Review on canine pyometra, oxidative stress and current trends in diagnostics
Rupali Rautela, Rahul Katiyar
1Division of Animal Reproduction, Indian Veterinary Research Institute, Izatnagar, Uttar Pradesh, 243122, India
2Division of Livestock Production, Indian Council of Agricultural Research Research Complex-North Eastern Hill Region, Umiam, Meghalaya, 793103, India
Keywords:Canine Inflammatory mediator Oxidative stress Pyometra
ABSTRACT Pyometra is one of the most important and common disorders in canine. The disease results in life threatening condition associated with septicemia and toxemia. The condition commonly occurs during luteal phase of estrous cycle, generally 4 weeks to 4 months of estrous cycle. The age, parity, breeds, administration of hormones are some predisposing factors of the condition. However, interaction between potentially pathogenic bacteria and hormonally primed uterus is believed to result in pyometra. The disease is initiated in the form of cystic endometrial hyperplasia, which later progresses into purulent uterine content following bacterial infection resulting in pyometra. The disturbance in antioxidant and prooxidant balance succeeding bacterial infection results in oxidative stress. The resultant stress further induces endometrial degeneration, immunosuppression and additionally aggravates the condition. An important clinical sign is malodorous, sanguineous or mucopurulent vaginal discharge with general symptoms such as vomiting, polyuria, polydipsia,depression, anorexia and occasionally fever. Till date, ultrasonography is the best diagnostic method while radiology and serological test are also helpful. Recently, the estimation of inflammatory mediators (acute phase proteins and prostaglandin metabolites) has appeared as marker for diagnosis of the pyometra. Determination of levels of inflammatory mediators has emerged as an important diagnostic method because it can be helpful in prognosis of the condition. The pyometra can be best treated by surgical manipulation (ovariohysterectomy);however, hormonal treatment can be given in less severe cases which aimed at reducing the effect of progesterone on the reproductive tract. Together, provision of antioxidants could be helpful in disease recovery along with the determined treatment. In this review, incidence,predisposing factors, etio-pathogenesis, oxidative stress associated with condition, diagnostic methods and treatment are discussed.
1. Introduction
Pyometra is an inflammatory condition of the endometrium resulting in the accumulation of pus inside the uterus. It is the most common and serious reproductive disorder that occurs during diestrus period in adult intact bitches[1,2]. Pyometra may be acute or chronic and is characterized by genital and systemic illness resulting in impaired homeostasis, hyperplasia of the endometrium,infiltration of inflammatory cells and accumulation of uterine exudates[3]. The condition succeeds following cystic endometrial hyperplasia (CEH) resulting in infertility either due to fertilization failure or early embryonic death[4]. The condition is thought to occur due to the invasion of opportunistic pathogen from vagina into the hormonally compromised uterus. The infection is established because of luminal accumulation of secretory fluids and the presence of numerous crypts and cysts where bacteria can proliferate along with local tissue degeneration. This association is reflected in the condition of cystic endometrium-pyometra complex. However, some reports suggest CEH and pyometra to be separate entity due to the differences in clinical and histological findings[5].
2. Incidence
The incidence of pyometra is reported to vary between 2.00%and 55.17%[6,7]. The incidence is very high in bitches of <10 years of age[8,9] and specifically between 6-8 years[7] (Table 1).

Table 1. Incidence of pyometra in various age groups of bitches.
3. Predisposing factors
A large number of factors like types of breed, age, parity and stage of estrous cycle may predispose pyometra in bitches. Among the breeds, Rottweiler, Saint Bernard, Chow Chow, Golden Retriever, Miniature Schnauzer, Irish Terrier, Spaniel and Collie are more susceptible, while German Shepherd, Daschund are less susceptible to pyometra[15,16]. In Indian conditions, breeds such as Spitz, Labrador, Alsatian, Doberman Pinscher, Boxer, Daschund,Rottweiler are more affected than Golden Retriever, Spaniel,Irish Terrier[7,17]. Other factors like parity also influence the risk of developing pyometra; being higher in nulliparous bitches[18]contributing about 75.00% to 77.78% of all pyometra cases[10,19].
