基于穿透电极的Electro-peroxone技术降解布洛芬
崔欣欣,林志荣,王会姣,余 刚,王玉珏*
基于穿透电极的Electro-peroxone技术降解布洛芬
崔欣欣1,林志荣2,王会姣1,余 刚1,王玉珏1*
(1.清华大学环境学院,北京 100084;2.赣南师范大学地理与环境工程学院,江西 赣州 341000)
利用网状玻碳电极(RVC)作为阴极,构建了一种基于穿透电极的electro-peroxone(E-peroxone)反应器,并系统研究了其对布洛芬的降解性能,考察了电流、流速等因素的影响,进行了能耗计算.结果表明,E-peroxone可以在30min内完全去除初始浓度为2.5mg/L的布洛芬,而电化学氧化和臭氧氧化去除率分别为59%和64%.曝入气体流速为250mL/min,气相臭氧浓度为8mg/L的条件下,电流为100mA,反应溶液流速为300mL/min时, E-peroxone技术去除布洛芬的效率最高,且能耗(EEO)仅为传统臭氧氧化技术的1/7(0.76kWh/m3-log5.30kWh/m3-log).提高流速可以强化穿透电极E-peroxone体系中的传质,从而强化布洛芬的去除,并降低EEO.
Elecrtro-peroxone;穿透电极;网状玻碳电极;布洛芬
药物和个人护理品(PPCPs)对饮用水供应、人体健康和生态系统构成潜在的威胁[1,2].污水处理厂传统的处理方法难以有效降解PPCPs,导致很多PPCPs及其代谢物质在二沉池出水中仍能被检出[2-6].高级氧化技术具有良好的矿化效果[7-22],被广泛应用于水中难降解污染物的去除.但也存在一些缺陷,如电化学技术受电流和传质限制[16],臭氧氧化具有明显选择氧化性且中间产物多、能耗高[18-20], O3/H2O2(peroxone)技术由于需外部投加H2O2导致安全性较低[17].将臭氧与电化学技术耦合的electro- peroxone(E-peroxone)技术,可将O2电化学原位转化为H2O2(式(1)),进而强化O3生成具有强氧化性的×OH(式(2)),无选择性地快速氧化各类污染物,能够显著提高臭氧难氧化污染物的去除效率,有望成为污水处理厂中高效去除PPCPs及其中间产物的深度处理工艺[23-33].
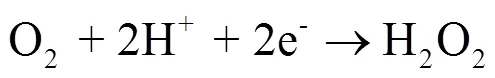
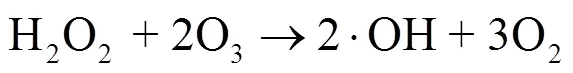
电化学氧化的反应机理决定了其受到污染物与活性物质在电极表面传质扩散的限制[16].目前E-peroxone技术中多采用平板电极,比表面积较小,污染物的传质效率较低.研究表明,使用多孔状的碳材料(如碳毡、碳布与碳纳米管等)充当电极,对有机污染物具有一定的吸附富集作用,且采用穿透电极模式可大大提高污染物及活性物质的传质扩散,从而提高污染物氧化速率[16,34-39].网状玻璃碳(RVC)的孔隙容积和比表面积巨大,流体流动阻力小, 导电性良好,有利于电化学反应过程中污染物的传质和转化[40].使用RVC电极作为E-peroxone系统中的穿透阴极,利用穿透电极流速越大,传质越好的特点,可强化O2、H2O2以及污染物在溶液与电极之间的传质,有望提高污染物去除效率和降低水处理能耗.因此,本研究构建了一种基于RVC穿透电极的E- peroxone系统,并对其去除水中布洛芬的性能进行了系统研究.
1 材料与方法
1.1 试剂与材料
采用典型的抗炎药物布洛芬作为目标污染物.为了准确检测污染物浓度变化以分析污染物的降解动力学和运行参数影响,在实验中采用了较高浓度的布洛芬初始浓度(2.5mg/L).实验所用布洛芬为分析纯级别,购于阿拉丁公司.实验中使用的其他试剂(如硫酸钠、磷酸氢二钠、硫酸等)均为分析纯,购于西陇公司.高效液相色谱(HPLC)所用流动相甲醇为色谱纯.试验所需所有溶液均由Thermo Scientific的高纯水系统产生的高纯水(阻抗18.2MΩ)配制.
1.2 实验装置
如图1所示,系统主要包括:内置阴阳电极的聚四氟柱形反应器、直流电源(LONG WEI PS-305DM)、臭氧发生器(Yanco INDUSTRIES LTD. OzoneLabTM Instrument OL80F/ DST)、臭氧检测仪、蠕动泵(LongerPump YZ1515x)等.反应器中采用RVC为阴极,钛镀钌铱为阳极,两电极平行放置,阴极在下、阳极在上,利用垫圈固定.采用半批次实验方式,利用蠕动泵使反应溶液以恒定流速流入反应器,并以恒定流速向反应器中曝气,进口处采用三通接头使气体和反应溶液同时进入反应器,反应器出水流回废水池.通过控制直流电源、臭氧发生器的启停可以分别对污染物进行单独臭氧氧化、单独电化学氧化以及E-peroxone技术处理.
