Qidong: a crucible for studies on liver cancer etiology and prevention
Jianguo Chen, Jian Zhu, Gaoren Wang, John D. Groopman, Thomas W. Kensler,4*
1Department of Epidemiology, Qidong Liver Cancer Institute, Qidong 226200, China; 2Department of Epidemiology, Tumor Hospital, Nantong University, Nantong 226361, China; 3Department of Environmental Health and Engineering, Johns Hopkins Bloomberg School of Public Health, Baltimore 21205, MD, USA; 4Public Health Sciences Division, Fred Hutchinson Cancer Research Center, Seattle 98109, WA 98109, USA
ABSTRACT Qidong (Jiangsu, China) has been of interest to cancer epidemiologists and biologists because, until recently, it was an endemic area for liver cancer, having amongst the highest incidence rates in the world. The establishment of the Qidong Cancer Registry together with the Qidong Liver Cancer Institute in 1972 has charted the patterns of liver cancer incidence and mortality in a stable population throughout a period of enormous economic, social, and environmental changes as well as of improvements in health care delivery. Updated incidence trends in Qidong are described. Notably, the China age-standardized incidence rate for liver cancer has dropped by over 50% in the past several decades. Molecular epidemiologic and genomic deep sequencing studies have affirmed that infection with hepatitis B virus as well as dietary exposure to aflatoxins through contamination of dietary staples such as corn, and to microcystins - blue-green algal toxins found in ditch and pond water - were likely important etiologic factors that account for the high incidence of liver cancer in this region. Public health initiatives to facilitate universal vaccination of newborns against HBV and to improve drinking water sources in this rural area, as well as economic and social mandates serendipitously facilitating dietary diversity, have led to precipitous declines in exposures to these etiologic factors, concomitantly driving substantive declines in the liver cancer incidence seen now in Qidong. In this regard, Qidong serves as a template for the global impact that a package of intervention strategies may exert on cancer burden.
KEYWORDS Liver cancer incidence; hepatitis B virus; aflatoxin; microcystin; screening; chemoprevention; mutational signature
Introduction
Geographical location and history of Qidong
Liver cancer is a malignancy in which geography and etiology are often linked, allowing the display of its distribution in the format of “geographical pathology”. Nowhere is this more apparent than along the eastern coast of China where a city(formerly called a county) has been known for decades to be amongst the areas with the highest liver cancer incidences in the world. Qidong (Figure 1) is an area of about 1,200 km2located at the eastern tip of the Jiangsu Province, at the mouth of the Yangtze River on the opposite bank of Shanghai, that forms a peninsula jutting out into the confluence of the Yellow Sea and the East Sea. There was nothing but river, some sand islands, and marshland areas in much of the region before the Qing dynasty was established(380 years ago). Fertile agricultural lands subsequently arose from depositions coming from the Yangtze River. A new county was established in March 1928 with the name of“Qidong”, meaning “open my east border”. By November 1989, Qidong was administratively reclassified to a countylevel city with Qidong County becoming Qidong City1. The depth of the yellow-red shading in the left panel of Figure 1 highlights the mortality rates for liver cancer by county in an initial nation-wide survey conducted between 1973 and 19752. Qidong, together with multiple counties surrounding Nanning (Fusui), Guangxi, and a few coastal counties in the southeast of China, had the highest incidence/mortality rates from liver cancer in the country, suggesting that they all shared common etiological risk factors.
Economy and environment

Figure 1 Liver cancer mortality in eastern China (left), eastern Jiangsu Province (middle) in the early 1970s and geographical location of Qidong City (right). Left, Atlas of Cancer Mortality2: white, data unavailable; grey, sparsely populated, < 2; light yellow, > 2; light orange, > 4;peach > 8; coral, > 16; salmon, > 32 per 105. Middle, tan, < 1 per 105/year; dark brown, > 50 per 105/year. Right, contemporary Qidong linked by bridges to Shanghai.
Traditionally, Qidong has been considered as agricultural land. The main agricultural crops produced in the area include corn, wheat, soybeans, peanuts, yams, and cotton.The climate is suitable for pears, peaches, oranges, and watermelons. Qidong is also known for its marine economy.It possesses 203 kilometers of coastline and 400 square kilometers of intertidal zone. Lüsi Fishery is one of the six largest fisheries in China, contributing to one-third of Jiangsu's annual total catch. Economic expansion over the last few decades has added numerous new sectors to the city's industry, including textile, mechanical, pharmaceutical,chemical, and civil construction industries. The city has been ranked 31st on the list of China's Top 100 Counties (countylevel cities, 2018) for its comprehensive economic strength and competitiveness. Environmental degradation in Qidong is limited, in part because of the overall modest and recent industrialization, although regional effects on air quality are evident. Importantly, the local municipal government has enacted stringent environmental rules over many years and has committed significant funds to protect the region's ecosystem. The city has been honored as a green (landscape garden) city of the Jiangsu Province since 2015. As seen in much of China, economic, social, and environmental changes over the past few decades have both positively and negatively impacted health outcomes of the Qidong population.
Population
Early settlers of Qidong were migrants who mainly came from both North and South China. Immigrants from the south came by crossing the Yangtze River. These two early migrant ethnic groups had verbal communication barriers and lived in two separate areas, in which migrants from the north lived to the west of the town while the ethnic group that crossed the Yangtze River lived to the east. This separation was the principal cause for today's two local dialects, the Tongdong Dialect (Lüsi region) that is close to the language used in the North of China, and the Qihai Dialect which is similar to the Chongming Dialect of the Wu language used in Jiangsu and Zhejiang. The population size increased very quickly from 0.61 M in 1949 to 0.78 M in 1959, and to 0.99 M in 1969, reaching a peak of 1.17 M by the end of 1997. According to household registrations of the latest census made in Qidong, population declined to 1.12 M at the end of 2016.
The overall population of Qidong has aged over the past decades, as is partially shown in the age-distribution pyramids in Figure 2. Given that aging is the largest predictive factor for most types of cancer, it is not surprising that the crude mortality rate from liver cancer has risen over the past decades. As discussed later, declines in agestandardized incidences of liver cancer are harbingers of improvements in health outcomes.
Health care
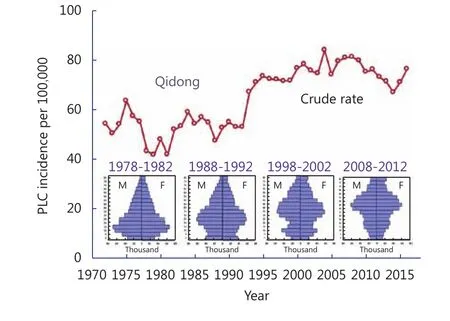
Figure 2 Crude incidence rates for primary liver cancer (PLC) and the population structure in Qidong over the periods of 1978-1982,1988-1992, 1998-2002, and 2008-2012.
