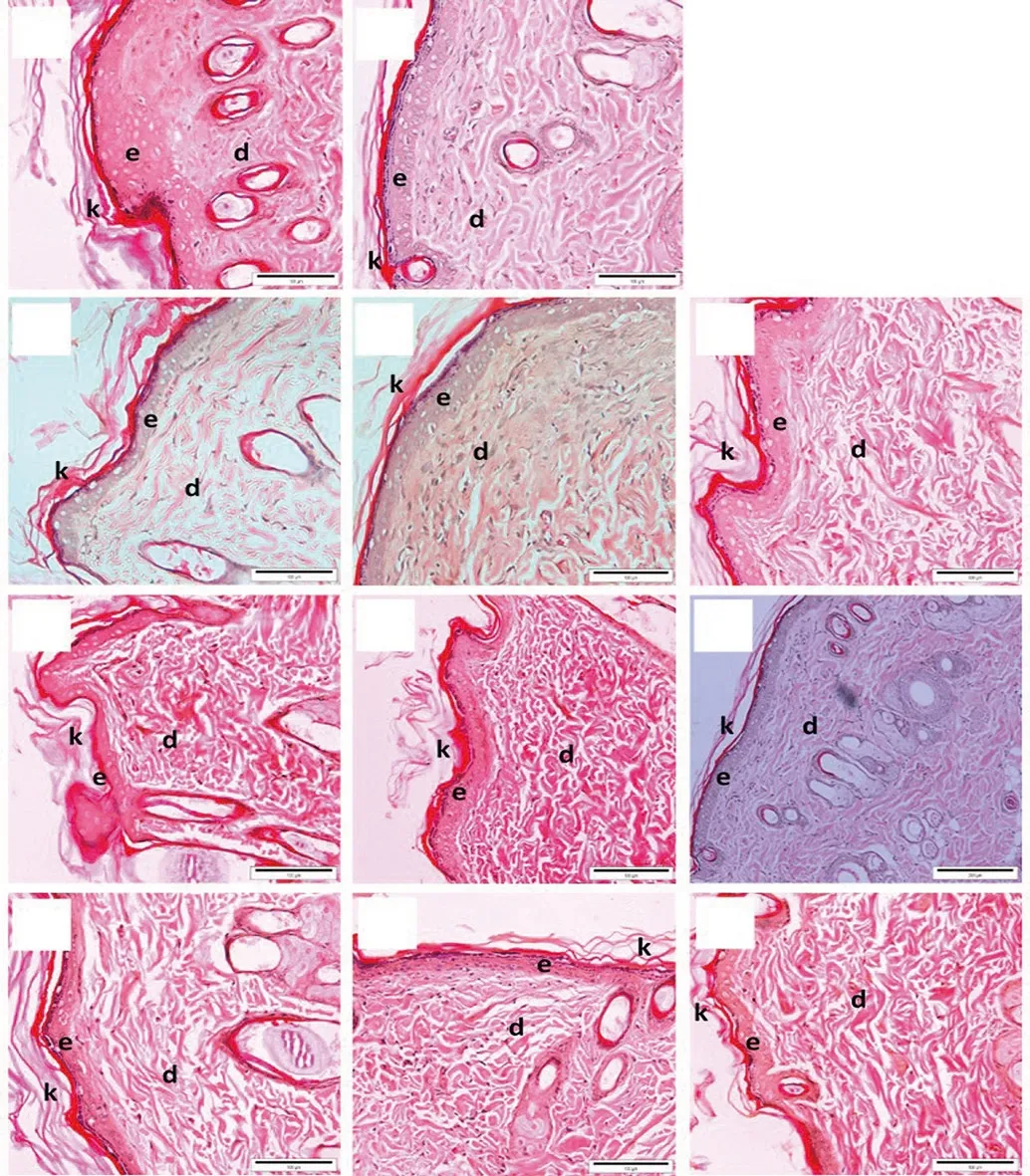
Figure 3. Light microscopic images of epithelization in the control and treated groups on day 12. A: PBS, B: 10SG, C: 1.5% of 50E, D: 3% of 50E, E: 6% of 50E,F: 1.5% of 95E, G: 3% of 95E H: 6% of 95E, I: 1.5% of hexane extract, J: 3% of hexane extract and K: 6% of hexane extract of A. longiloba (H&E stain, Bar = 100µm). e: epidermis, d: dermis, and k: keratin. Granulation tissue of positive control (10SG) reveals a well demonstrated and distinct epidermal and dermal layer,whereas in negative control group, the epidermal layer is not as definite as in the positive control. Granulation tissues from 50E and 95E of A. longiloba treatments reveal a distinct epidermal and dermal layer, which becomes thicker with increasing concentration of the extracts from 1.5% to 6%. However, the skin tissue of the group treated with hexane extract of A. longiloba shows thinner epidermal layer compared to the other extract treatments.