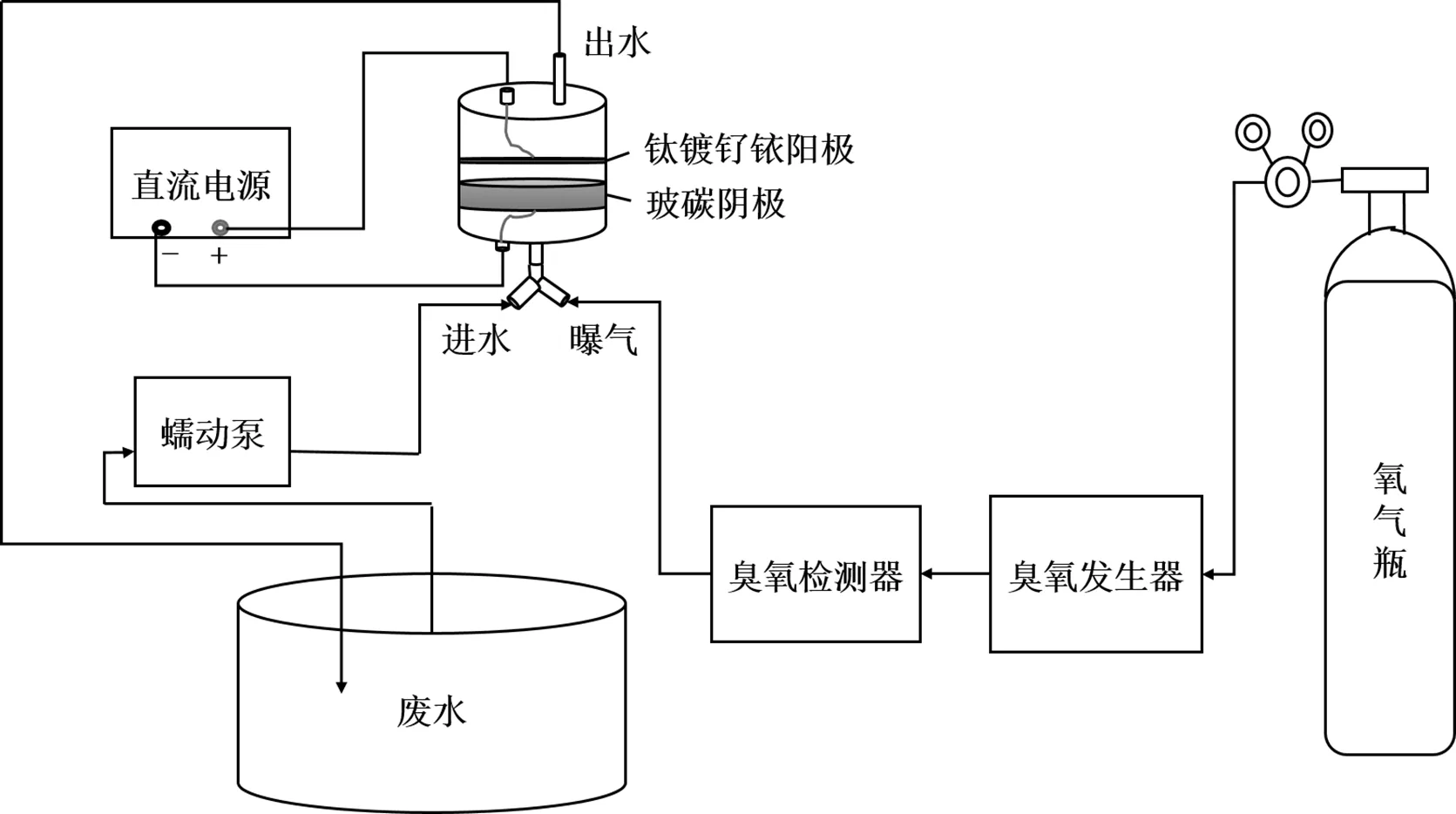
图1 穿透电极反应器示意
1.3 分析方法
溶液中过氧化氢浓度采用钛盐光度法测定,臭氧浓度采用indigo试剂法测定,布洛芬浓度通过高效液相色谱仪(Waters 2487 DualAbsorbance Detector; Waters 717 plus Autosampler; Waters 515HPLC Pump)测定[9].测定条件为:色谱柱Agilent TC-C18(2) (5μm,4.6mm×150mm);柱温30℃;检测波长220nm;流动相75%甲醇+25%高纯水(用2mmol/L醋酸铵和0.01%甲酸调节pH值,使pH = 4);流动相流速1mL/ min;进样体积50μL;运行时间10min.
1.4 运行参数
实验中运行参数如表1所示.

表1 实验运行参数
2 结果与讨论
2.1 RVC产过氧化氢性能研究
2.1.1 电流对RVC电产H2O2的影响 在E- peroxone过程中,H2O2的产生是影响处理效果的重要因素[41].在水处理过程中,废水在穿透电极反应器和水槽中循环流动,所曝入的O2进入水槽后与溶液充分接触混合.因此,溶解氧在整个处理过程中基本维持在与曝气中氧气浓度平衡的浓度(~42mg/L).由图2可知,在各电流条件下,反应体系中RVC电产H2O2的浓度与反应时间基本呈线性关系.当反应溶液流速为300mL/min时,电流由2.83mA/cm2提高至5.66mA/cm2时,20min后溶液中的H2O2浓度分别为 6.2和11.8mg/L,表明此电流范围内电化学产生H2O2的过程是受电流限制的.但是,当电流从5.66mA/cm2提高到14.15mA/cm2时,H2O2的浓度增长并不显著,表明电流超过5.66mA/cm2以后,电产H2O2的过程受到了O2向电极的传质限制.
RVC电产生H2O2的电流效率(CE(%))可由式(3)计算:

式中:为电化学反应转移的电子数目(本反应中=2);为法拉第常数(96486C/mol);H2O2为电化学反应过程中产生的H2O2浓度,mol/L;为反应溶液的体积,L;为由直流电源提供的通入电流的大小, A;为反应时间, s.