Health care and social welfare have developed rapidly in Qidong in the past several decades. There were very few private clinics or medical practitioners before the year 2000 in this region. At present, Qidong has seven city-level general hospitals (state-run), each one ranging from 200 to 1,200 doctors, nurses, and staff. There is a special office, as in most rural areas in China, in charge of the primary health care activities of the “New Rural Cooperative Medical System”and “Urban Residents' Medical Insurance” that serves most of the rural and urban population (over 95%). Facilities for treating patients with cancer are also available at ‘tumor wards' within each of the city hospitals, where both internal medicine and surgery are performed. The vast majority of cancer patients in this region were diagnosed and receive medical care in Qidong city or township hospitals.
Cancer registration
A population-based cancer registry (Qidong Cancer Registry)that includes all the region's residents was established in 1972, along with the founding of the Qidong Liver Cancer Institute (QDLCI). The main aim of cancer registration at that time was to promote epidemiological and etiological research on liver cancer and to monitor treatment outcomes.Data from the cancer registry and the mortality registration system (which was established in 1974 as one of the seven national rural sites for disease monitoring under the supervision of the Ministry of Health of the People's Republic of China) are now widely used not only for epidemiological research on liver cancer, but also for monitoring cancer incidence and mortality for all cancer sites and for evaluating the efficacy of cancer control programs3.Starting from the 1983-1987 period, cancer incidence and survival data from this registry have been published in the Cancer Incidence in Five Continents series4and have been used in both the World Cancer Report5and Cancer Survival in Developing Countries4,6.
Identification and classification of liver cancer
Liver cancer in this review refers to primary liver cancer(PLC, C22 as coded by the 10th version of International Classification of Diseases). Data provided here have been extracted from the Qidong Cancer Registry maintained by the QDLCI. The criterion for final diagnosis of PLC was based upon the “Diagnostic Criteria for Liver Cancer in China” of the Coordinating Group for the Research of Liver Cancer in China7. Briefly, final diagnosis was determined according to histopathological or clinical findings of malignancy, combined with imaging or tumor marker outcomes (e.g., α -fetoprotein). In the clinical practice of Qidong, B ultrasound/CT scan and/or plasma α-fetoprotein were widely used for the diagnosis of liver cancer. For a period of 45 years, the percentage of morphology verification(MV%) was 14.07%. The main type of liver cancer was hepatocellular carcinoma (HCC), which accounted for more than 95% of the liver cancer diagnoses. Cholangiocarcinoma(CC) and other types of liver cancer are not common in this area.
The epidemic of liver cancer
Incidence trends and rank
Overall, liver cancer is the most dominant form of cancer in the Qidong area. Between the years of 1972-2016, there were 32,556 cases, accounting for 27.79% of all cancers combined.Liver cancer has ranked first among all sites overall during the past 45 years, with an average crude incidence rate (CR)of 64.38 per 100,000 and an age-standardized rate by world population (ASRw) of 49.36 per 100,000. The truncated rate for 35-64 years of age was 113.35 per 100,000, and the cumulative rate for 0-74 years of age was 5.16%, with a cumulative incident risk of 5.03%. The CRs were already at a high level of about 50 per 100,000 in the early 1970s,increasing to over 70 per 100,000 after 1994, and reaching the highest rate of 83 per 100,000 in 2004. The CR has fluctuated over a range of 66.85 to 81.16 per 100,000 thereafter. While liver cancer had consistently been the number one type of cancer in the area, lung cancer overtook it since 2012.However, the ASRw of liver cancer for each year still ranks first among cancer sites (Table 1).
Incidence rate by gender
There are significant differences in incidence rates between men and women. Across this period of 45 years, there were 24,338 cases of liver cancer in males, and 8,218 cases infemales. The sex ratio was 2.96:1 by incident numbers, and 3.04:1 by CR. The CR and ASRw were 97.52 per 100,000 and 76.30 per 100,000 in males, and 32.09 per 100,000 and 23.43 per 100,000 in females, respectively. In the first two decades,the CRs ranged from 64 to 100 per 100,000; however, in most years since 1993, the CRs were over 100 per 100,000 in males.The CRs in females for the first two decades were relatively stable, from 17.88 to 26.14 per 100,000, but there has been an increase in the past two decades with rates ranging from 30.57 to 48.41 per 100,000.
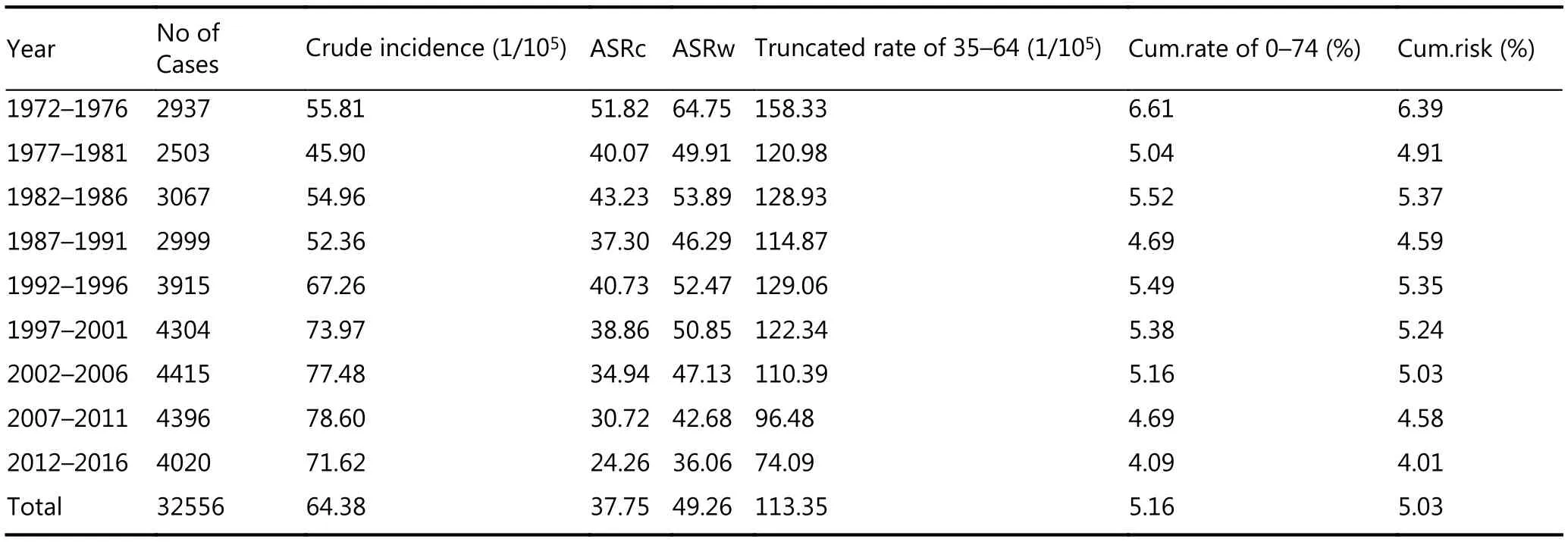
Table 1 Crude rate, ASR, truncated rate and cumulative rate for liver cancer incidence in Qidong, 1972-2016
Incident age and age-specific rate
While the average incident age for liver cancer is 53.09 (male 51.84, female 56.79) overall during the 45-year period, there has been a remarkable change beginning in 1990 in which the average incident age has increased from 48 to 60 years by 2014 in males, and from 50 to over 65 years by 2013 in females. This inflection to a higher incident age most certainly reflects dramatic changes in etiologic factors driving liver cancer risk in the Qidong population. The age-specific rate curve shows that the incidence rates increased with age from below 10 per 100,000 in childhood to over 100 per 100,000 in the age group of 35-, and reached a highest rate of 201.78 per 100,000 in the age group of 60-, which was sustained at this high level of about 190-200 per 100,000 thereafter in males. Females experienced a similar pattern in the rate curves; however, their rates were markedly lower than those of males, with a highest rate of near 100 per 100,000 in the age group of 75- (Figure 3).
