Methods of computed tomography screening and management of lung cancer in Tianjin: design of a population-based cohort study
Yihui Du, Yingru Zhao, Grigory Sidorenkov, Geertruida H. de Bock, Xiaonan Cui, Yubei Huang,Monique D. Dorrius, Mieneke Rook, Harry J. M. Groen, Marjolein A. Heuvelmans, Rozemarijn Vliegenthart, Kexin Chen, Xueqian Xie, Shiyuan Liu0, Matthijs Oudkerk, Zhaoxiang Ye
1Department of Epidemiology, University of Groningen, University Medical Center Groningen, Groningen 9713 GZ, The Netherlands; 2Center for Medical Imaging-North East Netherlands, University of Groningen, University Medical Center Groningen, Groningen 9713 GZ, The Netherlands; 3Department of Radiology,Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China; 4Department of Epidemiology and Biostatistics, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China; 5Department of Radiology,University of Groningen, University Medical Center Groningen, Groningen 9713 GZ, The Netherlands; 6Department of Radiology, Martini Hospital, Groningen 9728 NT, The Netherlands; 7Department of Pulmonary Diseases, University of Groningen, University Medical Centre Groningen, Groningen 9713 GZ, The Netherlands; 8Medisch Spectrum Twente,Department of Pulmonology, Enschede 7512 KZ, The Netherlands; 9Department of Radiology, Shanghai General Hospital,Shanghai Jiao Tong University School of Medicine, Shanghai 200080, China; 10Department of Radiology, Shanghai Changzheng Hospital, The Second Military Medical University Shanghai, Shanghai 200003, China
ABSTRACT Objective:European lung cancer screening studies using computed tomography (CT) have shown that a management protocol based on measuring lung nodule volume and volume doubling time (VDT) is more specific for early lung cancer detection than a diameter-based protocol. However, whether this also applies to a Chinese population is unclear. The aim of this study is to compare the diagnostic performance of a volume-based protocol with a diameter-based protocol for lung cancer detection and optimize the nodule management criteria for a Chinese population.Methods:This study has a population-based, prospective cohort design and includes 4000 participants from the Hexi district of Tianjin, China. Participants will undergo low-dose chest CT at baseline and after 1 year. Initially, detected lung nodules will be evaluated for diameter and managed according to a routine diameter-based protocol (Clinical Practice Guideline in Oncology for Lung Cancer Screening, Version 2.2018). Subsequently, lung nodules will be evaluated for volume and management will be simulated according to a volume-based protocol and VDT (a European lung nodule management protocol). Participants will be followed up for 4 years to evaluate lung cancer incidence and mortality. The primary outcome is the diagnostic performance of the European volume-based protocol compared to diameter-based management regarding lung nodules detected using low-dose CT.Results:The diagnostic performance of volume- and diameter-based management for lung nodules in a Chinese population will be estimated and compared.Conclusions:Through the study, we expect to improve the management of lung nodules and early detection of lung cancer in Chinese populations.
KEYWORDS Lung cancer; lung nodules; screening; computed tomography; China
Introduction
Lung cancer is the fourth most common cause of death and the most common type of cancer in China1. More than onethird of global lung cancer diagnoses (733.3 thousand in 2015) and lung-cancer-related deaths (610.2 thousand in 2015) occur in China2. The estimated age-standardized mortality rate in 2008 for lung cancer was 28.7 per 100, 000 in China, which is considerably higher than the world average (19.4 per 100, 000)3,4. The high mortality rate of lung cancer is mainly attributable to the known risk factors of tobacco smoking (68%), occupational agents (9.5%), and indoor radon (0.2%)5. Lung cancer is predicted to continue to be a major public health issue in the future because of large number of smokers (316 million according to the 2015 China Adult Tobacco Survey)6and population aging7.
Lung cancer is commonly diagnosed and treated at a relatively late stage, often after the first clinical symptoms occur8, and consequently has poor survival outcomes9,10.Numerous clinical trials conducted in the United States and Europe have provided growing evidence that screening with low-dose CT is efficient for early diagnosis of lung cancer11.The U.S. National Lung Screening Trial showed that screening with low-dose CT leads to a 20% lower risk of dying from lung cancer compared to chest radiography12.
