Falcaria vulgaris extract attenuates diabetes-induced kidney injury in rats
Jalili Cyrus, Roshankhah Shiva, Salahshoor Mohammad Reza✉
1Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
2Department of Anatomical Sciences, Medical School, Kermanshah University of Medical Sciences, Kermanshah, Iran
Keywords:Antioxidant Diabetes Falcaria vulgaris Kidney
ABSTRACT Objective: To assess the effects of Falcaria vulgaris (F. vulgaris) as an antioxidant on damage to kidney of diabetic rats.Methods: Diabetic rats were established via streptozotocin (60 mg/kg). Various doses of F. vulgaris extracts (50, 100 and 150 mg/kg) and streptozotocin + F. vulgaris extracts were administered via intraperitoneal (i.p) injection to 48 rats (n=8 per group) for 28 d.Subsequently, ferric ion reducing antioxidant power (FRAP) of renal tissue, thiobarbituric acid reactive species, blood glucose concentrations, insulin, nitrite oxide, the weight of animals,glomeruli characteristics and kidney function were evaluated.Results: Compared with the control rats, diabetic rats showed significant increase in malondialdehyde, blood urea nitrogen, creatinine, blood glucose, nitrite oxide contents in renal tissues, and glomerular diameter. Furthermore, tissue FRAP level, body weight, number of glomeruli and plasma insulin were markedly reduced in diabetic rats when compared with the control group (P < 0.05). However, in all F. vulgaris and F. vulgaris + streptozotocin groups,malondialdehyde level, blood urea nitrogen, creatinine, glomerular diameter, nitrite oxide,and glucose levels were decreased significantly; meanwhile, tissue FRAP level, body weight,glomeruli number and insulin serum level were increased, compared to the control diabetic group (P < 0.05).Conclusions: F. vulgaris extract alleviates renal damage in diabetic rats.
1. Introduction
Diabetes mellitus is one of the most common public metabolic illnesses associated with the absence of absolute or relative insulin[1].Increasing blood glucose, in the long run, can lead to damage to various organs and eventually death[2]. Due to diabetes, the kidneys suffer impaired function and tend to excrete protein. The kidneys also suffer from structural changes and dysfunction[3]. Increase in glomerular capillary growth, as a result of glomerular hypertrophy,is one of the primary responses to hyperglycemia caused by diabetes[4]. The diabetes is associated with oxidative stress due to the manufacture of reactive oxygen species (ROS) and it seems that an increase in oxidants in the body accelerates the development of symptoms of diabetes[5]. The ROS, known as free radicals, are oxidizing agents that are produced by oxygen metabolism and can react with proteins, lipids, sugars, and DNA[6]. Increasing ROS can cause lipid oxidation by oxidative stress[7]. Diabetes can increase blood glucose and lipids, as well as free radicals, and as a result,increase glomerular sclerosis and renal interstitial tissue damage.Nephropathy is the most common complication of diabetes[8].Flavonoids in plant composition exhibit oxidative stress-reducing properties by increasing glutathione, catalase, and superoxide dismutase and reducing lipid peroxidation[9,10]. Medicinal vegetation has been applicable for treating diabetes[11]. Falcaria vulgaris (F. vulgaris), as a medicinal plant, belongs to the Apiaceae(Umbelliferae) family and grows in the west and southwest of Iran as an annual wild plant[12]. This plant has a stem and its height is 30 cm on average. In the west of Iran, this plant has been traditionally used to treat skin ulcers, gastric disorders, including gastric ulcer,liver disease, renal stones and bladder[13]. Phytochemical researches on this medicinal vegetable have revealed the existence of saponin(50.9%) and tannins (39.8%)[14]. This plant contains protein, vitamin C, phytosterol, starchy resources and it is a source rich of tannins and ascorbic acid. Similar to antibiotics, it can be used for the recovery of skin sores[15]. Khazaei et al. showed that wound healing in the gastrointestinal tract occurred as a result of the antioxidant properties of F. vulgaris[16]. The main factor in F. vulgaris extract that reduces blood sugar is the presence of tannins and saponins[17].Also, the foremost elements in diverse portions of the plant show antioxidant action and neutralizing properties of free radicals[18].Considering that there has been no study on the effects of F. vulgaris on streptozotocin (STZ)-induced renal impairment, this study was designed for this purpose.