Annual percent change
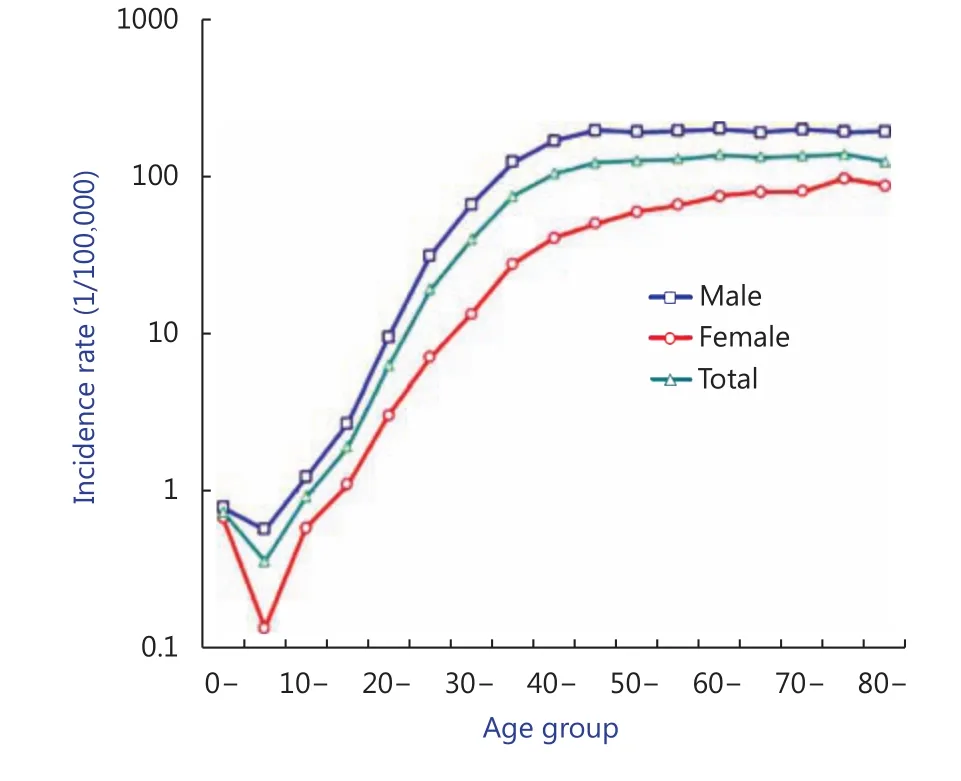
Figure 3 Age specific rates of liver cancer in Qidong, 1972-2016.
The percent changes (PC) of the CR and ASRw from 1972 to 2016 were 44.96% and -34.92%, respectively. The annual percentage changes (APC) were 1.21% and -0.90%,demonstrating a rapidly increasing trend in CR, certainly reflecting the aging of the Qidong population, and an encouraging sharp decrease in ASRw. Women had faster increases in the PC (63.13%) and APC (1.21%) of CR than men (38.68% and 1.01%, respectively); for ASRw, males showed a faster drop of -39.43% in PC and -1.09% in APC than did females (-28.03% and -0.90%).
Birth cohort incidence
The birth-cohort analysis shows fascinating trends for the incidence of liver cancer in Qidong residents of each age group who were born during different calendar years. Such analyses facilitate predictions for the future. Significant declines in the incidence curves can be seen for people under the age of 40, implying that the younger generation in this area has significantly altered its incidence rates from over 115 per 100,000 to 16 per 100,000 in the 35-39 age group; from 67 to 7 per 100,000 in the 30-34 age group; from 33 to near 2 per 100,000 in the 25-29 age group, and from over 10 to less than 1 per 100,000 in the 20-24 age group. However, for those people over the age of 65, the age-specific rates increased markedly; from 127 per 100,000 to 164 per 100,000 in the age group of 65-69, from 115 to 158 per 100,000 in the 70-74 age group, from 82 to 194 per 100,000 in the 75-79 age group, and from 48 to 241 per 100,000 in the age group over 80 years. As discussed later, substantive changes must have occurred in these younger birth cohorts. That is, risk factors that contributed to the occurrence of liver cancer must have been reduced in the younger generation or, on the contrary,hazard factors (other than aging) may have affected the elderly in this region (Figure 4).
Screening programs
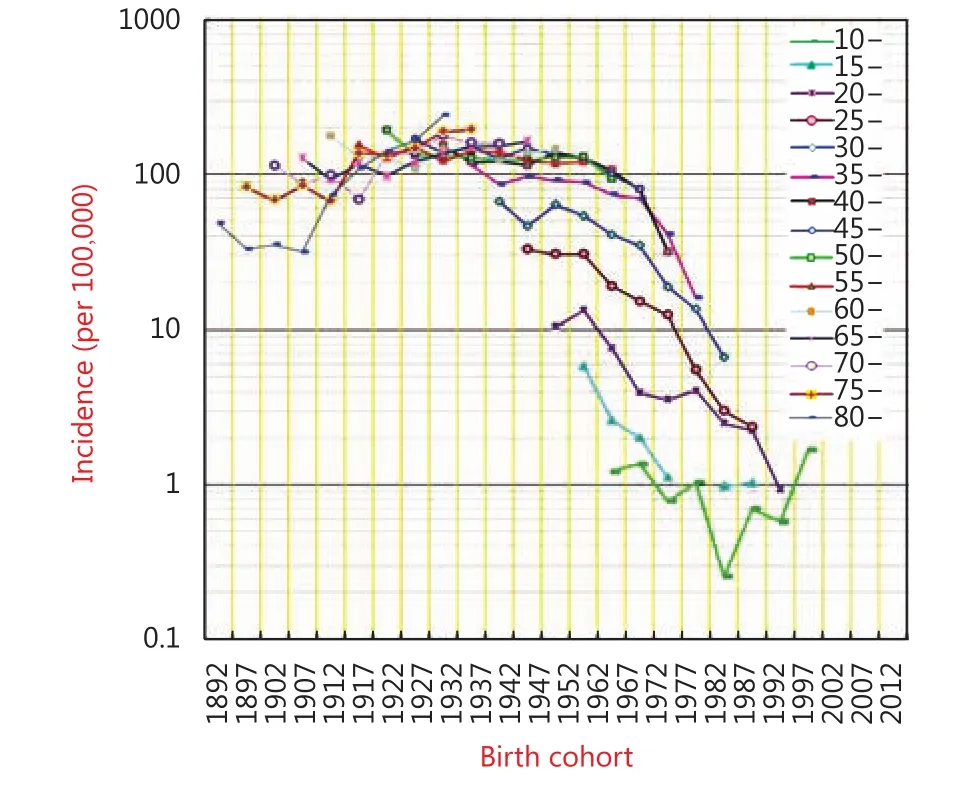
Figure 4 5-year birth cohort incidence rates for liver cancer in Qidong from 1972-2016.