Accordingly, there is an urgent need to implement an efficient lung cancer screening protocol in China, which is expected to reduce mortality and the burden caused by lung cancer. Although evidence exists for the role of CT in lung cancer screening12, investigations are currently underway to optimize nodule management and reduce the number of false-positive screening results13-15. Various lung cancer screening guidelines recommend nodule management based on manually measured nodule diameter16-18. However, a lung nodule management protocol based on nodule volume and volume doubling time (VDT) is more specific for early detection of lung cancer19compared to the diameter-based protocol of the American College of Chest Physicians, with a specificity of 94.9% vs. 90.0%, respectively, at a similar sensitivity of 90.9%13,20. We designed a study to be conducted in Tianjin, China, to validate the findings of a previous European study and demonstrate the diagnostic performance of a volume-based nodule management protocol as compared with a diameter-based protocol for the optimization of lung nodule management in a Chinese population.
Materials and methods
Participants
The study includes 4,000 participants who meet the following inclusion criteria: aged 40-74 years, resident in the Hexi district of Tianjin city for at least 3 years, and having no selfreported history of any malignant tumor. The inclusion criteria were assessed by a doctor in a community health service center (CHSC) during a face-to-face interview.
A recruitment campaign was conducted in the first quarter of 2017 by distributing advertisements such as video media(e.g., TV news), text media (e.g., newspapers), new media(e.g., WeChat), poster, leaflets, and free consultations, in which the participation criteria and time were announced.Between May and October 2017, 2,400 eligible residents visited the Yuexiu Street and Youyi Street CHSCs and agreed to participate. The remaining 1,600 volunteers were invited to visit the Yuexiu Street, Youyi Street, and Chentangzhuang Street CHSCs between April and September 2018.
Study design
In this population-based prospective cohort study,participants underwent a low-dose chest CT scan. Figure 1 presents an overview of the study design. At baseline, the first CT scan was performed in all participants and their data were collected. One-year after the baseline, a second CT scan will be performed and data will be collected again. Participants will be followed up for 4 years in total and any diagnosis of lung cancer and related information will be collected through the hospital information system and by contacting the participants or their relatives at the end of follow-up. This study is performed at Tianjin Medical University Cancer Institute and Hospital (TJMUCIH), and will be embedded in the larger NELCIN-B3 study21.