The condition develops only during diestrus period of estrous cycle,more specifically between 20-70 days after heat[20]. However, an early report indicated that the condition most often occurs within 4 weeks to 4 months post-estrus[15]. Except the above factors,the use of hormones for estrus suppression, uterine biopsies,scarification and uterine irritants such as suture material are also the other contributing agents of endometritis-pyometra complex[21,22].Besides, anatomical abnormalities of genital tract such as stricture and septum of vagina or vestibule are also reported to predispose the condition in young bitches[23].
4. Etiology
The etiology of canine pyometra is still unclear. However,the hormonal aberration followed by bacterial infection plays an important role in the etio-pathogenesis of the disease[24,25].Occurrence of the disease during the diestrus period indicates the prime involvement of progesterone in the course of the development[26,27]. Such involvement is further supported by the observation of reduced progesterone level in recovered bitches post treatment with the drugs reducing progesterone level or blocking its action[26,28]. During pro-estrus and estrus period, estrogen primes the uterus by increasing uterine sensitivity to the progesterone[29]. On the other hand, some early reports suggested concurrent function of both the hormones during course of development of disease[30,31].
The organisms isolated from the uterus of pyometra affected cases are similar as reported from the normal microflora of the vagina of healthy bitches[32,33]. Bacteria like Escherichia coli (E. coli),Staphylococcus aureus, Streptococcus spp., Pseudomonas spp., and Proteus spp. were isolated from the affected bitches[27,34]. Besides,bacteria causing sub-clinical urinary tract infection can also result in onset of pyometra[35] (Table 2).
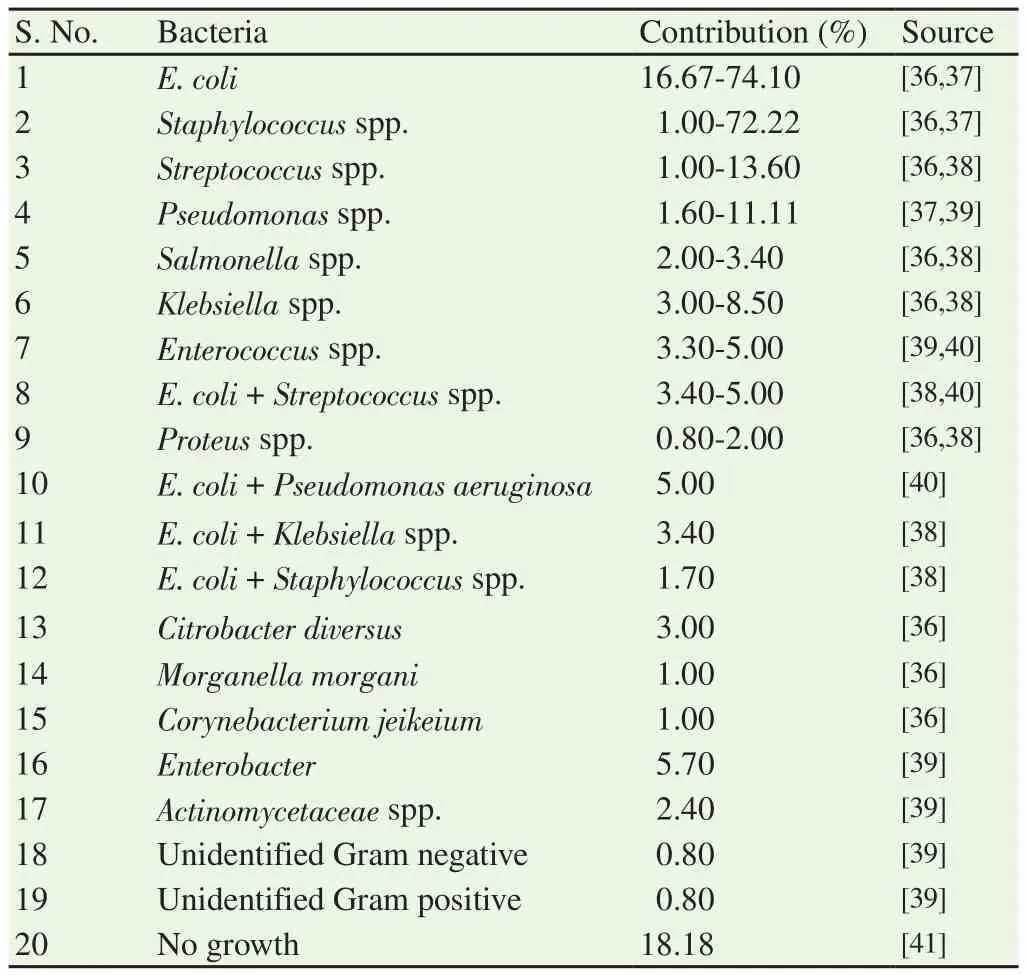
Table 2. Bacteria associated with canine pyometra.