2. Materials and methods
2.1. Materials
STZ was bought from Merck (Germany). Ether, formalin, ferric ion reducing antioxidant power (FRAP), sodium acetate, ferric chloride,iron sulfate, hematoxylin-eosin, and zinc sulfate powder were purchased from Sigma (USA). Commercial kits were obtained from Pars Azmoon (I.R. Iran). All other buffer additives and solvents were obtained from Merck (Germany).
2.2. Extract preparation
F. vulgaris vegetable was obtained from a native store (this plant was picked during spring and bought from western Iran). After confirmation by a botanist, the vegetable was washed. The leaves were desiccated in shadow for one week and ground by a grinder.One hundred gram of the powder was added to 70% ethanol. The acquired solution was reserved in a warm water bath (38 ℃) under shady state. Thereafter, the solution was progressively dispensed via filter paper and cleaned via a vacuum pump. It was then moved to a rotary device to obtain the additional solvent. The isolation procedure was persistent until the extract concentrated was obtained.The extract was dissolved in distilled water, sterilized after double filtration through a 0.2 µm filter and administered intraperitoneally in rats[19].
2.3. Animals
In this experimental study, 64 Wistar rats (male; eight weeks old;blood glucose concentrations 70-120 mg/dL) with a weight range of 230-250 g were bought from Razi Institute, Iran. They were placed in the animal household for one week prior to the start of the investigation. The housing conditions were under control in terms of temperatures (22±2) ℃, humidity (55%-60%), 12/12 h (light/dark). They were allowed to have free access to food and water and to be adapted to the location. All animals were treated in animal care center of Kermanshah Medical Sciences University, Kermanshah,Iran. All investigations performed in accordance with guidelines of National Institute of Health for the Care and Use of Laboratory Animals were approved by Research Deputy at Kermanshah University of Medical Sciences (receipt date: 9 February 2016,approved date: 17 February 2016) based on World Medical Association Declaration Ethic of Helsinki (Ethic number: IR.KUMS.REC.1394.383)[6].
2.4. Grouping
The rats were randomly separated into eight groups (n=8). Group 1, normal control group, received normal saline (1 mL/kg). Group 2,diabetic control group, was diabetic by STZ. Groups 3-5, F. vulgaris groups, were administrated with 50, 100 and 150 mg/kg of F.vulgaris extract, respectively with each dose given at 8:00 am daily.Groups 6-8, STZ + F. vulgaris groups, were diabetic, and treated with 50, 100 and 150 mg/kg of F. vulgaris extract, respectively. In groups 3-5, F. vulgaris extract was injected intraperitoneally for 4 weeks. The rats in groups 6-8, four weeks after induction of diabetes,received F. vulgaris daily (at 8:00 am every day) for 28 consecutive days[15,17].
2.5. Establishment of diabetic models
A single dose of STZ (Sigma) dissolved in saline (50 mg/kg) was administered intraperitoneally. The control group was given the similar volume of normal saline (1 mL/kg). Blood was taken from the animal’s cauda to confirm that they come to be diabetic 3 days after administration. This study was conducted by glucose oxidase enzyme with blood samples. The animals with a serum glucose level more than or equal to 300 mg/dL were regarded as diabetic[1].
2.6. Body weight measurement
The body weight of each animal was measured at 2 time points.Each rat was weighed using a digital scale (A&C / GF500). The animals’ weight was measured at the beginning and end of the study.The changes in animals’ weight in each group were computed via the following formula[1].
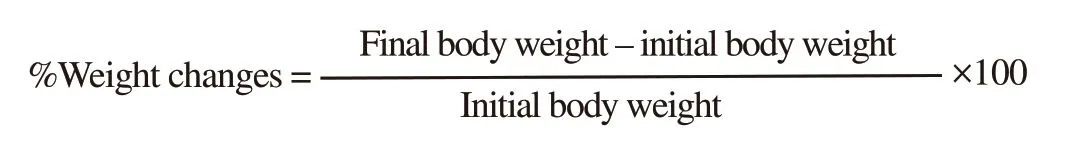
2.7. Blood glucose assay
The serum glucose level of animals was measured at three time points: starting point, 72 h after diabetes induction and 4 weeks after treatment. Serum glucose level of other groups (groups 3-5 and normal control group) was measured at the same time as diabetic groups. Twenty-four hours before blood sampling, all rats were kept fasting. Venous blood was drawn from the tail, and the samples were incubated for 15 min at 36 ℃ to accumulate. The isolated serum was stored at -20 ℃ until measurement of insulin, glucose and nitrite oxide (NO). Glucose oxidase technique was used to determine the serum glucose concentration[1].