CT image acquisition and reading
Participants undergo a CT scan in the Department of Radiology at TJMUCIH after an interview in one of the three regional CHSCs. All scans are performed using the same CT system: Definition AS (Somaton Definition AS 64, Siemens,Erlangen, Germany). The total radiation dose is a maximum of 2 mSv. Positioning of participants is head-first, supine,and with arms above the head. The CT scan is performed at inspiration with the following parameters: (a) spiral scan mode at 120 kVp and with a reference tube current of 35 mAs; (b) three reconstruction kernels: D45F and B80F at 1.0/0.7 mm thickness/increment and B30 at 2.0/1.0 mm thickness/increment.
CT images of each participant will be read twice independently by two groups of readers after the baseline and 1-year follow-up scans. The clinical management of participants will be based on the first reading.
First reading
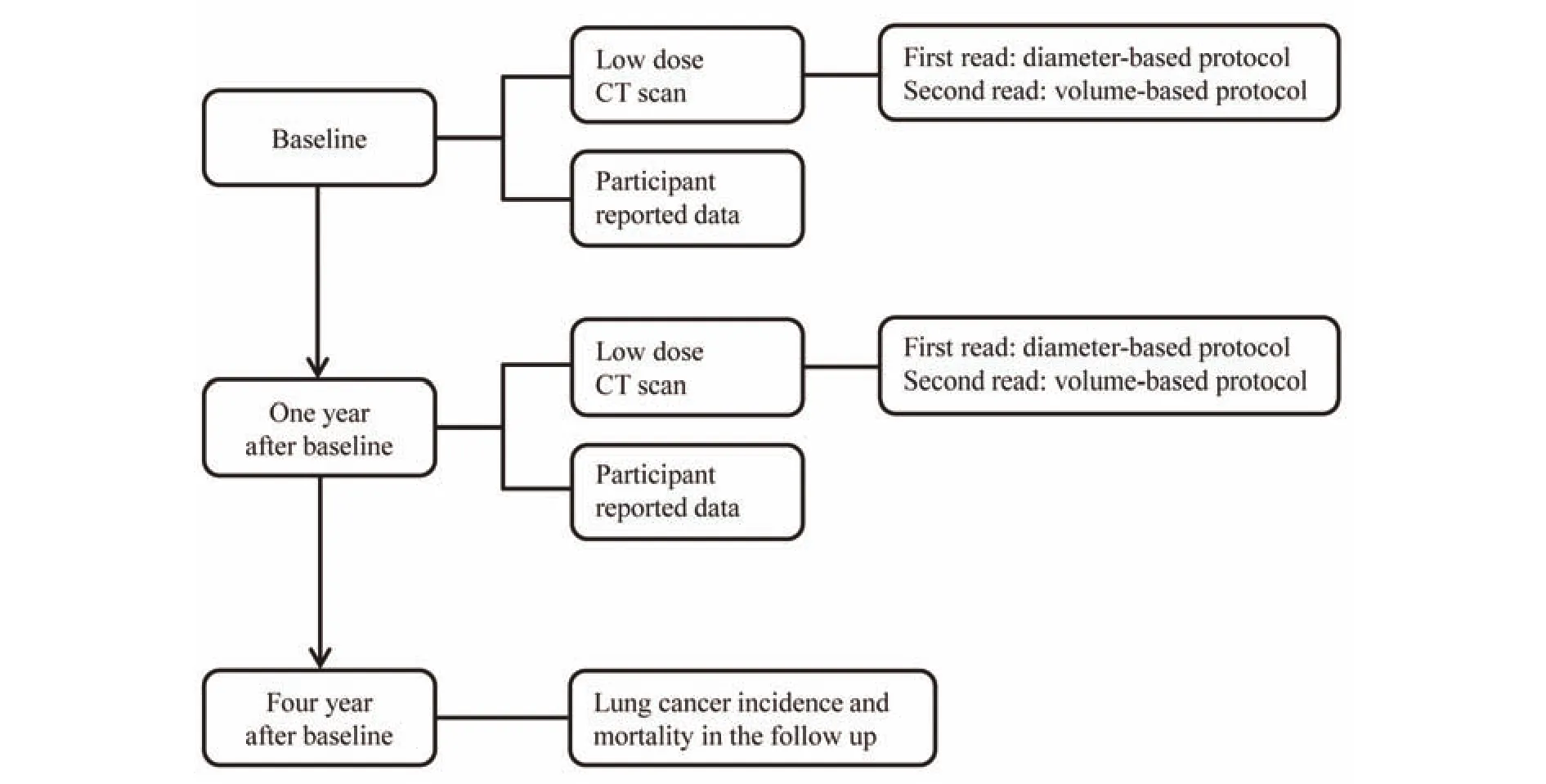
Figure 1 Design of methods of computed tomography screening and management of lung cancer in Tianjin.
The CT images obtained from the baseline and 1-year followup scans will be read by one of four specially trained Chinese resident radiologists and checked by one of two senior Chinese radiologists at TJMUCIH, using the Carestream Picture Archiving and Communication Systems (PACS) v.11.0. A complete list of the nodule parameters that will be collected is presented in Appendix 1. Lung Cancer Screening(version 2. 2018) in the Clinical Practice Guidelines in Oncology of the National Comprehensive Cancer Network(NCCN) will be used for the management of lung nodules16.This guideline recommends management of lung nodules based on their diameters. Participants with a detected solid or part-solid nodule ≤ 5 mm in diameter or non-solid nodule ≤19 mm in diameter will be referred to undergo follow-up thorax CT 1 year after the baseline. Participants with a detected solid nodule 6-7 mm in diameter or part-solid nodule ≥ 6 mm in diameter with a solid component ≤ 5 mm in diameter or a non-solid nodule ≥ 20 mm in diameter will be referred to undergo follow-up thorax CT 6 months after the baseline. Participants with a detected solid nodule 8-14 mm in diameter or a part-solid nodule ≥ 6 mm in diameter with a solid component 6-7 mm in diameter will be referred to undergo follow-up thorax CT 3 months after the baseline.Finally, participants with a detected solid nodule ≥ 15 mm in diameter or a part-solid nodule with a solid component ≥ 8 mm in diameter will be referred to a multidisciplinary team for clinical investigation and, if applicable, treatment.