5. Pathogenesis
The pathogenesis involves stimulation of the uterus by the estrogen which enhances the susceptibility of uterus. Abnormal estrogen level promotes over proliferation of endometrium and lengthens the period in which the uterine cervix remains open. This period is followed by prolonged interval of progesterone dominance during diestrus period. Progesterone causes endometrial proliferation with increased uterine glandular secretions and decreased myometrial contractions and closing of cervix[42]. These changes favor bacterial invasion to uterine lumen leading to pyometra[3,18]. These effects get cumulative after repeated estrous cycles, explaining the increased incidence in middle-aged to older bitches[23]. Further, progesterone induces development of endometrial receptors that allow the adhesion of the bacteria[43]. It is known that progesterone favors the expression of glycocalyx on apical cell surface of uterine epithelium. This is required for embryo recognition and implantation[43]. In CEH, these sugar residues are found in the uterine glandular regions together with epithelial structures and are binding targets for bacteria[44]. The adherence to endometrial glandular and epithelial cells is mediated by the fimbriae present on bacteria[45].
The hormonally compromised uterus becomes susceptible to infection by opportunistic bacteria that are derived from normal vaginal microflora. Bacteria proliferate in the lumen containing excessive secretary fluid. The presence of adhesive factors and cysts along with local tissue and leukocyte inhibition reducing local immunity favors bacterial growth. With each estrous cycle the effect gets cumulative resulting in the uterine pathology[15,46]. The lipopolysaccharide released from Gram-negative bacteria results in dysfunction of leucocytes. The lipopolysaccharide together with endotoxins released from Gram-positive bacteria[47] results in uncontrolled production of inflammatory mediators. These mediators cause irreversible damage to internal organs, sepsis and death of animal[27].
6. Oxidative stress and pyometra
The oxidative stress is a condition that occurs when there is an imbalance between pro-oxidants and antioxidants mechanisms of the body[48]. The imbalance is a consequence of an increased generation of free radicals and/or reduced physiological activity of antioxidant defenses against free radicals[49]. The oxidative stress has been reported to be associated with infectious conditions in various domestic animals including canines[41,50,51]. The high reactive oxygen species (ROS) level in animal makes them susceptible to infection due to poor immunity. These ROS damage epithelial cells barrier resulting in tissue injury, thereby making them susceptible to pathogen invasion. In addition, recently an association of oxidative stress and pyometra as a result bacterial infection has also been reported in equine species[52]. In response to infection and resultant inflammation, neutrophils activate oxidative metabolism resulting in“respiratory burst” and production of ROS. Generally, the ROS are involved in destruction of microorganisms. However, uncontrolled ROS production causes cellular damage in the surrounding tissue[53].The increased level of lipid peroxidation together with decreased level of antioxidants has been reported in pyometra affecting mare[52], rabbit doe[54] and bitch[55]. The reduced antioxidant levels indicate exhaustion of antioxidant system to counteract ROS leading to final state of oxidative stress.
6.1. Malondialdehyde (MDA)
ROS cause damage to cellular structure as a starter of lipid peroxidation. Various end products are formed during the radical induced decomposition of polyunsaturated fatty acid present on cell membrane. The MDA is one of the low-molecular weight end product formed during the oxidative damage[56,57]. It is one of the final and stable products of lipid peroxidation and therefore is a frequently used indicator of oxidative damage[58]. The high serum level of MDA in terms of thiobarbituric acid-reactive substances has been reported in pyometra affected than normal healthy bitches[59];however, the comparison was not significant. Similarly, high level of MDA has been reported in pyometra affected mares than healthy ones[52]. This decreased level of antioxidant in uterus of pyometra suspect high lipid peroxidation. In contrast, increased level of thiobarbituric acid-reactive substances in healthy than pyometra affected bitches has also been reported[55]. Therefore, more studies are required in this area.