2.8. Assay of serum insulin and biochemical test
Twenty-four hours after the last day of delivery of the extract,all rats were deeply anesthetized by intraperitoneal injection of ketamine HCl (90 mg/kg) and xylazine (15 mg/kg). Blood was taken from the heart without cutting the chest. The blood samples were coagulated, and centrifuged to separate the serum at 4 000 rpm for 10 min. Separated serum was kept at -70 ℃ for further determination.
Serum insulin was measured through ELISA method via the Monobind kit (USA, Sigma). The serum samples were defrozen(samples were taken at three time points similar to the procedure in blood glucose study). Prior to the start of the examination, the kits were exposed to laboratory temperature. The contents of a vial(10 mL) were diluted with purified water. The content of each vial was mixed with 2 mL distilled water. Twenty µL of the sample was transferred into the wells. Conjugated insulin solution (100 µL)was added to all wells and mixed afterwards. The surface of the wells was shielded with plate adhesive. The content of the plate was removed and 350 µL diluted irrigation solution was added to all wells. Thereafter, 6 µL of the substrate was added to all wells and incubated for 10 min. Fifty µL stop solution was added to all wells and plates were read by ELISA reader at 540 nm[20].
Creatinine and blood urea nitrogen (BUN) in serum were measured by an autoanalyzer (Access Random Liasys) using a kit (PARS–AZMON).
2.9. NO assay
NO was measured by Griess assay using microplate technique.Briefly, nitrite standards and sulfonamide solutions were prepared.The serum was deproteinized. The standard arch of nitrite concentration was designed. The optical density of the samples was evaluated via ELISA reader (Hyperion, Germany) at a wavelength of 460 nm[7].
2.10. Ferric reducing/antioxidant power and malondialdehyde (MDA) assays
In order to assess oxidative stress, the thiobarbituric acid reactive species were measured using MDA as the last product of lipid peroxidation in kidney tissue by performing colorimetric analysis.The absorbance was measured at 632 nm and tetraethoxypropane at serial concentrations was used as the outer standard. FRAP test was also performed, with 50 mL of nitrate buffer (Sigma, USA)and 6.5 mL ferric chloride (Sigma, USA). The red-colored complex absorbance was read in contradiction of a blank at 691 nm. Serial concentrations of FeCO2·6H2O (Sigma, USA) were applied as an exterior standard[21].
2.11. Histological analyses
Twenty-four hours after the last day of delivery of the extract,all rats were deeply anesthetized by intraperitoneal injection of ketamine HCl (90 mg/kg) and xylazine (15 mg/kg). The kidneys of each rat were immediately removed from the abdominal cavity for histopathology and fixed in a 10% formalin solution[6].Subsequently, the tissue was carefully washed and dehydrated via increasing concentration of ethanol. The tissue was then cleared in xylene, and embedded in paraffin. Serial sections were prepared by microtome (5 µm) (Leica RM 2125, Germany) from the tissues after molding of each sample, and marked with hematoxylin and eosin(H&E)[6]. From each kidney sample, 5 sections, 3 fields view from each slide, were obtained to examine the diameter of the glomerulus and glomerulus number using Olympus LX-96T-22T02 microscope linked to a PD21 Camera with 4.43-million pixel resolution(Olympus, Japan)[21].
2.12. Statistical analysis
Kolmogorov-Smirnov test was first conducted to confirm the data compliance of the normal distribution. One-way analysis of Variance(ANOVA) was performed for statistical analysis and Tukey post hoc test was used to determine the difference between the groups. SPSS Software (Ver.16) was used for data analysis and the results were expressed as mean ± standard error. P values < 0.05 was considered significant.
3. Results
3.1. Weight changes
The findings indicated differences in the mean index in terms of the percentage of the weight changes between groups. Significant increase was shown in the percentage of the body weight changes in the diabetic control group compared to normal control group (P <0.05). The percentage of the body weight changes in F. vulgaris groups(Group 3-5) was similar to the normal control group (P > 0.05). The percentage of the body weight changes in all STZ + F. vulgaris groups was decreased significantly compared to the diabetic control group (P< 0.05) (Figure 1).