计算发现,在反应过程中电流效率先下降然后逐渐稳定.这是由于反应初期RVC内有一定的氧气,电流效率相对较高,随着反应进行和氧气的消耗,其他副反应增多,导致产H2O2的电流效率逐渐下降并趋于稳定.此外,图2显示,当电流由2.83mA/cm2增长至7.07mA/cm2时,产H2O2的电流效率略有降低,但进一步提高电流至14.15mA/cm2会导致电流效率显著下降.这是由于电流增大到一定程度之后,RVC电化学还原O2产生H2O2的反应变为受O2的传质限制,增大电流不能促进阴极产H2O2的反应,反而会增强H2O2在阳极和阴极的分解反应[41],导致观察到的产H2O2电流效率下降.
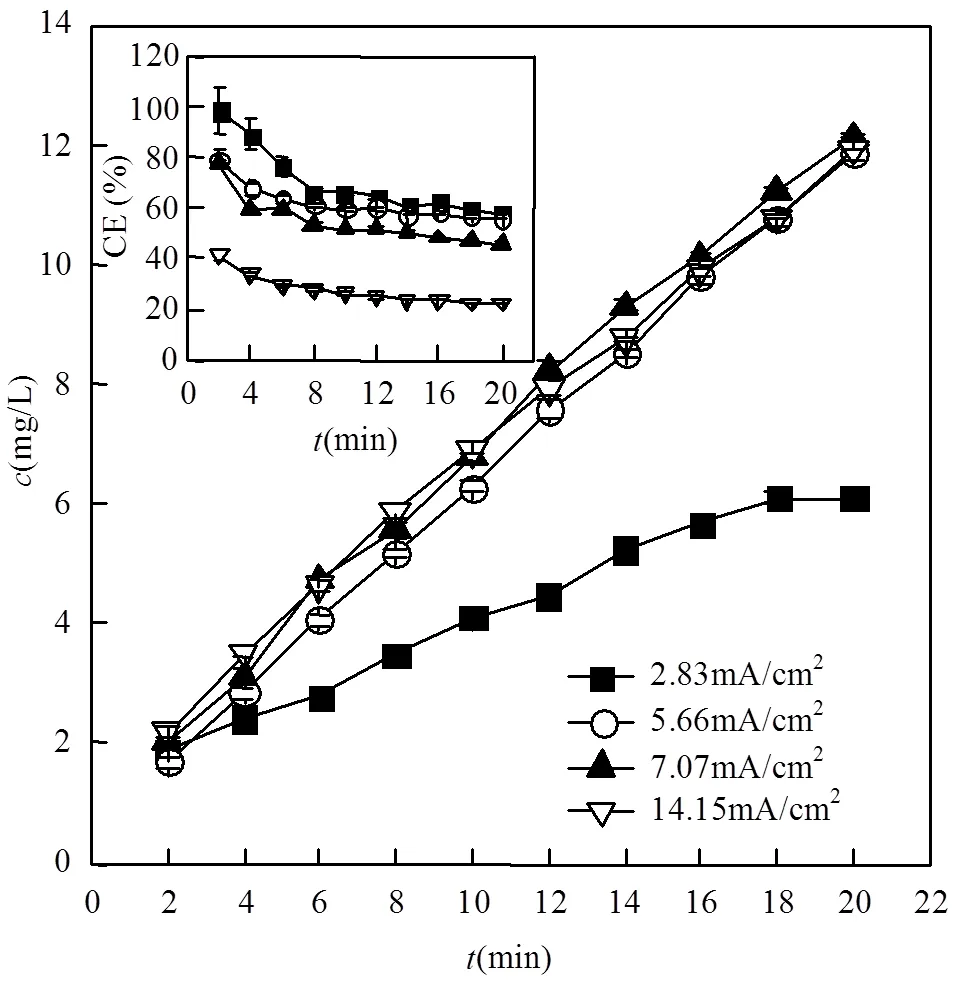
图2 电流对网状玻碳电极产H2O2的影响
内插图为产H2O2电流效率
2.1.2 进水流速对RVC电产H2O2的影响 由图3可知,产H2O2浓度随时间基本呈现线性增长趋势.进水流速增大,体系中H2O2浓度增加.外加电流为5.66mA/cm2,反应溶液流速分别为150和300mL/min时,反应20min后产H2O2浓度分别为9.0和11.8mg/L.这是由于反应溶液流速越大,对流增强,传质效果越好[43],有利于O2传质到电极并转化为H2O2.
RVC产H2O2电流效率随时间逐渐下降并趋于稳定.5.66mA/cm2条件下,反应溶液流速分别为150和300mL/min时,经过20min后,产H2O2电流效率分别为43%和56%.此结果表明,在穿透电极反应体系中,增大流速可改善电化学反应过程中氧气等活性物质的传质效果,从而提高电流效率.

图3 流速对网状玻碳电极产H2O2性能的影响
内插图为产H2O2电流效率
H2O2与O3的比例是影响×OH生成和污染物处理效果的重要因素.本研究中20min内曝入系统的O3剂量为0.083mmol/L,电流密度2.83~14.15mA/ cm2时产生的H2O2为0.072~0.143mmol/L, O3与H2O2物质的量比为5.84~11.57,如表2所示.与传统peroxone反应中报道的最佳O3与H2O2物质的量比(2:1)相比,E-peroxone系统的O3与H2O2物质的量比要高很多.这是由于在E-peroxone过程中,O3除了与H2O2反应生成×OH之外,还会在阴极发生还原反应产生O2或×OH等.本试验结果表明,电流密度为5.66~ 14.15mA/cm2, O3与H2O2物质的量比为6时,污染物去除效率较好.