6.2. Ascorbic acid (ASCA)
ASCA is a six-carbon ketolactone[60] which is considered as most important antioxidant in extracellular fluids[61]. It forms an important component of the non-enzymatic antioxidant defense system of the body as a chain breaking antioxidant[62,63]. The estimation of blood ASCA gives indirect assessment of antioxidant status of the body,since it is capable of regenerating other antioxidants to their active state[64]. The decreased level of ASCA has been reported in uterine infection in cow[65], buffalo[50]; however, in canines the reports are not available yet.
6.3. Superoxide dismutase (SOD)
SOD is one of the endogenous antioxidant enzyme which forms first line of defense against free radicals. The increased level of SOD also protects cells against toxic oxygen radicals during oxidative stress by scavenging both intra and extracellular superoxide radicals to form hydrogen peroxide and oxygen[66]. The decreased level of SOD has been found in uterine infection by various workers[52,65].
Similarly, low serum level of SOD in bitches and mares suffering with pyometra as compared to healthy ones has been reported[52,55,67,68] which indicates impaired antioxidant system.This is attributed to depletion of antioxidant enzymes in order to counteract the oxidative stress generated as a result of ROS.
6.4. Catalase (CAT)
CAT is a homotetramer with a molecular mass of 240 kDa and is widely distributed within the cell[69]. Catalase is believed to play an essential role in control of hydrogen peroxide (H2O2) generation. It catalyses the breakdown of H2O2into H2O and O2and requires iron as a cofactor[70]. The decreased serum level of CAT[55,68] indicates impaired antioxidant defense mechanism in pyometra affected bitches. Further, increased serum level of the enzyme following treatment provides indication of occurrence of oxidative stress in such bitches[67].
7. Diagnosis of pyometra
Canine pyometra can be diagnosed on the basis of case history,clinical examination, and laboratory analyses, often combined with radiography and/or ultrasonography of the uterus and ovaries[71].
7.1. Case history
Case history is important in preliminary diagnosis of pyometra[72].The definitive and important history includes history of estrus 4 weeks to 4 months back[15]. Often, there is a history that bitches have never been mated. The history includes onset of most important clinical signs such as polydipsia, polyuria, together with vomition,lethargy, depression and inappetance[18].
7.2. Clinical examination
The findings during clinical examination depend upon the patency of cervix. In open-cervix pyometra, the most important finding is presence of foul-smelling, sanguineous, mucopurulent discharge[73,74]. The discharge can be observed in 80% bitches suspected for pyometra[3]. Sometimes, the amount of discharge is very less and fastidious grooming makes it difficult to detect.Systemic signs such as vomition, depression, lethargy, polydipsia,polyuria are less in bitches with open-cervix pyometra as compared to close-cervix pyometra[27,31]. In contrast, closed-cervix pyometra is regarded as medical emergency[15]. Bitches are very ill at clinical presentation and show marked systemic signs and distended abdomen. Affected bitch are dehydrated, hypothermic due to onset of toxemia[75].
7.3. Systemic inflammatory response syndrome (SIRS)
The SIRS or sepsis is a syndrome associated with infectious,inflammatory, traumatic, or neoplastic foci that causes production and release of inflammatory mediators affecting body systemically.The condition occurs when circulatory shock is superimposed on sepsis and is often associated with canine pyometra[76]. In canines,SIRS can be clinically identified by the presence of any two of four criteria[77], as followings: heart rate >120 bpm, temperature>103.5 ℉ or <100.0 ℉, respiratory rate >20 breaths/min, white blood cell count >16 000/µL or <6 000/µL, band neutrophils >3%.
The above SIRS criteria may serve as screening test for clinical monitoring of the affected animals. These are not so accurate to be considered definitive but can be used as guidelines along with clinical judgement until specific markers for SIRS are identified[77,78].
7.4. Haematological findings
Leukocytosis and neutrophilia with shift to left and toxic degeneration of neutrophils and monocytes are the most common observation[1,79]. The blood leukocyte concentration increases to 15 000-60 000/mm3[20] and is more pronounced in closed-cervix pyometra. In chronic cases, normocytic and normochromic anemia reflects decreased erythropoiesis due to bone marrow affection[80].Thrombocytopenia may occur due to bone marrow affection by toxins[13].