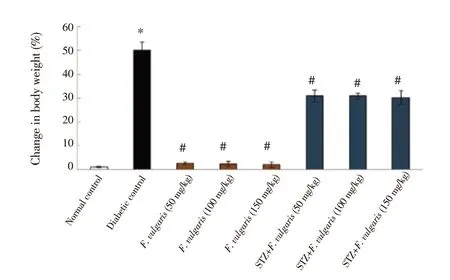
Figure 1. Mean body weight changes in different groups. *P < 0.05 vs.the normal control group. #P < 0.05 vs. the diabetic control group. STZ:streptozotocin.
3.2. Effect of F. vulgaris on blood glucose and insulin hormone
Mean of serum glucose level in diabetic control group was significantly increased compared to normal control group (P <0.05). The glucose level of F. vulgaris treated groups (Group 3-5)was similar to the normal control group (P > 0.05). Moreover,the glucose level of group 7 (STZ+ F. vulgaris 100 mg/kg) was improved significantly compared to the diabetic control group (P <0.05) (Figure 2).
The mean of blood insulin hormone was decreased significantly in the diabetic control group compared to the normal control group (P< 0.05). The mean of blood insulin hormone in all F. vulgaris treated groups (Group 3-5) was similar to that of normal control group (P >0.05). However, the insulin hormone in all STZ+ F. vulgaris groups was significantly enhanced in comparison with the diabetic control group (P < 0.05) (Figure 2).
3.3. Biochemical examination
The results of BUN and creatinine amount displayed a significant increase in diabetic control group (P < 0.05). However, these levels were not significantly different between F. vulgaris treated groups(Group 3-5) and normal control group (P > 0.05). Furthermore,BUN and creatinine in all STZ+ F. vulgaris groups were decreased significantly compared to the diabetic control group (P < 0.05)(Figure 3).
3.4. Histological examination
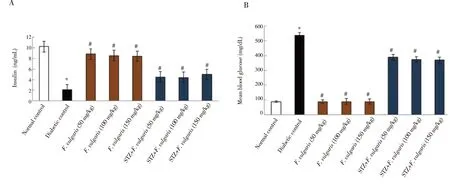
Figure 2. Effects of F. vulgaris on plasma contents of insulin (A) and glucose (B) in rats (n=8 for each group). *P < 0.05 vs. the normal control group. #P < 0.05 vs.the diabetic control group.
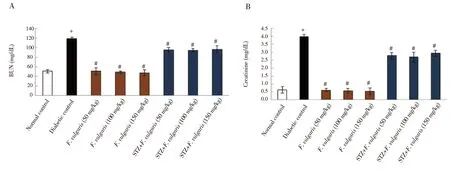
Figure 3. Effect of F. vulgaris on kidney biochemical factors. A: BUN; B: creatinine. *P < 0.05 vs. the normal control group. #P < 0.05 vs. the diabetic control group.
Diabetes caused a significant increase in the diameter of glomerulus tubule and a significant decrease in the glomeruli number compared to the normal control group (P < 0.05). But in terms of diameter of glomerulus and glomeruli number, F. vulgaris treated groups (Group 3-5) did not significantly differ from the normal control group (P >0.05). Additionally, F. vulgaris was found to decrease significantly the diameter of glomeruli and increase the number of glomeruli in diabetic rats (P < 0.05) (Figure 4).
Histological analysis presented normal renal construction in the normal control group and F. vulgaris treated groups (Group 3-5).STZ administration resulted in variable changes and renal injury.These abnormalities included extended space of Bowman’s capsule,glomerulus reduction, intertubular bleeding and enlarged diameter of distal and proximal tubules. After treatment with F. vulgaris in all doses, it was observed that F. vulgaris extract reduced renal damage caused by diabetes (Figure 5).
3.5. Oxidative stress assay
The oxidative stress analysis presented that the NO and MDA
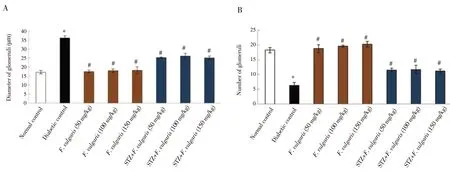
Figure 4. Glomerular diameter (A) and the number of glomeruli (B). *P < 0.05 vs. the normal control group. #P < 0.05 vs. the diabetic control group.