表2 E-peroxone过程中O3与H2O2的剂量
Table 2 Dosage of O3and H2O2during the E-peroxone process

2.2 E-peroxone技术处理布洛芬废水的研究
2.2.1 E-peroxone技术与电化学氧化、臭氧氧化技术处理布洛芬废水效果的比较 如图4所示,实验发现,电化学氧化技术和臭氧氧化技术在经过30min的处理时间后对溶液中布洛芬的去除率分别为59%和64%.而E-peroxone技术在5min时即可实现62%的布洛芬去除率,15min时布洛芬去除率可达93%,25min时布洛芬基本被完全去除.

表3 电化学氧化、臭氧氧化和E-peroxone技术中布洛芬降解的反应速率常数及能耗(EEO)比较
注:电化学氧化过程中,布洛芬的降解在15min后基本停止,降解动力学不符合一级动力学,无法计算EEO[45];“-”表示未添加.
电化学氧化过程初期布洛芬降解速率较快,但反应10min后,去除速率明显下降.这可能是由于在布洛芬降解过程中生成了更容易电化学氧化的中间产物,在阳极与布洛芬发生竞争反应,抑制了剩余布洛芬的降解[23,44].臭氧氧化对布洛芬的去除能力有限,这是由于布洛芬分子只有一个微活化芳香环且没有与O3反应的活性基团(O3= 9.6L/(mol×s))[12].与之相比,E-peroxone过程中产生的大量×OH可以快速地氧化布洛芬(·OH= 7.4×109L/(mol×s)).
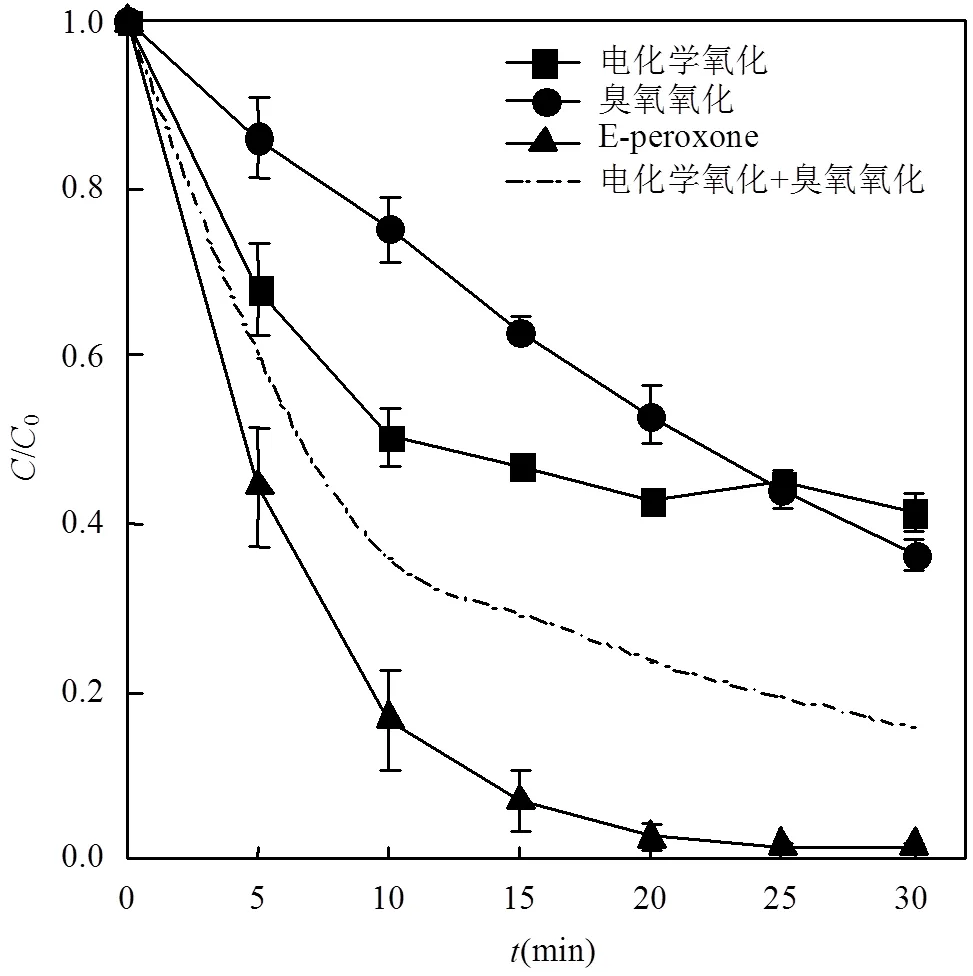
图4 单独电化学氧化、单独臭氧氧化和E-peroxone技术对布洛芬的降解情况
布洛芬初始浓度2.5mg/L;反应溶液体积400mL;气相臭氧浓度CO3= 8mg/L;气体流速250mL/min;反应溶液流速为300mL/min;电流5.66mA/cm2
对电化学氧化、臭氧氧化和E-peroxone过程中布洛芬的降解情况分别进行动力学拟合,其反应速率常数见表3.臭氧氧化与E-peroxone过程均符合一级反应动力学,电化学氧化过程分为0~10和10~30min两段分别进行一级动力学拟合.根据各处理过程的反应速率常数拟合电化学氧化与臭氧氧化加和的布洛芬降解曲线,如图4中虚线所示.可以看出,E-peroxone过程对布洛芬的去除效果明显优于电化学氧化加臭氧氧化,表明E-peroxone技术中电化学和臭氧氧化技术具有明显的协同作用,能够强化布洛芬的去除.增强因子(EF)被广泛用于评价处理过程的协同效应(式(4)).计算发现,E-peroxone过程对于电化学氧化和臭氧氧化具有明显协同作用,且处理10min后,由于单独电化学氧化受到抑制,协同作用明显增强,EF由1.68增长至4.19.