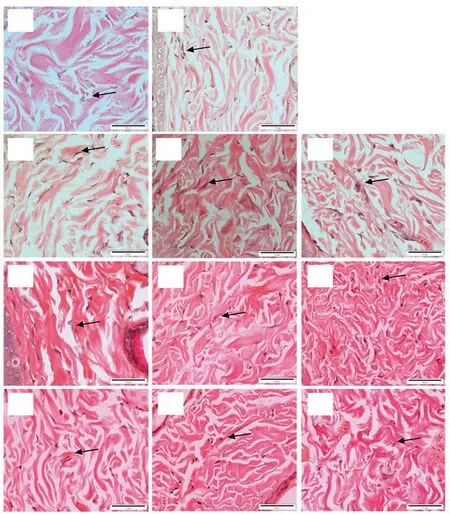
Figure 4. Light microscopic images of fibroblasts proliferation (arrows) in the control and treated groups on day 12. A: PBS, B: 10SG, C: 1.5% of 50E, D: 3% of 50E, E: 6% of 50E, F: 1.5% of 95E, G: 3% of 95E, H: 6% of E, I: 1.5% of hexane extract, J: 3% of hexane extract and K: 6% of hexane extract of A. longiloba(H&E stain, Bar = 50 µm). In negative control, tissue shows less number of fibroblast with large gap in between each fibroblast within the granulation tissue, while it shows high proliferation of fibroblast with intense distribution in the granulation tissue of positive control group. Compared with other extract treatment, the skin tissue treated with 6% of 95E of A. longiloba extract shows the better result in terms of fibroblasts proliferation.
In this study, the results show that the wound closure of the extracts treated group accelerates as the day of treatment increases,showing significant wound contraction. Immediately after the injury,hemostasis will take part and result in the formation of a fibrin clot at the site of wound[21]. The function of clot is to protect the injured area against pathogens and to stop blood loss. It consists of platelets embedded in a mesh of cross-linked fibrin fibers derived by thrombin cleavage of fibrinogen[22]. In this study, formation of clot can be seen on Day 1. At the same time, platelets assist in clotting also release cytokines such as platelet derived growth factor and transforming growth factor beta[20]. As the clot gets harder and dries out, it forms scab that functions in protecting the injured area[21]. After the scab falls off, new skin, known as granulation tissue characterized by pinkish color, can be seen in Day 6 for A.longiloba extract treated groups. However, for positive and negative control groups, the scar is still there (Day 6), meaning that it takes longer time to fall off than with A. longiloba extracts treated groups.Granulation tissues consist of different ranges of collagen but higher proportion in type 3 collagen produced by fibroblast and new blood vessels[5]. The accelerated wound contraction in the treatment may be due to increase of fibroblast proliferation in wound granulation tissue[9]. After that, by the process called tissue remodeling, the granulation tissue slowly turns into a mature connective tissue that ultimately restores the connective tissue structure and function[23].Percentage of wound contraction with 6% of 50E treatment showed higher percentage of wound contraction with 82.50%, followed by 6% of 95E treatment, 10SG, 6% of hexane extract and lastly by PBS with 82.32%, 82.30%, 79.37% and 69.86%, respectively. However, it is important to study the histology of the skin for the prediction and optimization of wound healing through H&E stain, and Masson’s Trichrome staining in the granulation tissue.
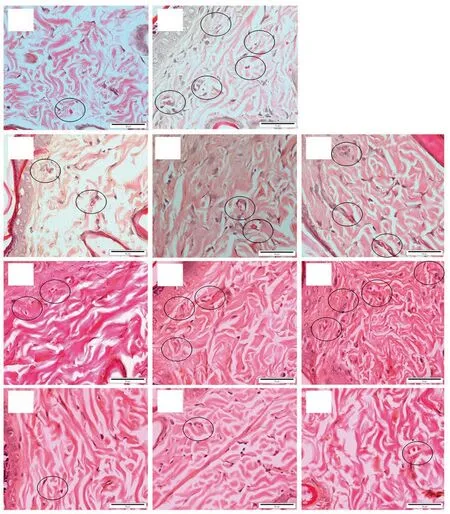
Figure 5. Light microscopic images of vessel formation (circle) in the control and treated groups on day 12. A: PBS, B: 10SG, C: 1.5% of 50E, D: 3% of 50E, E: 6%of 50E, F: 1.5% of 95E, G: 3% of 95E, H: 6% of 95E, I: 1.5% of hexane extract, J: 3% of hexane extract and K: 6% of hexane extract of A. longiloba (H&E stain,Bar = 50 µm). A large number of new vessels with intense distribution are shown in granulation tissue of positive control, while fewer new vessels are observed in negative control. In comparison with other A. longiloba extract treatments, the skin tissue treated with 6% of 95E of A. longiloba extract demonstrates more new vessels in the granulation tissue.

Figure 6. Light microscopic images of collagen organization in the control and treated groups on day 12. A: PBS, B: 10SG, C: 1.5% of 50E, D: 3% of 50E, E: 6%of 50E, F: 1.5% of 95E, G: 3% of 95E, H: 6% of 95E, I: 1.5% of hexane extract, J: 3% of hexane extract and K: 6% of hexane extract of A. longiloba (Masson’s Trichrome stain, Bar = 200 µm). Dense composition and well-organized arrangement of collagen fibrils are observed in skin tissue of positive control and 6% of 95E extract treated group, while collagen fibrils that are loosely arranged in an irregular manners and presented with large gaps between each fibril unit are found in negative control.