Screening for liver cancer has been pursued as a risk reduction strategy in the Qidong area since the early 1970s.By measuring serum α-fetoprotein (AFP) levels, more than two million adults (aged 15 and over) in the general population of Qidong have been screened. About 1/3 of patients with liver cancer detected by these screening efforts were “early phase” cases. Some benefited from surgical treatment, which was the only effective therapy during that period8. However, in the 1980-1990s, the screening program was terminated because of cost-benefit considerations. After this dormant period for mass screening for liver cancer in the general population, a new understanding of targeting screening for people within high-risk groups [i.e., individuals positive for HBsAg (hepatitis B surface antigen)] was proposed and practiced during the 1990-2000s by using both AFP detection and B-scans9. As a consequence, earlier diagnosis of liver cancer was achieved. Furthermore, in 2006,Qidong became one of the regions joining the national screening program for liver cancer in high-risk groups by means of AFP detection and B-scans, which has shown better outcomes - more than 38% 5-year survival in patients who participated in the biannual screening10. Seemingly, earlier detection has resulted in more opportunities for suitable treatments.
Liver cancer prevention
Risk factors for liver cancer in Qidong
Unlike many cancers, the etiological factors that drive the risk of developing liver cancer are well characterized,especially those pertaining to the Qidong area. Important risk factors in this regional epidemic include infection with hepatitis B virus (HBV), exposure to aflatoxins in the diet,and to microcystins in the drinking water. Areas where viral hepatitis is endemic also have a high incidence of liver cancer.The prevalence of infection with HBV in Qidong, measured by HBsAg, is currently about 9%-10%10. At least 90% of primary liver cancers in Qidong are associated with HBV infection8,11. In a prospective study of 31-year follow-up with 355,305 person-years in Qidong, the relative risk of developing liver cancer in HBsAg carriers was found to be 11.70 (95%CI: 9.06-15.19), compared with non-carriers12.
Hepatitis C virus (HCV), to date, has not been observed as an important risk factor in this region12,13, unlike other areas of the world such as the US, where it is currently driving increasing rates of liver cancer. Perinatal infection accounts for a substantial fraction of HBV carriers. The typically long delay between initial infection with HBV and the development of liver cancer indicates the multifactorial nature of this cancer and the role of persistent viral infection.This timeframe also highlights the need for comprehensive universal vaccination programs against HBV for newborns.
Aflatoxins, naturally occurring mycotoxins that are found in contaminated foods such as corn, peanuts, and other groundnuts, are potent hepatocarcinogens in animal models and have been classified as Group 1 human carcinogens by IARC14. Early surveillance studies in the Qidong countryside during the 1970s indicated a high frequency of contamination of the dominant dietary staple, corn, with aflatoxins8. In some years, 99% of the sampled maize exceeded the US FDA/China FDA action level of 20 ppb for this foodstuff. Ducks raised in the region, also fed with corn,were observed to have a high incidence of liver cancer,perhaps serving as sentinel species for the impact of moldy corn on the human Qidongese population. We measured serial levels of aflatoxin adducts with albumin over two 12-week periods: September-December 1993 (wave 1) and June-September 1994 (wave 2) in 120 individuals. Aflatoxin B1(AFB1)-albumin adducts were detected in all but one of the 792 serum samples15. Using linear regression models on the samples collected weekly during each wave, the mean aflatoxin-albumin adduct levels increased (P<0.05) during the 12 weeks of wave 1 and decreased (P<0.05) over the 4 months of wave 2, indicating some seasonality in the exposure. Neither HBsAg nor gender modified the baseline mean or the temporal trend.
Microcystins, hepatotoxic polypeptides produced by blooms of blue-green algae, act as hepatic tumor promoters in animal models16,17. Their levels can be quite high in ditch and pond water. Exposures to microcystins were greatly reduced in Qidong by the late 1970s through local government efforts to provide deep well water and later on,pipe tap water to all villages. Such well/tap water is largely devoid of microcystins. Epidemiological surveys in the 1980s suggested that the risk of developing liver cancer in Qidong was very dependent upon the source of drinking water and the likelihood of exposure to these microcystin tumor promoters (Figure 5). Up to a 12-fold higher risk of liver cancer was associated with using ditch water compared to deep well water as drinking source8,18.
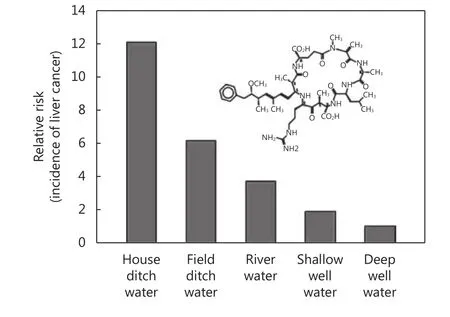
Figure 5 Effect of source of drinking water on the relative risk of developing liver cancer in Qidong area. Adapted from reference 62.
HBV and aflatoxin biomarkers in human studies
Aflatoxin adducts formed with DNA and proteins are the direct products of (or surrogate markers for) damage to critical cellular macromolecular targets. Such biomarkers have been used extensively to assess exposure as well as cancer risk in liver cancer endemic areas across the world. In a similar vein, HBsAg serves as a biomarker of infection of hepatocytes with HBV. This serum biomarker appears before the onset of symptoms, reaches peak levels during overt disease, and often declines to undetectable levels 3-6 months after infection.