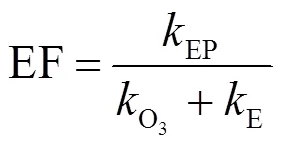
为探究×OH在布洛芬降解中的作用,根据布洛芬浓度变化曲线对×OH暴露量进行反算(式(5)),由图5可见,臭氧氧化与E-peroxone过程中×OH暴露量随时间基本呈线性增长趋势(2= 0.994~0.997),表明在该过程中×OH浓度基本保持稳定,其中,E-peroxne过程中×OH稳态浓度约为0.387×10-9mmol/L,约为臭氧氧化过程(0.077×10-9mmol/L)的5倍.因此,E- peroxone技术可以显著地强化布洛芬的去除.
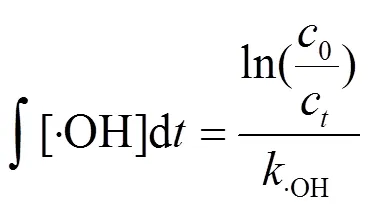
布洛芬初始浓度2.5mg/L;反应溶液体积400mL;气相臭氧浓度CO3= 8mg/L;气体流速250mL/min;反应溶液流速为300mL/min;电流5.66mA/cm2
2.2.2 电流对E-peroxone过程布洛芬处理效果的影响 如图6所示,随着电流的增大,E-peroxone技术对布洛芬的去除速率相应增加.电流为2.83mA/cm2时经过30min的处理时间布洛芬去除率为83%,5.66~14.15mA/cm2时在20min基本可实现布洛芬的完全去除.这是因为增大电流可以提高电极反应的速率,增加反应过程中原位产生的H2O2,并进而强化臭氧转化产生更多的×OH,高效地降解布洛芬分子.
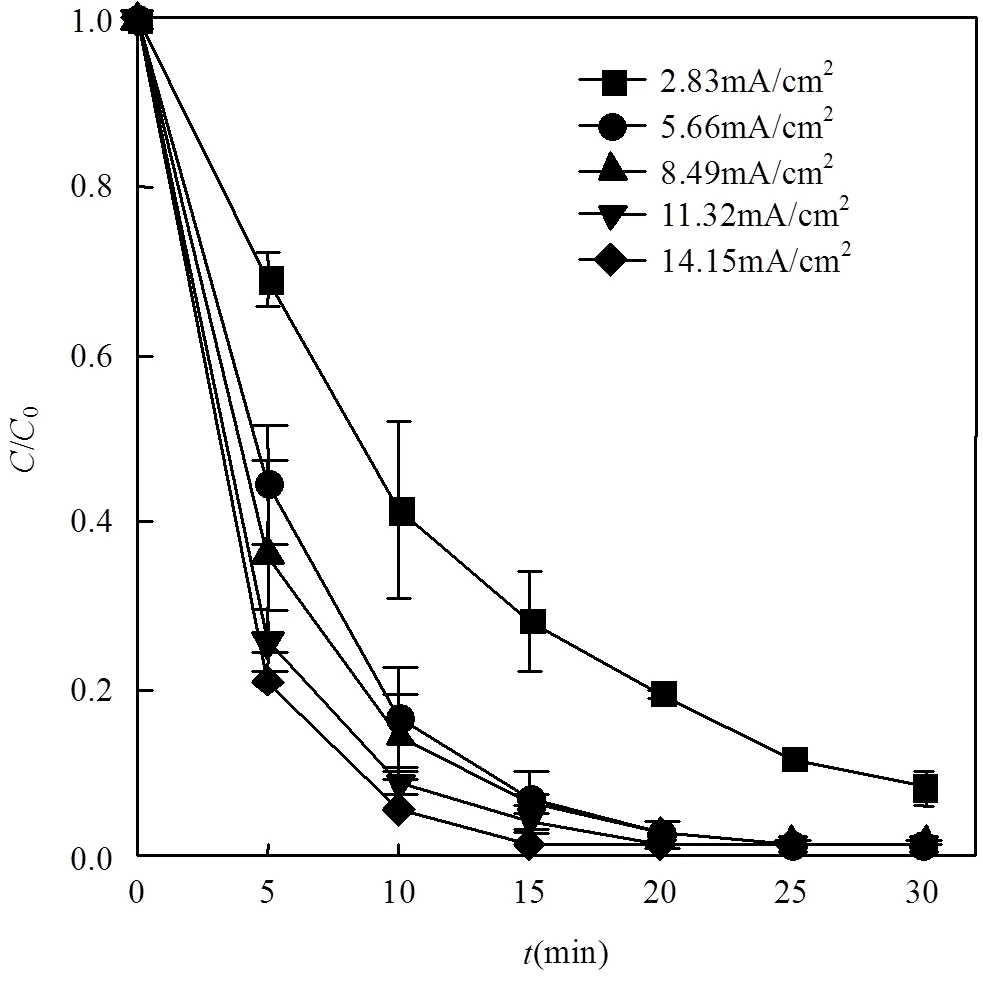
图6 不同电流条件对布洛芬废水处理效果的影响
布洛芬初始浓度2.5mg/L;反应溶液体积400mL;臭氧浓度CO3= 8mg/L;气体流速为250mL/min;反应溶液流速为300mL/min

图7 不同反应溶液流速条件对布洛芬废水处理效果的影响
布洛芬初始浓度2.5mg/L;反应溶液体积400mL;臭氧浓度CO3= 8mg/L;气体流速为250mL/min;电流为5.66mA/cm2
2.2.3 流速对E-peroxone过程布洛芬处理效果的影响 如图7所示,反应溶液流速由30mL/min逐步提高到300mL/min的过程中,布洛芬的降解速率逐步加快.反应溶液流速为300mL/min时,反应10min基本达到88%的去除效率,20min时基本实现布洛芬的完全去除.这表明,在穿透电极E-peroxone系统中,提高流速可以增强反应体系中的对流传质,可以强化污染物和活性物质在电极和溶液间的传质,促进电化学过程的进行,从而提高污染物的去除效率.