7.5. Blood biochemistry
The most consistent finding in pyometra is elevated levels of alkaline phosphatase, which can be present in 50%-75% cases[18].Together with this, increased bilirubin and cholesterol levels indicate intrahepatic cholestasis. Renal malfunction is also a common finding in severe cases and can be evident as alteration in acidbase balance and electrolyte levels in the body. Increased serum alanine aminotransferase concentrations reflect hepato-cellular damage either due to toxemia or diminished hepatic circulation due to dehydration. Hyperproteinemia occurs as a result of increased production of gamma-globulins in response to antigen[78,81].
7.6. Ultrasonography
Ultrasonography reveals an enlarged uterus with convoluted, tubular horns containing anechoic or hypoechoic fluid with thickened endometrium[82,83]. The luminal contents are usually homogenous and echo-dense due to pus particles[84]. The presence of cystic structures with thickened endometrium is diagnostic for CEH with or without pyometra[23]. Recently, blood flow in uterine artery using Doppler ultrasonography has been used for diagnosis of pyometra.A high blood rate and low vascular resistance has been reported in bitches suffering from pyometra than normal diestrus ones[85].
7.7. Radiography
In radiography, detection of soft tissue opacity mass in the caudal abdomen causing the cranial displacement of small intestine and dorsal displacement of colon can be observed[86]. The radiography can be used as an aid in diagnosing pyometra in the bitch, but generally is inconclusive. This is due to similar radiographic characteristics of mucometra and uterine torsion with pyometra[87,88].
7.8. Histological examination
Histological examination of affected uterus represents cystic endometrial glands, increased endometrial thickness, aggregation of inflammatory cells and bacterial colonies in the glandular and uterine luminal areas. The endometrial hyperplasia occurs due to cystic deformation of glands and glands deformation along with fibroblast proliferation in stroma, inflammatory reaction in CEH and endometritis/pyometra cases, respectively. These stromal fibroblasts are expected to play a part in pathogenesis of endometritis/pyometra because of their sex hormone receptor pattern[89]. The inflammatory cells observed include neutrophils, macrophages and lymphocytes.Similarly, the degenerated endometrial cells are characterized by large cytoplasmic vacuoles, reduced size with degenerative morphology of nucleus[90].
7.9. Inflammatory mediators
Inflammatory mediators are the biochemical mediators that are released during inflammation. The inflammatory mediators attract and activate phagocytic cells to remove microbes,promote tissue repair and resolution of inflammation[91]. In general, the inflammatory mediators in general circulation act as indicator for systemic inflammation. The blood concentration of inflammatory mediators such as acute-phase proteins (APP) and prostaglandin (PG) metabolites reflects the intensity of infection or inflammation[92,93]. The release of inflammatory mediators is initiated by endotoxin from Gram-negative bacteria during canine pyometra[94-96]. On invasion of E. coli, the lipopolysaccharide(endotoxin) is released from the cell wall of the organism causing dysfunction of neutrophils and macrophages leading to excessive synthesis and release of pro-inflammatory cytokines and secondary inflammatory mediators[27,97]. The pro-inflammatory cytokines further induce the biosynthesis of APP in hepatocytes to regulate the inflammatory response[98]. Similarly, endotoxins are potent stimulators of PGs’ release[93,99]. In a word, blood concentration of inflammatory mediators reflects the intensity of infection or inflammation[92,93,100].
7.9.1. APP
An APP is an inflammatory cytokine that removes the damaging factor, limits inflammation, and restores homeostasis of the body[101]. Infection triggers increased production of proinflammatory cytokines from macrophage and neutrophils as immunological response which further induces transcription factors for APP[102,103]. The blood concentration of APP reflects the level of inflammation[104] and involves the earliest changes that occur during infection or inflammation in animals including canine[101]. Hence, it could be helpful in diagnosis and prognosis of disease condition in the species[105,106]. The plasma concentration of APP may increase(positive APP) or decrease (negative APP) by at least 25% during inflammation[107]. The changing pattern in the production from hepatocytes is responsible for such altered concentration of APP in the blood[108].