Second reading
During the second reading, each scan will be interpreted again by radiologists with experience in lung cancer screening, who will be blinded to the first reading. Semiautomated volumetry software (MM Oncology Syngo. via VB10B, Siemens Healthcare, Forchheim, Germany) will be used to measure the volume and evaluate the other parameters (diameter, location, edge, shape, density,calcification, attachment) of the lung nodules (Appendix 1).Solid and part-solid lung nodules
Based on the results of the second reading, solid and partsolid lung nodules (solid component visible in a mediastinal window setting) will be reclassified according to the reference values from the European volume-based lung nodule management protocol11and the management of the nodules will be modeled on this protocol. According to the European volume-based protocol, the solid and part-solid lung nodules will be reclassified into three groups: nodules with a volume< 100 mm3(negative results), nodules with a volume of 100-300 mm3(indeterminate results), and nodules with a volume > 300 mm3(positive results)11,13. In case of part-solid nodules, only the volume of the solid component will be measured.
Solid and part-solid lung nodules detected at baseline will be managed as follows: (1) for negative results: a CT scan 1 year after the baseline; (2) for those with intermediate-sized nodules: a follow-up CT scan 3 months after the baseline to calculate the VDT for further assessment; and (3) for those with a positive result: referral to a multidisciplinary team for further clinical investigation and, if applicable, treatment.
The assessment of intermediate-sized nodules is based on VDT, which will be calculated based on the 3-6-month or 1-year follow-up scans. A fast VDT (< 400 days) indicates a positive screening result, a VDT of 400-600 days indicates an indeterminate screening result, and a VDT ≥ 600 days indicates a negative screening result.
In the volume-based nodule management model,participants with negative and indeterminate results will undergo a CT scan 1 year after the baseline. Solid and partsolid nodules newly detected at the one-year follow-up scan must be followed up 1-3 months later.
Non-solid lung nodules
Non-solid nodules can be further classified into homogenous non-solid nodules (also considered pure ground glass nodules) and heterogeneous non-solid nodules.Heterogeneous non-solid nodules have solid components in a lung window setting but not in a mediastinal window setting. When the diameter of the solid part observed in a mediastinal window setting exceeds 0 mm, the nodule should be classified as part-solid and managed accordingly22.
No validated protocol currently exists for the volumebased management of non-solid nodules. In recent years however, software for the semi-automated volumetric analysis of non-solid nodules has been improved, thereby enabling a volumetric approach to non-solid nodules, similar to the volume-based management of solid and part-solid nodules.
In the second reading, we will assess the volume of nonsolid nodules by using semi-automated volumetry in MM Oncology Syngo. via. The mass will be calculated using the formula M = V x (HUmean+ 1000)/1000, where M is mass(mg/mL), V is volume (mm3), and HUmeanis the mean attenuation in Hounsfield units.
For both homogenous and heterogeneous non-solid nodules with a volume < 1,125 mm3, regular follow-up scans after 12 months are recommended to evaluate appearance of the solid components. When the volume exceeds 1,125 mm3,follow-up at 6 months is recommended23.
On follow-up CT, both volume and mass will again be assessed and VDT and mass doubling time (MDT) will be calculated. If follow-up CT reveals solid components (visible in a mediastinal window setting) in a previously non-solid nodule, the result will be considered positive result and referral will be recommended.
No validated cut-off currently exists for the assessment of growth in non-solid nodules. The doubling times of growing non-solid (pure ground glass) nodules are expected to be a median VDT of 1,832 days and a median MDT of 1,556 days24. However, this includes benign entities as well as the spectrum of pre-malignant and malignant adenocarcinoma.VDT- or MDT-based management must be established in the future, possibly in an approach combining both growth and morphologic features. We propose a further 24 months of follow-up for non-solid nodules that do not show any growth(increase in volume and/or mass < 25%) or any newly appearing solid components (visible in a mediastinal window setting).
Self-reported participant data
To assess the health conditions of the participants and explore the risk factors of lung nodules or lung cancer, the participants' self-reported data will be collected at baseline and after 1 year by using epidemiological questionnaires before the low-dose CT scans. At baseline, data about general characteristics, risk factors of lung cancer, and health status of the participants were collected by trained staff during faceto-face interviews. The following questionnaires were used for the interview: (1) A questionnaire intended to obtain data about the participant's general characteristics, medical history, and known risk factors of lung cancer including behavioral, environmental, and occupational risk factors; and(2) the EQ5D-3L questionnaire25,26. One year after the baseline investigation, data on modified risk factors and health status will be collected again to determine any changes in behavior and health conditions by using a simplified questionnaire.