2.2.4 动力学拟合与反应速率常数计算 根据动力学拟合与计算,在E-peroxone过程中,布洛芬的降解为伪一级反应,其不同条件下的反应速率常数如表3所示.在E-peroxone过程中,随着外加电流增大,布洛芬降解的反应速率常数相应增大.此外,随着反应溶液流速增大,布洛芬降解的反应速率常数也相应增大.由此可以看出,穿透电极E-peroxone体系具有流速越大、传质越好的特点,在提高单位时间内处理水量的同时不会降低污染物的去除效率,在水量波动时能够很好地保证出水水质.
2.2.5 能耗计算 去除1m3水中某种污染物90%的浓度所消耗的能量(EEO, kWh/m3-log)被广泛用于比较各种技术的能耗和经济性[45].表3显示了臭氧氧化和E-peroxone技术中去除布洛芬的EEO(式(6)和(7))[33].

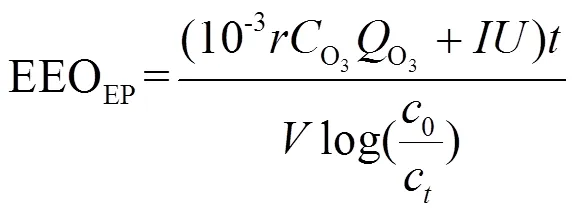
式中:是产生O3的能耗(15kWh/kg);CO3为曝入的混合气中气相臭氧的浓度,mg/L;O3为曝入气体的流速,L/min;为反应时间,h;为溶液体积,L;0与C分别为时间= 0和时刻的污染物浓度,mg/L;为外加电流,A;为平均电极电势,V.
在E-peroxone技术中,EEO随电流和流速的增大均呈减小趋势,电流为14.15mA/cm2,流速为300mL/min条件下EEO最小,为0.76kWh/m3-log.臭氧氧化技术的EEO为5.30kWh/m3-log,约为E-peroxone技术能耗的7倍.
以上结果表明,E-peroxone技术能比传统臭氧技术更加高效低耗地降解布洛芬.此外,与其他技术相比,E-peroxone技术处理也更加高效.杨丽娟等[46]利用Fenton法在40min实现布洛芬86%的去除,朱宏等[47]利用铁碳微电解法可在120min达到80%的布洛芬去除率,苏海英等[48]利用g-C3N4-10/TiO2复合材料光催化降解布洛芬在120min实现81.3%的去除率,活性污泥法处理24h最高只达14.76%的去除率[49]等.而E-peroxone技术在20min即可基本实现布洛芬的完全降解,是一种高效的处理技术.
在今后的研究中,将对低浓度布洛芬的降解进行研究,并对其降解途径和中间产物进行进一步分析.
3 结论
3.1 RVC产H2O2性能受电流和溶液流速影响.提高电流可以加快H2O2的产生速率,但超过一定电流范围后,会受到氧气的传质限制,并引起H2O2自分解增强,电流效率下降.流速增大,氧气等活性物质传质增强,有利于H2O2的产生.电流5.66mA/cm2,溶液流速300mL/min条件下,反应20minRVC产H2O2电流效率为56%.
3.2 E-peroxone技术基本可实现布洛芬的完全降解,且反应体系中布洛芬的降解受电流和溶液流速影响.电流增大,布洛芬降解速率越快,去除速率越高. 流速300mL/min条件下,电流密度14.15mA/cm2时布洛芬降解的反应速率常数最大,为0.284min-1.流速增大,布洛芬的降解更迅速,去除效率更高.电流密度5.66mA/cm2条件下,流速300mL/min时布洛芬降解速率常数最大,为0.173min-1.
3.3 E-peroxone技术进行水处理的EEO明显低于臭氧氧化技术,且流速越大,能耗越低.在溶液体积为400mL,气体流速为250mL/min、臭氧浓度8mg/L的情况下,最佳运行条件为,电流14.15mA/ cm2,溶液流速300mL/min,此时E-peroxone技术的能耗为0.76kWh/m3-log,仅为臭氧技术的1/7.
[1] Daughton C G, Ternes T A. Pharmaceuticals and personal care products in the environment: agents of subtle change [J]. Environmental Health Perspectives, 1999,107(Suppl 6):907-938.
[2] Esplugas S, Bila D M, Krause L G, et al. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents [J]. Journal of Hazardous Materials, 2007,149(3): 631-642.
[3] Carballa M, Omil F, Lema J M, et al. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant [J]. Water Research, 2004,38(12):2918-2926.
[4] Oller I, Malato S, Sánchez-Pérez J A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination--a review [J]. Energy Environmental Protection, 2012,409(20):4141-4166.
[5] 赵 琦,何小娟,唐翀鹏,等.药物和个人护理用品(PPCPs)处理方法研究进展[J]. 净水技术, 2010,29(4):5-10. Zhao Q, He X, Tang C, et al. Research progress on treatment processes of pharmaceuticals and personal care products (PPCPs) [J]. Water Purification Technology, 2010,29(4):5-10.
[6] Rossner A, Snyder S A, Knappe D R U. Removal of emerging contaminants of concern by alternative adsorbents [J]. Water Research, 2009,43(15):3787-3796.
[7] Brillas E, Sirés I, Oturan M A. Electro-Fenton process and related electrochemical technologies based on Fenton's reaction chemistry [J]. Chemical Reviews, 2009,109(12):6570.
[8] Wang Y, Li X, Zhen L, et al. Electro-Fenton treatment of concentrates generated in nanofiltration of biologically pretreated landfill leachate [J]. Journal of Hazardous Materials, 2012,229-230(3):115-121.