Several cohort studies have examined the relation of HBV infection with aflatoxin exposure and liver cancer. Data obtained from cohort studies have the greatest power to determine a true relationship between exposure and disease outcome because one starts with a healthy cohort, obtains biomarker samples, and then follows the cohort until a significant number of cases are obtained. A nested study within the cohort can then be designed to match cases and controls. Two major cohort studies with aflatoxin biomarkers have demonstrated the important role of viral and chemical carcinogens in the etiology of liver cancer. The first study,comprising more than 18,000 men in Shanghai, examined the interaction of HBV and aflatoxin biomarkers as independent and interactive risk factors for liver cancer19,20.The nested case-control data revealed a statistically significant increase in the adjusted relative risk of 3.4 for those liver cancer cases where urinary aflatoxin biomarkers were detected. For HBsAg-positive (a marker of infection with HBV) people, the relative risk was 7, but for individuals with both urinary aflatoxins and positive HBsAg status the relative risk was 59. These results strongly support a causal relationship between the presence of the chemical, the viralspecific biomarkers, and the risk of HCC. However, the underlying mechanisms driving this multiplicative interaction have not been fully resolved.
Subsequent cohort studies in Taiwan have substantially confirmed the results from the Shanghai investigation. Wang et al.21examined liver cancer cases and controls nested within a cohort and found that in HBV-infected people there was an adjusted odds ratio of 2.8 for detectable compared with nondetectable aflatoxin-albumin adducts and of 5.5 for high compared with low levels of aflatoxin metabolites in urine. In a follow-up study, there was a dose-response relationship between urinary AFM1levels and risk of liver cancer in chronic HBV carriers22. Similar to the Shanghai study, the liver cancer risk associated with AFB1exposure was more striking among HBV carriers with detectable aflatoxin-N7-guanine in urine. Studies by others in Qidong revealed that HBV hepatitis is ubiquitous in Qidong HCC cases, whereas HCV contributes little to this risk. Moreover, in a 13.25-year follow-up on a cohort of 145 men with chronic HBV hepatitis in Qidong, the relative risk from aflatoxin exposure was found to be 3.5 (1.5-8.1) and it was observed that even modest levels of aflatoxin exposure tripled the risk of HCC in HBV-infected men11.
Mutational signatures
Exposures to exogenous and endogenous carcinogens can lead to characteristic mutational signatures that bear witness to the underlying mutagenic processes. There have been numerous studies conducted in Qidong to measure mutational burden, signature, and temporality in one-off and longitudinal cohort studies. Non-target genes [e.g.,hypoxanthine guanine phosphoribosyltransferase gene (HPRT)], target genes (e.g., TP53) and effector genes (e.g.,HBV viral genome) have been interrogated. In an early study,we determined somatic mutation frequencies in human HPRT23. Ninety healthy subjects from Daxin were assigned as low or high exposure to AFB1, according to a dichotomization of their levels of aflatoxin-albumin adducts around the population mean. HPRT mutant frequency was determined in individuals by a T cell clonal assay. An odds ratio (OR) of 19.3 (95%CI: 2.0~183) was demonstrated for a high HPRT mutation frequency in individuals with high aflatoxin exposure compared with those with low aflatoxin exposure.
The relationship between aflatoxin exposure-driven mutation and development of HCC has been further highlighted by molecular biological studies on a tumor suppressor gene commonly mutated in many human cancers.Two simultaneously published studies showed the first linkage between aflatoxin exposure and a specific mutation in TP53, one based upon Qidong samples24,25. Subsequently,many studies on liver cancer mutations in populations exposed to high levels of dietary aflatoxin have found high frequencies of G-C to T-A transversions, with clustering at codon 249. In contrast, few mutations in codon 249 have been found in liver cancer from Japan and other areas where there was little exposure to aflatoxin. Initially, we evaluated genetic alterations in 24 liver resection specimens from Shanghai and Qidong26. HBV was integrated in all patient samples. Alterations of TP53 were present in 95% of the cases. All seven liver cancers from Qidong and three out of five from Shanghai had the aflatoxin-associated point mutation with a G to T transversion at codon 249 (R249S).Similarly, Szmanska et al.13detected R249S mutations in 11 out of 16 (64%) liver cancers from Qidong.
In a follow-up study utilizing serial samples from a longitudinal collection of plasma samples from the same cohort of 1,638 high-risk individuals in Qidong, we compared results from plasma DNA and DNA sequencing for specific mutations in 25 liver tumors using short oligonucleotide mass analysis (SOMA)27. Mutations were detected in 10 samples. Analysis of 20 additional plasmatumor pairs showed that 11 tumors and 6 plasma samples contained the specific mutation. Ten plasma samples from healthy individuals were all negative. Jackson et al.28further explored the temporality of detection of this mutation in plasma before and after clinical diagnosis of liver cancer in the same patient. Sixteen patients diagnosed with liver cancer between 1997 and 2001, with plasma samples collected before and after liver cancer diagnosis, were selected for the study.In samples collected prior to liver cancer diagnosis, 22% of the plasma samples had detectable levels of the codon 249 mutation. The codon 249 mutation was detected in 45% of all plasma samples following diagnosis of cancer. Further,persistence of this mutation in plasma once it became measurable was statistically significant in repetitive samples following diagnosis. Nearly one-half of the patients were positive for this marker at least 1 year, and in one case 5 years, prior to diagnosis. Using PCR amplification rather than SOMA, Huang et al.29reported that the R249S mutation in TP53 was detected at a much higher frequency in plasma of liver cancer patients from Qidong than in specimens from cirrhotic or healthy controls also residing in Qidong. This promise of a specific early diagnostic marker for liver cancer has, however, not been developed in subsequent years.
Whole genome sequencing in recent years has provided new insights into the contribution of mutagenic exposures to liver cancer development. Zhang et al.30sequenced 49 liver tumors collected between 1990 and 2016 in Qidong in order to refine genetic features associated with aflatoxin exposure.The dominant pattern was G>T transversions. The TP53 mutation frequency was very high (81.6%) and the major genotype was the R249S hotspot mutation. Additional genes harboring mutations included TERT, AXIN1, CTNNB1 and ADGRB1. The aflatoxin mutational signature, highly represented in the Qidong samples, was observed at low frequencies in tumors from the US (3.5%) and France(1.7%). A study by Huang et al.31combined the analysis of experimentally induced mutational signatures in human cells and mice with those of the Qidong tumor set and found remarkable similarity amongst the aflatoxin-experimental systems but less so in comparison to the human tumors. The animal and cell line studies appear not to reflect the complexities of the HBV-aflatoxin interactions seen in Qidong.