Follow-up
Follow-up evaluation will be performed after 4 years to identify participants with a confirmed diagnosis of lung cancer. Participants diagnosed with lung cancer will be identified through the hospital information system during the follow-up period. In case participants are diagnosed with lung cancer in another hospital, participants or their relatives will be contacted by phone to obtain diagnostic information 4 years after the baseline investigation. Therefore, a diagnosis of lung cancer can be clinically confirmed using either medical records or self-reports of the participants or their relatives.
All participants will be invited to attend a follow-up interview with qualified staff from the Department of Epidemiology at TJMUCIH. The staff will conduct these follow-up interviews with both active and passive approaches. Phone calls will first be made to inquire about the patient's diagnosis of lung cancer, vital status, and any further examination undergone after the initial screening. If there is no response to phone calls, the hospital information system at TJMUCIH will be searched for matches to identify patients recently admitted to receive medical care. In the case of deceased patients, family members will be asked to provide the date and cause of death. Established cancer registries and death registries in Tianjin will also be used as a supplement to identify any diagnosis of lung cancer and the vital status of the patient.
Information will be collected using a short questionnaire,mainly including the diagnosis date, hospital, type and stage of lung cancer, surgery, cost of treatment, survival status, and cause of death. Lung cancer will be classified as screeningdetected lung cancer or interval lung cancer. The former refers to lung cancer detected during the first year after a positive screening test and the latter refers to lung cancer detected during the first year after a negative screening test.
Outcomes
The primary outcome is the diagnostic performance of the European volume-based management protocol as compared to the diameter-based protocol. The secondary outcomes are(1) the difference between the classifications of lung nodules according to the diameter-based and volume-based protocols, (2) the frequency of risk factors of lung cancer and the overall health score among participants with and without lung nodules as well as the prevalence and the incidence of lung nodules, (3) the incidence of confirmed lung cancer and related mortality and its influencing factors, and (4) the expected reduction in time to diagnosis and number of follow-up CT scans when incorporating volume-based management.
Data de-identification and storage
For each participant, a random number will be generated and linked to an existing participant-number from the hospital records. All the information used for the identification of a participant (name, participant number, date of birth) will be removed from the dataset. The file containing the information necessary for linkage of the randomly generated numbers and the participant-numbers will be stored separately on a dedicated NELCIN-B3 server at TJMUCIH and backed up on an encrypted USB drive.
Data analyses
The diagnostic performance of the volume-based and diameter-based protocols will be assessed by calculating the sensitivity, specificity, positive predictive value, and negative predictive value for the first and second rounds of screening.The sensitivity will be calculated by dividing the number of true-positive screening results (screening-detected lung cancers) by the number of true positive and false negative screening results (interval cancers detected during the same period). The specificity will be calculated by dividing the number of true negative screening results by the number of true negative and false-positive screening results. The positive predictive value will be calculated by dividing the number of true-positive screening results by the number of true positive and false-positive screening results. The negative predictive value will be calculated by dividing the number of true negative screening results by the number of true negative and false negative screening results. The differences in lung nodule classification between the volume-based and diameter-based protocols will be tested using the McNemar-Bowker test. Relevant risk factors related to the presence of lung nodules will be identified using univariate analysis and multivariate logistic regression. The relationship between the presence of lung nodules and the overall health status will be tested using multiple linear regression. The expected reduction in time to diagnosis and number of follow-up CT scans for the true positive results will be calculated for volume-based management.
Further research
In this population-based cohort study, we will test and validate the efficacy of a volume-based protocol for the management of screen-detected lung nodules in a Chinese population in terms of reducing false-positive results. If the protocol demonstrates superiority in reducing false-positive results, we intend to optimize the management of solid and part-solid lung nodules detected using low-dose CT in a Chinese context. Furthermore, we intend to identify the relevant risk factors of lung cancer to optimize the criteria for defining high-risk groups that might benefit from CT screening in a general Chinese population and to evaluate the potential cost-effectiveness of low-dose CT screening for lung cancer in China.
Discussion
Low-dose CT screening for lung cancer can reduce the mortality of the disease12. However, one of the downsides of CT screening is the detection of large numbers of benign non-calcified nodules, potentially obtaining a high number of false-positive results.
The routine evaluation of low-dose CT images for lung cancer in China has been based on assessing the diameter of lung nodules18,27. In the current diameter-based protocol, a large proportion of benign nodules require further work-up,which may increase the radiation risk from more frequent and higher-dose CT evaluations, the risk of complications from invasive evaluations, and financial and psychological burdens28. Lung nodule management based on volume and VDT has proven effective in reducing the false-positive rate of low-dose CT screening in a European population11.However, findings from European studies cannot necessarily be directly translated to a Chinese population. To explore the efficacy of the European CT screening protocol in China, we designed this population-based cohort study as part of the NELCIN-B3 project aiming to test and validate the diagnostic performance of a European management protocol for lung nodules by modeling volume-based management in a Chinese population.