[9] 袁 实.电催化臭氧水处理技术的开发和研究 [D]. 北京:清华大学, 2014.Yuan S. The development of a novel Electro-peroxone technology for water and wastewater treatment [D]. Beijing: Tsinghua University, 2014.
[10] Klavarioti M, Mantzavinos D, Kassinos D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes [J]. Environment International, 2009,35(2):402-417.
[11] Matilainen A, Sillanpää M. Removal of natural organic matter from drinking water by advanced oxidation processes [J]. Chemosphere, 2010,80(4):351-365.
[12] Huber M M, Canonica S, Gunyoung Park A, et al. Oxidation of pharmaceuticals during ozonation and advanced oxidation processes [J]. Environmental Science & Technology, 2003,37(5):1016-24.
[13] Sirés I, Brillas E. Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review [J]. Environment International, 2012,40(40):212-229.
[14] Panizza M, Cerisola G. Direct and mediated anodic oxidation of organic pollutants[J]. Chemical Reviews, 2009,109(12):6541.
[15] Moreira F C, Soler J, Fonseca A, et al. Electrochemical advanced oxidation processes for sanitary landfill leachate remediation: Evaluation of operational variables [J]. Applied Catalysis B Environmental, 2016,182:161-171.
[16] Ji Y, Wang J, Jia J P. Improvement of electrochemical wastewater treatment through mass transfer in a seepage carbon nanotube electrode reactor [J]. Environmental Science & Technology, 2009, 43(10):3796-802.
[17] Rosal R, Rodríguez A, Perdigónmelón J A, et al. Removal of pharmaceuticals and kinetics of mineralization by O3/H2O2in a biotreated municipal wastewater [J]. Water Research, 2008,42(14): 3719-3728.
[18] Lee Y, Gerrity D, Lee M, et al. Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: use of kinetic and water specific information [J]. Environmental Science & Technology, 2013,47(11):5872-5881.
[19] Lee Y, Kovalova L, Mcardell C S, et al. Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent [J]. Environmental Science & Technology, 2013,47(11):5872-5881.
[20] Quero-Pastor M J, Garrido-Perez M C, Acevedo A, et al. Ozonation of ibuprofen: a degradation and toxicity study [J]. Science of the Total Environment, 2014,466-467(1):957-964.
[21] Tekle-Röttering A, Jewell K S, Reisz E, et al. Ozonation of piperidine, piperazine and morpholine: Kinetics, stoichiometry, product formation and mechanistic considerations [J]. Water Research, 2016,88(2): 960-971.
[22] 李启彬,张爱平,李 民,等.O3/H2O2降解垃圾渗滤液浓缩液的氧化特性及光谱解析[J]. 中国环境科学, 2017,37(6):2160-2172. Chen W, Zhang A, Li M, et al.Decomposition of organics in concentrated landfill leachate with ozone/hydrogen peroxide system: Oxidation characteristics and spectroscopic analyses [J]. China Environmental Science, 2017,37(6):2160-2172.
[23] Li X, Wang Y, Yuan S, et al. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process [J]. Water Research, 2014, 63(7):81-93.
[24] Bakheet B, Yuan S, Li Z, et al. Electro-peroxone treatment of Orange II dye wastewater [J]. Water Research, 2013,47(16):6234-6243.
[25] Yuan S, Li Z, Wang Y. Effective degradation of methylene blue by a novel electrochemically driven process [J]. Electrochemistry Communications, 2013,29(10):48-51.
[26] Qiu C, Yuan S, Li X, et al. Investigation of the synergistic effects for p-nitrophenol mineralization by a combined process of ozonation and electrolysis using a boron-doped diamond anode [J]. Journal of Hazardous Materials, 2014,280(280C):644-653.
[27] Li Z, Yuan S, Qiu C, et al. Effective degradation of refractory organic pollutants in landfill leachate by electro-peroxone treatment [J]. Electrochimica Acta, 2013,102(21):174-182.
[28] Frangos P, Wang H, Shen W, et al. A novel photoelectro-peroxone process for the degradation and mineralization of substituted benzenes in water [J]. Chemical Engineering Journal, 2016,286:239-248.
[29] Wang H, Yuan S, Zhan J, et al. Mechanisms of enhanced total organic carbon elimination from oxalic acid solutions by electro-peroxone process [J]. Water Research, 2015,80:20-29.
[30] Li Y, Shen W, Fu S, et al. Inhibition of bromate formation during drinking water treatment by adapting ozonation to electro-peroxone process [J]. Chemical Engineering Journal, 2015,264:322-328.
[31] Bakheet B, Qiu C, Yuan S, et al. Inhibition of polymer formation in electrochemical degradation of p-nitrophenol by combining electrolysis with ozonation [J]. Chemical Engineering Journal, 2014, 252(5):17-21.
[32] Wang H, Bakheet B, Yuan S, et al. Kinetics and energy efficiency for the degradation of 1,4-dioxane by electro-peroxone process [J]. Journal of Hazardous Materials, 2015,294:90-98.
[33] Yao W, Wang X, Yang H, et al. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process [J]. Water Research, 2016,88:826-835.
[34] Gonzálezgarcía J, Bonete P, Expósito E, et al. Characterization of a carbon felt electrode: structural and physical properties [J]. Journal of Materials Chemistry, 1999,9(2):419-426.
[35] Gonzálezgarcía J, Vicente Montiel A, Aldaz A, et al. Hydrodynamic behavior of a filter-press electrochemical reactor with carbon felt as a three-dimensional electrode [J]. Industrial & Engineering Chemistry Research, 1998,37(11):4501-4511.