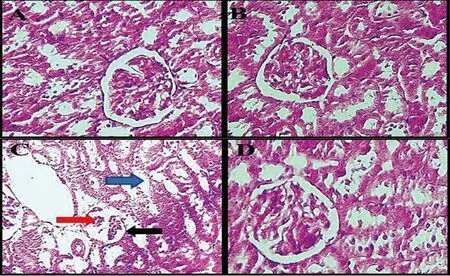
Figure 5. Histological changes of the kidneys (hematoxylin-eosin, × 100). A, Normal kidney structure in the normal control group. B, Normal kidney structure in the F. vulgaris group (150 mg/kg). Glomeruli with Bowman’s capsule and distal and proximal tubules are observed in a specific range. C, in the diabetic control group, increase in Bowman’s capsule space (black arrows), glomerular shrinkage (red arrow), and increase in the diameter of distal and proximal tubules (blue arrow) are found. D, Normal kidney structure in F. vulgaris plus streptozotocin group (150 mg/kg).were significantly increased in the diabetic group compared to normal control group (P < 0.05). Whereas, these levels were declined significantly after treatment with F. vulgaris at all doses compared to the diabetic control group (P < 0.05). Moreover,diabetes significantly reduced the renal tissue FRAP of the diabetic control group compared to the group of normal control (P < 0.05).Administration of F. vulgaris at three doses improved significantly the FRAP in renal tissue of diabetic rats (P < 0.05) (Figure 6).
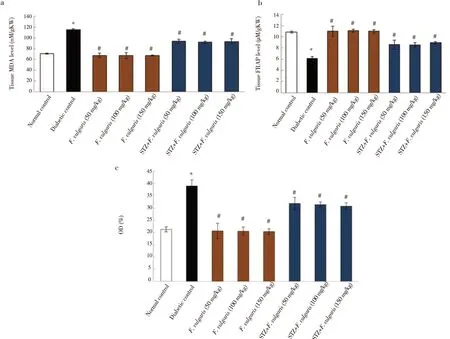
Figure 6. Effect of F. vulgaris on: (a) kidney MDA level; (b) tissue FRAP level; (c) NO level. *P < 0.05 vs. the normal control group. #P < 0.05 vs. the diabetic control group. FRAP: ferric ion reducing antioxidant power; MDA: malondialdehyde.
4. Discussion
Diabetes mellitus is the most common endocrine disorder and diabetic nephropathy is one of its long-term symptoms. In this case, the kidney undergoes many morphological changes and glomerulopathy is the most important structural change in the kidney which results in an increase in the thickness of the glomerular base membrane and enlarged mesangial volume[22]. The role of oxidative stress and consequently the production of free radicals in diabetic patients and their involvement in the pathogenesis of diabetes are extremely important[5]. In this research, the effect of F. vulgaris hydroalcoholic extract on histopathological changes in the renal tissue of STZ induced diabetic rats was studied.These results showed that diabetes increased the thickness of the Bowman’s capsule and decreased the number of glomeruli, while for diabetic group that received F. vulgaris extract, the diameter of the capsule was decreased and the number of glomeruli was increased compared with the diabetic control group. Therefore, it was assumed that STZ-induced diabetes through oxidative stress stimulates lipid peroxidation, increases lipid metabolism, degrades DNA, destroys cell membranes and ultimately leads to cell death[23].The consequences of the current study are in line with the outcomes of Alabdan et al. who showed that diabetes increases the expression of Bax protein and decreases the activity of the bcl2 anti-apoptotic protein in diabetic mice[24]. It seems that diabetes increases the production of ROS and superoxide by activating apoptotic pathways, which leads to a reduction in the number of glomeruli and ultimately an increase in the size of the Bowman’s capsule[25].Dong et al. showed that saponins can suppress the expression of apoptotic factors[26]. It appears that F. vulgaris increased the number of glomeruli and decreased the diameter of Bowman’s capsules compared to the diabetic group. The results of the current study also showed that serum levels of NO were significantly enhanced in the diabetic group, but with the addition of hydroalcoholic extract,a decrease was observed. Oxidative stress conditions, including diabetes, can express the inducible nitric oxide synthase (iNOS)mRNA and thus increase the amount of NO as an oxidizing agent[17].Increase in NO can induce apoptosis in diabetic rats via increasing the expression of bax gene and reducing the expression of bcl2 and bcl-xl genes[27]. Cytokines production may cause impairment in function and decrease the viability of insulin-producing beta cells by stimulating the iNOS enzyme and producing high levels of NO[28]. The outcomes of a research by Szkudeski et al. showed that induction of hyperglycemia by STZ causes the destruction of islets of langerhans beta cells by oxidant production and inappropriate NO production, and this result is in agreement with the outcomes of the current study[29]. Further, it has been assumed that saponins in the extract of F. vulgaris inhibit the synthesis of iNOS[17]. This result was confirmed by Jang et al. who showed that treatment with saponins extracted from Platycodon grandiflorus inhibited significantly the expression of iNOS protein[30]. It was observed that insulin levels were significantly increased and blood glucose decreased after treatment with F. vulgaris extract compared to the diabetic control