Recently, much attention has been paid to the use of APP as reliable inflammatory marker in both human and animal studies[109,110]. In dogs, the main APPs constitute C-reactive protein (CRP), serum amyloid A (SAA) component, haptoglobin (Hp) and albumin. It has been evidenced that the levels of APP in peripheral blood provide valid indication of the inflammatory state of the uterus and thus provide future direction to assess the value of those markers in prognosis of pyometra[111].
7.9.2. CRP
The CRP is an acute-phase serum protein synthesized primarily in the liver as part of acute-phase response[112]. The chief stimulus responsible for synthesis of CRP is interleukin (IL)-6 and the synthesis is further enhanced by IL-1β[113]. The primary role of CRP is to regulate the acute inflammation together with protective action against bacterial infection[114]. The CRP is considered as classical APP as it appears in high concentration during early stage of any kind of infection. However, it remains undetectable during normal healthy condition. Similarly, CRP increases markedly during pyometra and can be used as prognostic marker for the disease[115].Further, decrease in CRP after ovariohysterectomy in pyometra affected bitches is associated with good clinical outcome[116].Therefore, it has been used for detection of acute infection and also to check the treatment response[114].
7.9.3. SAA
The SAA is another APP released from liver during inflammation in response to cytokines such as IL-6, IL-1, tumor necrosis factor,interferon-γ, and transforming growth factor-β[117]. The concentration of SAA increases dramatically during tissue inflammation and injury up to 1 000-fold greater than normal[118]. The SAA attracts phagocytes to the site of inflammation, increases expression of inflammatory cytokines and prevents apoptosis of neutrophils and thus involves resolution of inflammation[119]. On the other hand,SAA is a precursor of protein amyloid A, an insoluble product that gets deposited in internal organs and increases risk of organ failure and death[117].
The SAA is reported to be valuable in identification of malignancy associated with inflammation in humans[120,121]. Similarly, in canines SAA is reported to be more specific marker for systemic inflammation[122,123] and hence can also be used for diagnosis and prognosis of diseases in veterinary medicine. Recent study has reported high level of SAA in pyometra affected than healthy bitches[124] and queen[125]. From the above, it appears that SAA can be used an indicator of severity of inflammation together with the prognosis of the disease condition.
7.9.4. Hp
The Hp is an APP associated with scavenging of hemoglobin released from red blood cells. The IL-6, IL-1, and tumor necrosis factor are the major inducers of the protein[126]. The level of Hp increases to many folds during local or systemic infection and trauma or inflammation. The Hp is known to quench ROS released from neutrophils and hence protect the cells from oxidative damage[127].Also, Hp inhibits cyclooxygenase enzymes in order to restrict the inflammation[128]. Previous reports have indicated its association with infectious and non-infectious inflammatory diseases[129-131].The increased level of Hp in pyometra has been reported in bitch[124]and queen[125] indicating a good candidate for routine diagnostic for the condition.
7.9.5. Albumin
Albumin is considered to be a negative APP as the serum concentrations decrease in inflammation and/or infection. It likely occurs due to decreased production by the liver and/or increased vascular permeability that may lead to extravasal accumulation of albumin or loss through kidneys[13]. This hypoalbuminemia has been associated with endotoxin released from Gram-negative bacteria[132]and bacteremia during canine pyometra[133]. The decreased concentration of serum albumin in pyometra affected bitches[124]and queen[125] has been reported. Therefore, albumin might have a diagnostic value in dogs with pyometra as a negative prognostic marker for survival but require future studies[71].
7.9.6. PG metabolites
PG is considered as a reliable and sensitive marker of endotoxemia and inflammatory condition[116,134]. The systemic release of PG can be assessed by measuring its more stable main circulating metabolite that is PG-metabolite. The prognostic value of PG metabolites is reported to be indicator of sepsis and morbidity of pyometra affected bitches. Earlier studies have reported an increase in PG-metabolite concentration in pyometra affected bitches as compared to healthy ones[78,124]. Therefore, it is suggestive to develop test for rapid detection of PG-metabolite which would be clinically very useful in the detection of severity of the condition. Further, it would enable clinicians to decide whether to postpone surgery or emergency ovariohysterectomy is required[116] or medical treatment is sufficient as a treatment.