[36] Liu Y, Liu H, Zhou Z, et al. Degradation of the common aqueous antibiotic tetracycline using a carbon nanotube electrochemical filter [J]. Environmental Science & Technology, 2015,49(13):7974- 80.
[37] Gao G, Pan M, Vecitis C D. Effect of oxidation approach on carbon nanotube surface functional groups and electrooxidative filtration performance [J]. Journal of Materials Chemistry A, 2015,3(14):7575- 7582.
[38] Yue Z R, Jiang W, Wang L, et al. Surface characterization of electrochemically oxidized carbon fibers [J]. Carbon, 1999,37(11): 1785-1796.
[39] Gao G, Zhang Q, Hao Z, et al. Carbon nanotube membrane stack for flow-through sequential regenerative electro-Fenton [J]. Environmental Science & Technology, 2015,49(4):2375.
[40] Friedrich J M, Ponce-De-León C, Reade G W, et al. Reticulated vitreous carbon as an electrode material [J]. Journal of Electroanalytical Chemistry, 2004,561(1/2):203-217.
[41] Kuo C H, Li Z, Zappi M E, et al. Kinetics and mechanism of the reaction between ozone and hydrogen peroxide in aqueous solutions [J]. Canadian Journal of Chemical Engineering, 2010,77(3):473-482.
[42] Zhou W, Gao J, Ding Y, et al. Drastic enhancement of H2O2electro- generation by pulsed current for Ibuprofen degradation: strategy based on decoupling study on H2O2decomposition pathways [J]. Chemical Engineering Journal, 2017,338:709-718.
[43] Liu H, Vecitis C D. Reactive transport mechanism for organic oxidation during electrochemical filtration: Mass-transfer, physical adsorption, and electron-transfer [J]. Journal of Physical Chemistry C, 2016,116(1):374–383.
[44] Ambuludi, Loaiza S, Oturan, et al. Kinetic behavior of anti- inflammatory drug ibuprofen in aqueous medium; during its degradation by electrochemical advanced oxidation [J]. Environmental Science & Pollution Research International, 2013,20(4):2381-2389.
[45] Bolton J R, Bircher K G, Tumas W, et al. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven systems (IUPAC Technical Report) [J]. Pure & Applied Chemistry, 2001,73(4):627- 637.
[46] 杨丽娟,胡 翔,吴晓楠.Fenton法降解水中布洛芬[J]. 环境化学, 2012,31(12):1896-1900. Yang L, Hu X, Wu X. Degradation of ibuprofen by Fenton oxidation [J]. Environmental Chemistry, 2012,31(12):1896-1900.
[47] 朱 宏,胡 翔.铁炭微电解法降解布洛芬的研究 [J]. 环境工程学报, 2013,7(5):1735-1738. Zhu H, Hu X. Study on degradation of ibuprofen by iron-carbon micro-electrolysis [J]. Chinese Journal of Environmental Engineering, 2013,7(5):1735-1738.
[48] 苏海英,王盈霏,王枫亮,等.g-C3N4/TiO2复合材料光催化降解布洛芬的机制[J]. 中国环境科学, 2017,37(1):195-202. Su H, Wang Y, Wang F, et al. Preparation of g-C3N4/TiO2composites and the mechanism research of the photocatalysis degradation of ibuprofen [J]. China Environmental Science, 2017,37(1):195-202.
[49] 汤 迎,虢清伟,洪澄泱,等.活性污泥去除4种典型药品的研究[J]. 工业水处理, 2016,36(2):63-66. Tang Y, Guo Q, Hong C, et al. Research on the removal of four kinds of typical medicine by activated sludge [J]. Industrial Water Treatment, 2016,36(2):63-66.
Effective degradation of ibuprofen by flow-through electro-peroxone process.
CUI Xin-xin1, LIN Zhi-rong2, WANG Hui-jiao1, YU Gang1, WANG Yu-jue1*
(1.School of Environment, Tsinghua University, Beijing 100084, China;2.Collage of Geographical and Environmental Engineering, Gannan Normal University, Ganzhou 341000, China)., 2019,39(4):1619~1626
By combining conventional ozonation with in situ electro-generation of hydrogen peroxide (H2O2) to enhance ozone (O3) transformation to hydroxyl radicals (×OH), the electro-peroxone (E-peroxone) treatment can significantly enhance the oxidation of ozone-refractory pollutants. A flow-through E-peroxone system was established using a reticulated vitreous carbon (RVC) as the cathode. The effects of main operational parameters (e.g., current and flow rate) on ibuprofen abatement were evaluated systematically. The results showed that the E-peroxone process could completely abate ibuprofen (initial concentration 2.5mg/L) in a synthetic solution in 30min, whereas conventional ozonation and electrolysis could only abated 64% and 59% of ibuprofen, respectively. The electrical energy consumption per log-order removal (EEO, kWh/m3-log) of ibuprofen by ozonation was 5.30kWh/m3-log, but was only 0.76kWh/m3-log by the E-peroxone process under the conditions of 100mA, 250mL/min gas flow rate, 8mg/L ozone and 300mL/min solution flow rate. Increasing the solution flow rate to increase the kinetics of electrode mass transfer, the rate of ibuprofen abatement could be further enhanced in the flow-through E-peroxone process. These results suggest that flow-through E-peroxone process may provide an effective and energy-efficient alternative for the abatement of refractory pollutants in water treatment.
electro-peroxone;flow through;reticulated vitreous carbon;ibuprofen
X522
A
1000-6923(2019)04-1619-08
2018-09-17
国家重大科技专项(2017ZX07202-001)
*责任作者, 副教授, wangyujue@tsinghua.edu.cn
崔欣欣(1993-),女,河北保定人,清华大学硕士研究生,主要研究方向为新兴污染物与高级氧化技术.