group. It seems that the F. vulgaris extract prevents beta cell degradation, reduces NO levels and possesses inhibitory effect of STZ on insulin secretion[15]. Also, saponins in F. vulgaris extract can reduce glucose and increase insulin in the serum by inhibiting intestinal glucose uptake and enhancing the function and release of insulin from beta cells that have not been injured by STZ[15]. Yu et al. showed that saponins in astragaloside reduced significantly serum glucose in diabetic rats, and this is aligned with the results of the current study[31]. As regards the weight of rats, the results of the current study displayed that the mean of weights at the end of the study period in the diabetic control group had a significant reduction as compared with the normal control group. But in the F. vulgaris plus STZ groups, there was a significant increase in weight compared with the diabetic control group. During type 1 diabetes, since the cells cannot use blood sugar due to defective production of insulin, therefore, the diabetic animal will be very thin[32]. These results suggest that the improvement of weight loss in the groups treated with F. vulgaris extract can be attributed to its effect on increased insulin production and absorption[15]. The finding of Nimenibo-Uadia showed that administration of the extract of Vernonia amygdalina (the content of alkaloids, saponins and flavonoids) to diabetic animals resulted in the recovery of their lost weight. Thus, these results agree with the findings of the present research[33]. Based on the outcomes of the present study, BUN and creatinine levels in the diabetic control group displayed a significant rise as compared with the control normal group. However, in F.vulgaris plus STZ groups, there was a significant decrease in BUN and creatinine levels. Increased BUN and creatinine indicates glomerular damage, which leads to a decrease in renal excretion of these materials[34]. Diabetes seems to increase the production of ROS, causing damage to oxygenated mediators, altering glomerular filtration and increasing the permeability of the membrane[35].Saponins in F. vulgaris can decrease the effects of diabetes on BUN serum levels and creatinine in animals by inducing antioxidant enzymes, for example catalase and superoxide dismutase, and reducing ROS production[36]. The result of Usoh et al. displayed that administration of Gongronema latifolium (the content of saponins,tannins, alkaloids) reduced significantly creatinine, urea, BUN, in the blood serum of diabetic rats induced by STZ, which is similar to the findings of this study[37]. The results in this study similarly displayed that F. vulgaris is capable of decreasing lipid peroxidation and enhancing anti-oxidant capacity of renal tissue, therefore reducing oxidative stress. According to these conclusions, a great number of studies had revealed anti-oxidant properties for F. vulgaris[15-17].Therefore, it seems that F. vulgaris with its anti-oxidant property could decrease MDA and increase FRAPs in the treatment groups through preventing the production of ROS. The results of a study by Salahshoor et al. indicated that treatment of F. vulgaris in diabetic animals caused a significant increase in total antioxidant capacity and inhibition of oxidative stress production compared with diabetic group[17]. It was found that the extract of F. vulgaris can prevent the development of diabetic nephropathy.
In conclusion, the present study displayed that hydro-alcoholic extract of F. vulgaris can significantly improve renal impairment caused by STZ. Therefore, the extract of F. vulgaris could be considered as a potential and novel therapeutic agent. But,supplementary investigation in animal models is necessary to get additional conclusive indication for the molecular communication among F. vulgaris and diabetes mechanism leading to progressive renal destruction.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Funding
This research was supported by the Research Council of Kermanshah University of Medical Sciences in 2018 (Grant No.95201).
 Asian Pacific Journal of Tropical Biomedicine2019年4期
Asian Pacific Journal of Tropical Biomedicine2019年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimalarial activities of butanol and ethylacetate fractions of Combretum nigricans leaf
- GC-MS analysis and anti-mosquito activities of Juniperus virginiana essential oil against Anopheles stephensi (Diptera: Culicidae)
- Antioxidant compounds and capacities of Gac (Momordica cochinchinensis Spreng) fruits
- Keladi candik (Alocasia longiloba Miq.) petiole extracts promote wound healing in a full thickness excision wound model in rats
- Epidemiology, clinical features and transmission of re-emerging arboviral infection chikungunya