The contribution of HBV to the pathogenesis of liver cancer is multifactorial and is further complicated by the identification of mutant variants in HBV that may alter its contributions to the carcinogenic process. In many cases of liver cancer in Asia and Africa, a double mutation in the HBV genome, an adenine to thymine transversion at nucleotide 1762 and a guanine to adenine transition at nucleotide 1764(1762T/1764A), has been found in tumors and bestows a significant increased risk of liver cancer32. Several studies in Qidong have found that the HBV double 1762T/1764A mutation was not only detectable in plasma samples at the time of HCC diagnosis but could be measured in some individuals up to 15 years prior to diagnosis33,34. We measured levels of the HBV double 1762T/1764A mutation in plasma samples from a matched case-control investigation of 345 men who died of HCC and 625 controls who were nested within a cohort of male HBsAg carriers35. Matched preserving OR were used as a measure of association and 95% confidence CI, as a measure of precision. A total of 278(81%) of the cases were positive for the HBV 1762T/1764A mutation compared to 250 (40%) of the controls. The matched preserving OR of 6.72 (95%CI: 4.66~9.68) strongly indicated that cases were significantly more likely than controls to have the mutation. Plasma levels of DNA harboring the HBV mutation were on average 15-fold higher in cases compared to controls. Tu and colleagues36have also compared the complete sequence of HBV isolated from 20 liver cancer and 35 non-cancer patients in Qidong. These results implicate A2189C and G2203W as new predictive markers for liver cancer. The OR were 3.99 (95%CI:1.61~9.92) and 9.70 (95%CI: 1.17~80.58), respectively, for A2189C and G2203W. A separate longitudinal study demonstrated that six point mutations, including T1653,V1753, T1762, A1764, T1766, and A1768 were found to occur more frequently in cancer than in non-cancer patients.T1653 and V1753 were risk factors independent of the double mutation for liver cancer37. In the same cohort, Qu et al.38observed that pre-S deletions and pre-S2 start codon mutations were independently associated with the development of liver cancer. The longitudinal nature of the analysis indicated that these mutations were not present at the beginning of infection but arose de novo during the progression of hepatic disease. These authors have also observed a significant biological gradient of cancer risk by the number of mutations in the viral C gene38. Nonetheless, a causative role of any of these mutations in hepatocarcinogenesis remains to be established.
Approaches to prevention of liver cancer
Multiple strategies for reduction and prevention of HCC in high-risk populations need to be developed and implemented. While there is an intrinsic appeal towards primary prevention strategies, such as vaccination against HBV and reduced contamination of dietary staples with aflatoxins, these approaches are not without their challenges.Since transmission of HBV often occurs from mother to child, it is necessary to vaccinate high-risk populations early in life prior to viral infection. Even with vast economic resources being brought to bear and assuming complete coverage with a vaccine that is totally successful, it will require a number of generations to achieve overall prevention. Attempts to reduce dietary aflatoxin exposure through improved storage conditions and diligent monitoring of levels of contamination in the developing world are strategies that will also require considerable investment in the infrastructure of food production,processing, and distribution. In the developed world, the consumer supports a substantial price for foods with reduced levels of aflatoxins. Such capacity and infrastructure are not possible for subsistence farmers in the low-income regions of the world. These constraints of time, money, and effort make it plausible to consider additional approaches, such as chemoprevention, towards reducing the risk of liver cancer from unavoidable exposures to aflatoxins. In general, such an approach, given the epidemiology of liver cancer in these high-risk areas of the world, would seem more suited to have an immediate impact on the current generation of at-risk individuals and could provide benefit until more comprehensive and complex primary prevention strategies can be put in place. However, chemoprevention for the mitigation of risk from environmental exposures should not be considered a substitute for primary prevention. Qidong has been a study area for evaluating the safety and efficacy of both forms of prevention strategies.
HBV vaccination
HBV vaccination is an exceedingly important strategy for primary prevention of liver cancer. Pilot vaccination programs were conducted in some Qidong townships from 1983 to 1984, partially funded by the USA and UK. After the safety, immunogenicity, and efficacy of HBV vaccination was determined, a population-based, cluster randomized,controlled trial was carried out between 1985 and 1990. The overall coverage of Qidong newborns was perhaps 30%during this period, when about 40 thousand newborns were randomly assigned to the vaccination group (received 3-doses of HBV vaccine) and some 38 thousand neonates were randomly assigned to the control group (received neither a vaccine nor a placebo)39,40. Vaccination programs languished in the rural townships of Qidong during the 1990s, largely due to limited access to the vaccine and its prohibitive cost relative to annual wages. Beginning in 2002, a subsidized HBV vaccination program was instituted by the Chinese government and by 2006 the fees associated with the 3 injections were also covered. So far, the neonatal HBV vaccination has been found to significantly decrease HBsAg seroprevalence, and hepatic diseases in childhood and young adulthood in the Qidong area have been reduced39. However,while the reduced incidence or mortality of liver cancer may be anticipated over a long period, there is still no evidence at present to indicate that vaccination has contributed to the reduction of rates seen in this area, as shown in earlier birth cohort curves in Figure 4.
Changing rates for liver cancer in Qidong:unexpected success for primary prevention
A package of postharvest measures (hand sorting, drying on mats, sun drying, storage in natural fiber bags and on wooden pallets, and use of insecticides) has been used in West Africa in feasibility studies to restrict aflatoxin contamination of groundnut crops41. While effective at reducing aflatoxin exposures post-harvest, as measured by serum aflatoxin-albumin adduct concentrations in residents of control and intervention villages, the long-term implementation of such strategy has not been initiated. In a similar approach, ad hoc hand sorting of harvested corn and peanuts by farmers and distributors to remove moldy items had been a common sight in the rural townships of Qidong.However, devising strategies for sustained implementation and large-scale dissemination of the educational and technical skills required for population-scale benefit remain huge challenges in this arena of primary prevention.