The prediction or prognosis of disease using various laboratory parameters is currently difficult and hence requires future exploration.To date, no studies are available to estimate the increase level of APP(except albumin) and PG-metabolite in pyometra affected bitches at pre-treatment. Therefore, studies are required to be focused on identification of clinical biomarkers with high sensitivity and specificity to predict the future outcome of the pyometra[71]. Also, it will be valuable to facilitate the decision making for optimal therapeutic regime and to minimize complications post-treatment[116].
8. Treatment
8.1. General treatment
The general treatment including use of antibiotics, fluid therapy along with this oral supplementation of antioxidants could be helpful. Broad-spectrum antibiotics should be administered concomitantly with specific treatment protocol. It is necessary to determine anti-microbial activity against the bacteria isolated from vaginal discharge before initiation of antibiotic therapy. However,earlier clinical evidences have suggested cephalosporins, potentiated sulfonamides or amoxicillin either alone or in combination with clavulanic acid effective[18,135]. It is recommended to re-evaluate the animal by ultrasonographic examination 2 weeks after the completion of the treatment and if vaginal discharge, fever or neutrophilia is still present, a prolonged course of antibiotic therapy is recommended[18].The bitches should be medically stabilized with appropriate intravenous fluid therapy. The fluid therapy is essential in order to ensure correction of dehydration, minimum renal toxic effects[75].The required fluid therapy can be assessed by 60 mL/kg + % dehydration ×body weight/100[42]. The complications associated with septicemia and uremia are common; therefore, attention should also be given to plasma electrolytes and acid-base status of the animal.
Immunosuppression is a common finding in various bacterial and viral infections including canine pyometra[42,136]. This may be induced due to endotoxemia as a positive relation between endotoxins and reduced immune function has been reported[136].Therefore, it is necessary to consider immunostimulants (in form of antioxidants) in the therapeutic protocol to manage immunocompromised bitches affected with pyometra.
8.2. Specific treatment
The specific treatment includes surgical therapy by ovariohysterectomy or medical therapy using hormones.
8.2.1. Surgical management
The ovariohysterectomy is considered as the most common and safe treatment of canine pyometra[137]. Even though ovariohysterectomy is considered safest, post-surgical mortality is reported to be 5%-25%[71,138]. The effects of anesthesia and surgical procedure may be hazardous in bitches suffering with severe organ malfunctions[139].Post-surgical complications that may arise include peritonitis, septic shock, disseminated bacterial infection and hemorrhage[140]. The metabolism of anesthetic agents, hypothermia due to blood loss and tissue manipulation during surgery are the factors resulting in oxidative damage in the body. The previous studies have reported the increased serum level of MDA and decreased level of antioxidants(glutathione,β-carotene and retinol) after ovariohysterectomy in healthy bitches[141,142]. This reduced level of antioxidants and increased level of lipid peroxidation indicate the state of oxidative stress after undergoing surgical manipulation.
8.2.2. Medical management
Medical or hormonal therapy can be given if the condition is not life-threatening or owner desires to breed the bitch in the future. Some objectives should be considered while deciding the hormonal protocols[18] and these are: 1) prevention of the effect of progesterone; 2) promotion of the expulsion of uterine content; 3)inhibition of bacterial growth or their lysis.
8.2.2.1. Dopamine agonist/Anti-prolactin drugs
Prolactin is luteotropic in bitch hence use of anti-prolactin results in rapid reduction in the level of blood progesterone[143]. Dopamine agonists such as Bromocriptine and Cabergoline are substantially used for the treatment of pyometra. Bromocriptine at 20 µg/kg or Cabergoline at 5 µg/kg can be used either alone or in combination with PG.
8.2.2.2. Progesterone receptor antagonist/ Antiprogestin
Antiprogestin includes aglepristone that competitively binds with progesterone receptors and blocks its biological activity. Injection of aglepristone at 10 mg/kg subcutaneously on day 1, 2 and 8 or on day 15 has been reported to be effective in both open and closedcervix pyometra, if uterine lumen is still visible on ultrasonographic examination[42]. Previous report indicated 100% effectiveness,95.7% and 100% complete evacuation of uterine content within 14 and 21 days post-treatment, respectively. Previous study also reported that the drug can solely be responsible for evacuation of uterine content[137]. It has been assumed that aglepristone blocks the progesterone receptors and increases the myometrial contractibility.The myometrial contractibility occurs due to the secretion of endogenous PG as a consequence of inflammation[144].