The proliferative phase is characterized by angiogenesis, collagen deposition, granulation tissue formation, epithelialization and wound contraction[7]. The results of histological analysis in this study indicate that the topical application of 95E of A. longiloba results in the production of a distinct epidermal and dermal layer and the epithelial lining with keratin contained a large amount of fibroblast,collagen depositions, and angiogenesis, which promotes wound healing. This results are in the line with the results of Zahra et al[24]and Al-Henhena et al[25] who showed that granulation tissues treated with ethanolic plant extract showed distinct epithelial cells, more fibroblast, collagen and the formation of angiogenesis.
The effect of wound healing in granulation tissue may be attributed to increased collagen formation and angiogenesis[26]. New vesselformation, known as angiogenesis, refers to the generation of new micro vessels structures from pre-existing vessels adjacent to the wound[27]. It is triggered when the haemostatic plug has formed as platelets which release TGF-β, platelet-derived growth factor and fibroblast growth factor[2]. One of the functions of angiogenesis in granulation tissues is to improve circulation to the wound site, thus providing oxygen and nutrients essential for the healing process which includes re-epithelialization[25,27]. Collagen is considered as predominant extracellular protein in the granulation tissue synthesized by fibroblast cells in the wound area that function in providing strength and integrity to tissue matrix[5,20]. Immediately after injury, fibroblasts in the wound edges begin to proliferate and approximately by day 4, they start to migrate into the provisional matrix of the wound clot, where they synthesize a collagen-rich matrix, including collagens, proteoglycans, and elastin through a process termed as fibroplasia[27].
The histology results from this study showed that 6% of 95E A. longiloba showed the significant results for epithelialization formation, fibroblast, angiogenesis and collagen distribution in the granulation tissue after 12 d compared to other groups.Epithelialization is an essential component of wound healing to ensure successful wound repair[28]. It is defined as healing by the growth of epithelium completely covers the wound[29]. In this study, the results from light microscopy indicate that 6% of 95E of A. longiloba has positive effects on the epithelialization process in wound healing. It is observed that there is a full bridging of the epithelial lining with keratin layers. In this study, it is also shown that there is a high proliferation number of fibroblast, new vessel distribution and well-organized arrangement of collagen fibrils in 6% of 95E of A. longiloba compared to other treated groups.These results are in agreement with the study done by Latif et al[8], showing that histological analysis of the wounds treated with 3% of Alocasia denudata stem juice contained a high number of fibroblast proliferation, collagen synthesis, new vessel formation and structured epithelium layer, which results in better wound healing effect. The current study showed that A. longiloba petiole extracts have the wound healing potential and the 6% of 95E is found to be more effective compared to other extracts. This result might be due to the variety of compounds present in the 6% of 95E extract which can provide more anti-inflammation, antioxidant and antimicrobial activity that contributes in the acceleration of wound healing. In fact, alcoholic solvent can penetrate the cell membranes efficiently and permit the extraction of high amounts of phytochemicals from the plants material[30]. PBS showed the lowest wound contraction because it is a vehicle control that undergoes normal wound healing compared to 6% of 95E extract that contains phytochemicals which can improve wound healing.
In conclusion, 6% of 95E petiole extract of A. longiloba showed a remarkable wound healing activity and has the potential to be developed as an effective natural wound healing agent. This preliminary studies give ideas to proceed with main study. Therefore,it is necessary to conduct further studies with different purified constituents in order to understand and validate the complete mechanism of wound healing action of A. longiloba petiole extract.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The authors would like to thank the Ministry of Higher Education Malaysia for the financial funding with Fundamental Research Grant Scheme (FRGS) (Grant Number: R/FRGS/A07.00/00710A/002/2016/000374). We would like to acknowledge Universiti Malaysia Kelantan as well as Animal Research and Service Centre (ARASC USM) and Central Research Lab (CRL) -Universiti Sains Malaysia, Health Campus for providing laboratory facilities.
FundingThis work is supported by Fundamental Research Grant Scheme(FRGS) (Grant Number: R/FRGS/A07.00/00710A/002/2016/000374).
 Asian Pacific Journal of Tropical Biomedicine2019年4期
Asian Pacific Journal of Tropical Biomedicine2019年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimalarial activities of butanol and ethylacetate fractions of Combretum nigricans leaf
- GC-MS analysis and anti-mosquito activities of Juniperus virginiana essential oil against Anopheles stephensi (Diptera: Culicidae)
- Antioxidant compounds and capacities of Gac (Momordica cochinchinensis Spreng) fruits
- Falcaria vulgaris extract attenuates diabetes-induced kidney injury in rats
- Epidemiology, clinical features and transmission of re-emerging arboviral infection chikungunya