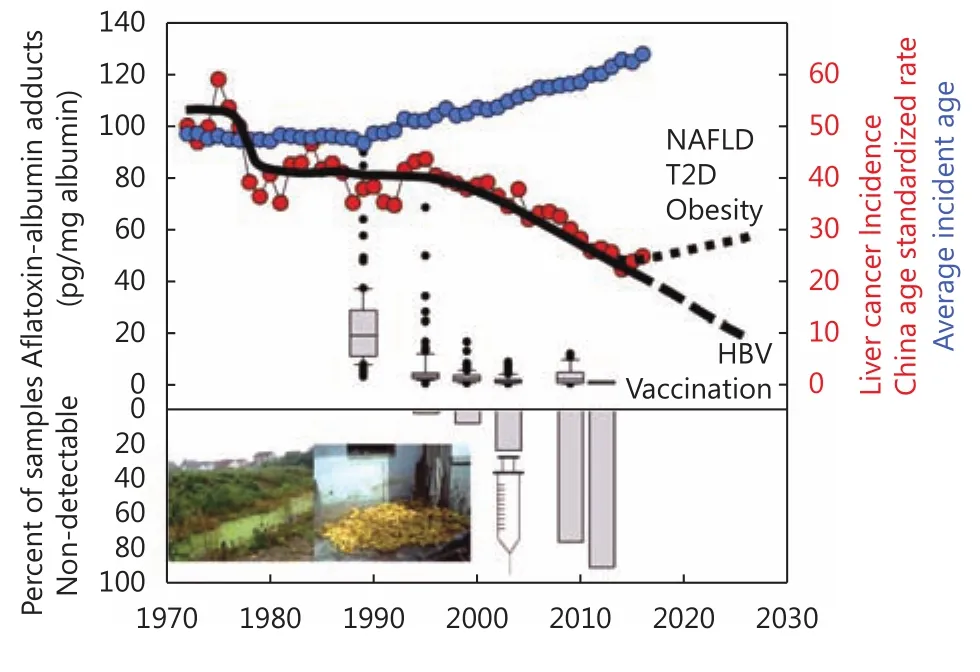
Figure 6 Timeline and prediction of liver cancer incidence and involved factors. Public health initiatives to provide alternatives to algal contaminated drinking water sources in the 1970s and implementation of universal HBV vaccination of newborns in the 2000s along with economic and social changes leading to dietary diversity and dramatic reductions in aflatoxin exposures in the 1980s and 1990s have led to reduced exposures to important etiological factors and subsequent changes in liver cancer incidence and average incident age in Qidong. Emerging etiological risks such as non-alcoholic fatty liver disease (NAFLD),type II diabetes (T2D) and obesity may reverse this trend in future years. Data are from references 42 (aflatoxin albumin adducts) and 63 (China age-standardized incidence and average incident age).
Utilizing the availability of serum samples collected over nearly a quarter century, changes in aflatoxin exposure patterns have been documented in Qidong (Figure 6). In the mid-1980s, Deng Xiaoping's “Socialism with Chinese Characteristics” reforms led to the dismantling of communes and to major agricultural changes that opened up a market economy. The immediate impact in Qidong was a rapid dietary switch from corn to rice consumption. Rice is typically contaminated with aflatoxins at much lower levels than corn or groundnuts. As shown in Table 1 and Figure 4,the population-based cancer registry of Qidong documented a more than 50% reduction in the incidence rates of liver cancer occurring across birth cohorts from the 1960s to the mid-1980s in Qidongese people less than 40 years of age,although they were all born before the inception of universal vaccination of newborns against HBV. Median serum levels of the aflatoxin-albumin adduct biomarker decreased from 19.3 pg/mg albumin in 1989 to undetectable (<0.5 pg/mg) by 2009 and beyond42. A population-attributable benefit of 65%for reduced liver cancer mortality was estimated for the government-facilitated switch of dietary staple from corn to rice; 83% of this benefit was for those infected with HBV.Food policy reforms in China thus resulted in a dramatic decrease in aflatoxin exposure, which, independent of HBV vaccination, reduced liver cancer risk. The extensive HBV vaccine coverage now in place augurs even greater risk reductions in the future.
Continuing economic growth is leading to a market basket diversity that has reduced the exposure to aflatoxin coming from a single source, susceptible to high levels of contamination. Access to a greater number of types of foods will lower the risk of exposure by lowering the intake of foods contaminated with mycotoxins43. With a global perspective, a recent monograph from IARC outlines a number of these economic changes along with the development of fungal resistant strains of seeds and the potential use of biocontrol techniques to limit aflatoxin exposure in regions where economies are in transition from low to middle income44.Collectively, all of these primary prevention strategies will help to mitigate aflatoxin toxicities in humans.
Human chemoprevention trials
Chemoprevention is a strategy for the secondary prevention of cancer. This approach entails the use of drugs, dietary supplements or functional foods to retard, block, or reverse the carcinogenic process in particular individuals or target populations. Such interventions can serve to alter cell fate, by either preventing cells from acquiring carcinogen-mediated damage to the genome and epigenome or by impeding proliferation of preneoplastic cells or, alternatively,accelerating their apoptosis. One successful strategy for cancer chemoprevention is modulation of carcinogenmetabolizing enzymes, leading to diminished DNA damage and enhanced elimination. Inducers of conjugating enzymes such as dithiolethiones and sulforaphane inhibit tumorigenesis of environmental carcinogens including aflatoxins and air pollutants in various animal models.
Oltipraz, a substituted 1,2-dithiole-3-thione, was originally developed by the pharmaceutical industry as a possible treatment for schistosomiasis and was extensively evaluated in clinical trials in the early 1980s. In extensive preclinical evaluation by the U.S. National Cancer Institute and others,oltipraz was found to be effective as an anticarcinogen in many animal models45. Therefore, aflatoxin biomarkers were used as intermediate endpoints in a chemoprevention trial of oltipraz in Qidong46. This was a placebo-controlled, doubleblind study in which participants were randomized to receive placebo or 125 mg oltipraz daily or 500 mg oltipraz weekly.Median levels of a detoxication product - aflatoxinmercapturic acid (a glutathione conjugate derivative) - were elevated six-fold in the 125-mg group, but were unchanged in the 500-mg group. Increased formation of aflatoxinmercapturic acid reflects induction of aflatoxin conjugation through the actions of oltipraz on the expression of glutathione transferases. The apparent lack of induction in the 500-mg group was thought to reflect a masking effect caused by diminished aflatoxin-8,9-epoxide formation for conjugation through the inhibition of CYP1A2 in this highdose group. Although the oltipraz clinical trial demonstrated the proof of principle for the elevated flux through pathways leading to aflatoxin detoxication in humans, the practicality of using a drug-based method for prevention in the economically developing world is limited due to cost,availability, and culture-based aversion to the use of drugs.Thus, there was a need for other innovative approaches.
Many foods have high levels of enzyme inducers that enhance aflatoxin detoxication and elimination from the body. The anticarcinogenic properties of chlorophyllin, a water-soluble derivative of chlorophyll, have been demonstrated in a number of animal models; its initial characterization arose from the inhibition of liver cancer development in aflatoxin-treated trout47. Although the primary mode of action is thought to be the sequestration of aflatoxin by chlorophyllin through formation of a 1:1 complex leading to impaired absorption in the gut,experimental data have characterized some enzyme-inducing properties that may also contribute to its mechanism of action48. In a randomized, double-blind, placebo-controlled chemoprevention trial also conducted in Qidong,chlorophyllin was determined to alter the disposition of aflatoxin49. One hundred and eighty healthy adults were randomly assigned to ingest 100 mg of chlorophyllin or a placebo three times a day prior to each meal for 4 months.The primary end point was modulation of levels of urinary aflatoxin-N7-guanine adducts collected during 3 months into the intervention. Adherence to the study protocol was outstanding, and no adverse events were reported.Chlorophyllin consumption at each meal led to an overall 55% reduction in median urinary levels of this aflatoxin biomarker compared with those of subjects taking placebo.