To the best of our knowledge, studies examining the application of volume-based management in a Chinese population are lacking. We will compare two well-defined protocols for lung nodule management in CT screening for lung cancer (the European volume-based protocol and an American NCCN diameter-based protocol) in a Chinese population. Moreover, the secondary interpretation of CT images will be performed with blinding to the primary interpretation. Furthermore, the population-based design allows further exploration of the risk factors of lung cancer.The results also have the potential to be adapted to other urban contexts in China. Therefore, we believe the results of this highly feasible study will greatly contribute to the optimization of early detection of lung cancer in China.
This study has some limitations. First, lung cancer diagnosis could be established in another hospital, preventing access to the clinical records of the participants. In such cases,we will contact the participants; however, recall bias is unavoidable. Second, the inclusion criteria of patients having no history of malignant tumour will be evaluated through a face-to-face interview. Thus, patients who are unaware of a previous malignant tumour diagnosis may be erroneously included.
Ethical approval and consent to participate
The NELCIN-B3 project in China is led by Shanghai Changzheng Hospital, which is affiliated with Second Military Medical University. TJMUCIH is a hospital participating in the NELCIN-B3 project. The ethical approval for NELCIN-B3 project was issued by the committee on ethics of the leading institute, namely the Committee on Ethics of Biomedicine Research of Second Military Medical University. According to Chinese legislation, if ethical approval is provided by the leading institute of a clinical project, other participating institutes must recognize the conclusion of the ethical committee of the leading institute;hence, acquiring the ethical approval of each institute is unnecessary29. Each participant will be asked to read and sign an informed consent form that introduces the screening project, its benefits, potential harm, and confidentiality.
Acknowledgements
This work is a part of NELCIN-B3 project. The NELCIN-B3 project is funded by The Royal Netherlands Academy of Arts and Sciences (Grant No. PSA_SA_BD_01) and Ministry of Science and Technology of the People's Republic of China,National Key R & D Program of China (Grant No.2016YFE0103000). Tianjin Medical University Cancer Institute and Hospital offers free chest CT scans for all participants for the purpose of this study. Dr. Du thanks for the financial support from China Scholarship Council (CSC file No. 201708340072).
Conflict of interest statement
No potential conflicts of interest are disclosed.
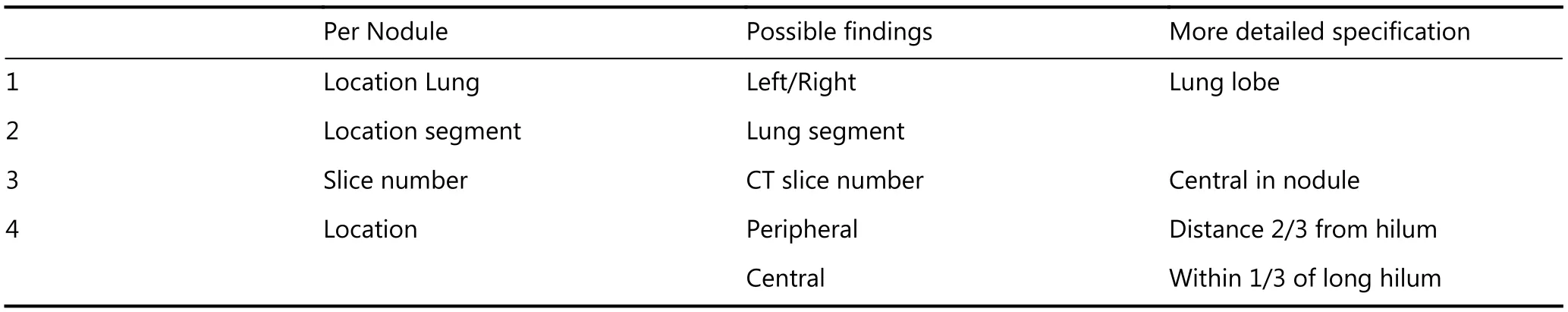
Appendix 1. Nodule parameters to be collected
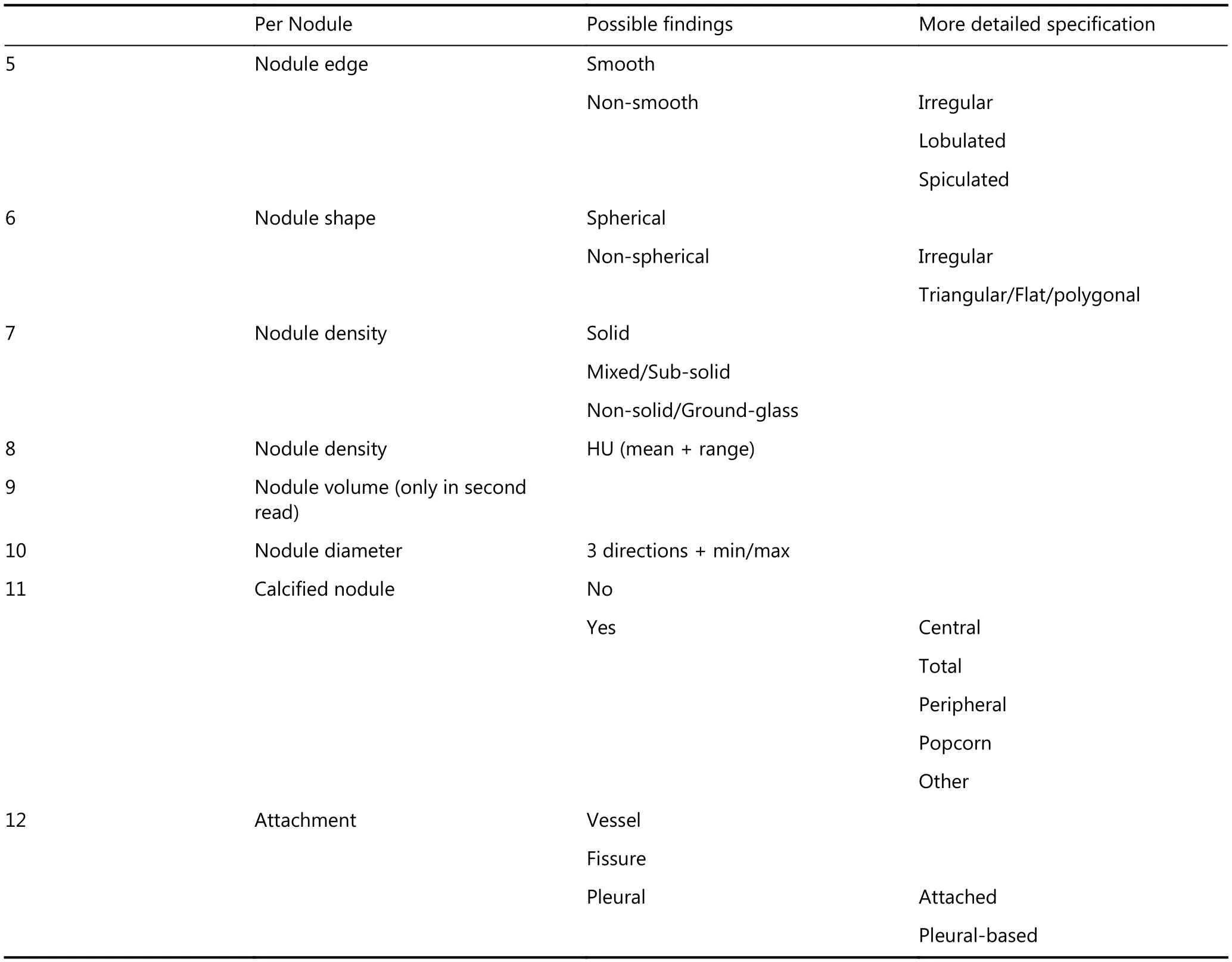
Per Nodule Possible findings More detailed specification 5 Nodule edge Smooth Non-smooth Irregular Lobulated Spiculated 6 Nodule shape Spherical Non-spherical Irregular Triangular/Flat/polygonal 7 Nodule density Solid Mixed/Sub-solid Non-solid/Ground-glass 8 Nodule density HU (mean + range)9 Nodule volume (only in second read)10 Nodule diameter 3 directions + min/max 11 Calcified nodule No Yes Central Total Peripheral Popcorn Other 12 Attachment Vessel Fissure Pleural Attached Pleural-based
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Circular RNAs and human glioma
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