8.2.2.3. PG
PG has both luteolytic and uterotonic properties which were utilized for the treatment of pyometra. Therapeutic protocol includes intramuscular injection of 100-250 µg/kg of natural or 10 µg/kg body weight of synthetic PG for 5-7 days. Second series of treatment can be given 10 days later if complete evacuation of pus and uterine diameter has not decreased on ultrasound examination. The efficacy of the treatment has been reported to be 75%-90%[74]. However,side-effects are associated with the use of natural PG which include panting, salivation, vomiting, straining, diarrhoea, pyrexia[145] and risk of uterine rupture in closed-cervix pyometra[146]. These can be prevented by administration of atropine 15 min prior to PG injection,use of synthetic PG or progressive administration of PG dosage.Earlier study has reported the injection of 0.10 mg/kg on day 1, 0.20 mg/kg on day 2 and then 0.25 mg/kg to check the sideeffects[74]. Also, intravaginal infusion of PG at 0.15 mg/kg(0.30 mL/10 kg) has been found successful in 86.6% cases[78]. No side-effect has been found through this protocol and hence generates new therapeutic possibilities. However, further trials for validation are recommended[18,147].
8.2.2.4. Drugs in combination
The drugs or hormones in combination can be used to increase the efficacy of the treatment. Two subcutaneous injections of aglepristone at 10 mg/kg 24 h apart followed by a second injection 8 days later or third injection after 15 days if required can be given on combination with PG at 1 µg/kg from 3 to 7 days of treatment. The combination increases the success rate from 60% (when aglepristone was used alone) to 84% on day 90 post-treatment[42]. Similarly,combination of dopamine agonist and PG has synergistic action on luteolysis[148,149]. Cabergoline at 5 µg/kg once a day orally for 7 days can be combined with PG injections. The protocol resulted in decreased serum progesterone and cervical relaxation within 24-48 h as compared to single PG treatment[18].
9. Conclusion
Despite being various treatment regimens followed to resolve the condition, the mortality rate is considerable in canine pyometra.The generation of oxidative stress is also a part of concern due to its affection in systemic functions resulting mortality of animal. Further it should not be ignored that the susceptibility of animal to pyometra could also be due to poor antioxidant mechanism of the body. The lack of data in available literature emphasizes more studies on association between oxidative stress and pyometra. Additionally, the marker for detection of the presence of SIRS in canine pyometra is also needed. The APP and PG metabolites are considered as good candidates. However, there is a need to settle on their threshold values in order to differentiate pyometra with other systemic condition. The studies on marker based of prognosis of pyometra should be focused which could be helpful to decide whether immediate surgical intervention is required or medical therapy would be sufficient. Further, future studies on association between the biomarker and oxidative stress are required to assess prognosis of the condition. An early diagnosis followed by appropriate intervention is requisite. More researches in the area to develop treatment regimens should be emphasized and levels of biochemical markers should be estimated in order to evaluate the treatment response. Also, there is a need to advocate immunotherapy along with specific treatment of pyometra to improve outcome of the disease condition.
Conflict of interest statement
The authors declare that there is no conflict of interest.
 Asian Pacific Journal of Reproduction2019年2期
Asian Pacific Journal of Reproduction2019年2期
- Asian Pacific Journal of Reproduction的其它文章
- Effect of combination of Gynura procumbens aqueous extract and Trigona spp. honey on fertility and libido of streptozotocin-induced hyperglycaemic male rats
- Effect of Vitex agnus-castus plant extract on polycystic ovary syndrome complications in experimental rat model
- Improvement of Phaseolus vulgaris on breastfeeding in female rats
- Oestrous cycle of Wistar rats altered by sterol and triterpenes rich fraction of Adansonia digitata (Linn) root bark - A scientific rationale for contraceptive use
- Comparison of p38 MAPK, soluble endoglin and endothelin-1 level in severe preeclampsia and HELLP syndrome patients
- Secondary sex ratio of assisted reproductive technology babies