In a more recent chemoprevention trial in humans, a beverage made from hot water infusions of 3-day old broccoli sprouts - a broccoli “beverage” or “tea” - containing defined concentrations of glucoraphanin (a stable precursor of the anticarcinogen sulforaphane), was evaluated for its ability to alter the metabolic disposition of aflatoxin50. In this study, 200 healthy adults drank beverages containing either 400 or <3 μmol glucoraphanin nightly for 2 weeks. Urinary levels of aflatoxin-N7-guanine were similar in individuals in the two intervention arms. However, in a secondary analysis,measurement of urinary levels of sulforaphane metabolites indicated striking inter-individual differences in bioavailability. This outcome likely reflects individual differences in the rates of hydrolysis of glucoraphanin to sulforaphane by the intestinal microflora of the study participants. Accounting for this variability, a significant inverse association was observed for the uptake and subsequent urinary excretion of sulforaphane and aflatoxin-N7-guanine adducts in individuals receiving broccoli sprout beverage. This preliminary study illustrates the potential use of an inexpensive, easily implemented, food-based method for prevention in a population at high risk for aflatoxin exposures.
Follow-up studies have employed this intervention strategy in Qidong with good success towards enhancing the detoxication of air pollutants over a several month timeframe51-53. This strategy of “frugal” or “green”chemoprevention assumes broad relevance, as environmental contamination becomes an emerging public health crisis in many areas of the economically developing world54. Many,but certainly not all, environmental carcinogens can be detoxified through the molecular pathways induced by agents such as oltipraz, sulforaphane, and even newer molecules.
Perspective
Emerging risk factors
Today liver cancer is the second leading cause of cancer deaths worldwide, accounting for 8.1% of the global burden of cancer55. Remarkably, while most cancer diagnoses occur in the sixth or seventh decade of life, in the case of liver cancer there is a profound overrepresentation of cases identified before the age of 50. Since liver cancer has a poor five-year survival, this disease contributes to a large fraction of life-year loss due to cancer across the globe. As stated previously, liver cancer is somewhat unique because many of the risk factors that contribute to the overwhelming majority of cases have been identified. As described in this review, this knowledge base has been utilized for the design of a number of prevention strategies in high-risk populations such as that found in Qidong. Nonetheless, globally, there will be approximately 100 million liver cancer deaths in the 21st century.
The three major HCC risk factors that have impacted the Qidong population, HBV, aflatoxin, and microcystins, are now being aggressively addressed using a number of economic investment and public health prevention tools. If there were no other risk factors for the development of liver cancer, then it could be predicted that the decline in incident cases would continue over the next several decades, perhaps leading to the classification of liver cancer as a “rare” form of cancer in Qidong. Striking declines have already been seen in other populations for gastric and lung cancers,demonstrating that some forms of cancers are not always inevitable. Unfortunately, in the case of liver cancer there are emerging risk factors that portend a reversal of the progress made to date unless new forms of interventions are implemented in the near future.
Over the past 10 years, in both men and women in the United States there has been a 2% to 3% rise in liver cancer deaths per year, despite nearly universal HBV vaccination and generally low dietary aflatoxin exposures56,57. Some of this increase in the United States is clearly related to the hepatitis C virus infection epidemic that started in the 1970s and has been systematically studied over the last several decades. Recent success in the therapeutic cure of hepatitis C viral infection in people bodes well for lowering the impact of this factor as a major contributor to liver cancer mortality.Fortunately, hepatitis C infection rates in Qidong remain low and, at least at present, the use of these expensive new therapies will not be widespread or needed.
The World Cancer Report, 20145outlined that over 11% of the liver cancer cases worldwide are attributable to alcohol consumption, with a higher burden in men compared to women. Clearly, alcohol consumption continues to rise in many populations, as economic development gives access to a wider spectrum of alcoholic products available to a larger number of individuals. Nonetheless, the greatest concern for the reversal of the decline in liver cancer in Qidong lies with the emerging impact of obesity and resultant type II diabetes.Qidong, like many areas around the world, has been subject to increasing obesity in both men and women along with the resultant increase in type II diabetes cases. Recent data have found that nearly 10% of the adult population in East Asia has type II diabetes58,59. The prevalence of obesity and diabetes facilitates the development of fatty liver disease and cirrhosis, which are major biological precursors to the development of liver cancer60. While there have been relatively few successful interventions in populations to blunt obesity and diabetes, this need will be a major public health imperative for the next several decades.
A final issue for which the Qidong Cancer Registry will be an essential tool is the monitoring of the male to female ratio of liver cancer that historically has been 2:1 to 3:1 in this population. Current data from men and women suffering from high obesity and type II diabetes in Central America have shown a 1:1 ratio of liver cancer development,potentially suggesting a steeper rise in the incidence of this disease in women compared to men61. Thus, screening and intervention strategies will have to be more broadly applicable to the general population rather than focusing on the historic high-risk group of men.
Lessons from Qidong studies
The establishment of a cancer registry along with the founding of the Qidong Liver Cancer Institute in 1972 has provided an opportunity to examine the roles of chemical and viral factors, and importantly their interactions, in the etiology of liver cancer. The prescience of the medical teams of Shanghai and Jiangsu of founding the first cancer registry in a rural area of China is remarkable. As a consequence, this registry has charted the impact of social, economic, and environmental changes on the trajectory of liver cancer in this “hotspot” region63. The promises and challenges of primary and secondary prevention modalities, including universal HBV vaccination, early detection screening, dietary change, and chemoprevention, have literally played out on the fields of Qidong. Longitudinal cohort collections of serum and urine samples, sometimes linked to tumor specimens, have provided exceptional granularity to molecular epidemiological studies probing etiology and preventive efficacy. Moreover, the principles developed during the molecular, clinical, and population-based studies in Qidong have broad applicability throughout the world in the quest to reduce the global burden of cancer. The world owes a debt of gratitude to the thousands of Qidongese who have participated in the screening, vaccination, and chemoprevention studies over the past decades, leading to improved insights into the etiology and preventive strategies against liver cancer.
Acknowledgements
This work was supported by grants from the US National Institutes of Health (Grant No. R01 CA196610 and R35 CA197222) and Chinese National Key Projects (Grant No.2008ZX10002-015, 2008ZX10002-017, 2012ZX10002009,2018ZX10732202-001). We thank our colleagues at the Qidong Liver Cancer Institute, the Qidong People's Hospital,the Shanghai Cancer Institute and Johns Hopkins University who have assisted in our studies. We thank all the colleagues from Shanghai, Jiangsu, and Beijing (Medical Teams or Institutions) for their groundbreaking contributions in the investigations and research on liver cancer; and thank the leadership of the Qidong City government for fostering our collaborations, and most importantly, we thank the many residents of Qidong as well as the local doctors for their dedicated participation in these studies. They have been the enablers to probe the etiology and prevention of liver cancer.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Circular RNAs and human glioma
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
- Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer
