Circular RNAs and human glioma
Jinglei Liu, Kun Zhao, Nunu Huang, Nu Zhang
Department of Neurosurgery, The First Affiliated Hospital of Sun Yat-sen University, Guangdong Provincial Key Laboratory of Brain Function and Disease, Guangzhou 510080, China
ABSTRACT As a newly discovered type of RNA, circular RNAs (circRNAs) are widespread throughout the eukaryotic genome. The expression of circRNAs is regulated by both cis-elements and trans-factors, and the expression pattern of circRNAs is cell type- and diseasespecific. Similar to other types of non-coding RNAs, functions of circRNAs are also versatile. CircRNAs have been reported previously to function as microRNA (miRNA) sponges, protein sponges, coding RNAs or scaffolds for protein complexes.Recently, several circRNAs have been reported to play important roles in human malignancies, including glioma. Here, we reviewed several reports related to circRNAs and glioma, as well as the potential diagnostic and therapeutic applications of circRNAs in brain cancer. In general, some circRNAs, such as circSMARCA5 and circCFH, are found to be expressed in a gliomaspecific pattern, these circRNAs may be used as tumor biomarkers. In addition, some circRNAs have been found to play oncogenic roles in glioma (e.g., circNFIX and circNT5E), whereas others have been reported to function as tumor suppressors (e.g.,circFBXW7 and circSHPRH). Furthermore, circRNA is a good tool for protein expression because of its higher stability compared to linear RNAs. Thus, circRNAs may also be an ideal choice for gene/protein delivery in future brain cancer therapies. There are some challenges in circRNA research in glioma and other diseases. Research related to circRNAs in glioma is comparatively new and many mysteries remain to be solved.
KEYWORDS Circular RNA; glioma; miRNA sponge; translation; protein scaffold; miRNA target
Introduction
Circular RNA (circRNA) is a newly discovered type of RNA reported to be associated to many human diseases, including glioma. In this review, we sought to summarize the studies related to circRNAs, including their tissue-/disease-specific expression pattern, diverse functions, expression, functional roles, and therapeutic potential in human glioma. Finally, we discussed the current challenges of circRNA research in glioma and some other essential aspects.
Fascinating RNA world
As important intermediate linkers in the central dogma,RNAs have multiple biological functions. RNAs can not only transmit genetic information (such as mRNAs and tRNAs)stored in DNA, but they can also directly store genetic information (such as some RNA viruses)1. In addition, RNAs can also exhibit independent biological activities, including binding to and regulating the function of other biological macromolecules (such as DNA or proteins), and they can also function as enzymes (such as ribozymes)2. There are two categories of RNAs, including coding RNAs and noncoding RNAs (ncRNAs), depending on their protein-coding abilities.The coding RNAs are usually referred to as mRNAs, while non coding RNAs comprise of many subtypes, such as tRNAs, rRNAs, small nucleolar RNAs (snoRNAs), miRNAs,Piwi-interacting RNAs (piRNAs), long noncoding RNAs(lncRNAs), and circRNAs, among others3. The functions of noncoding RNAs are diverse and cover almost all common RNA functions3. As RNA research continues to develop, the boundaries between coding RNA and noncoding RNA are becoming increasingly blurred. Some previously known noncoding RNAs have been found to be able to translate proteins4. For example, toddler is a micropeptide encoded by a noncoding RNA (ENSDARG00000094729 in zebrafish),which plays an important role in zebrafish embryonic signaling5. Some RNAs that were originally considered as coding RNAs have been found to function as noncoding RNAs too, and are thus termed as coding and noncoding RNAs (cncRNAs)6. A typical example of a cncRNA is p53 mRNA, which can not only translate p53 protein in the ribosome but can also directly bind to MDM2 and regulate its function7. Therefore, the functions of RNAs are diverse,and our understanding of RNA functions needs to be improved. It is also necessary to understand the functions of RNAs, especially newly discovered RNA molecules, in many areas.
Major types of circRNAs
CircRNAs are generated from back-splicing of primary transcription products. In normal RNA splicing process,exons edited into final RNA products are arranged in the order of host gene. For circRNAs, the downstream exons are connected to the upstream exons, as shown in Figure 1.There are several types of circRNAs. Most circRNAs shared the same exon sequences corresponding to the host gene.Several circRNAs carry some intron sequences of flanking introns, and are thus termed exon-intron circRNAs(EIciRNA)8. Further, some circRNAs could also contain only intron sequences, termed as ciRNA9.
Brief history of circRNA
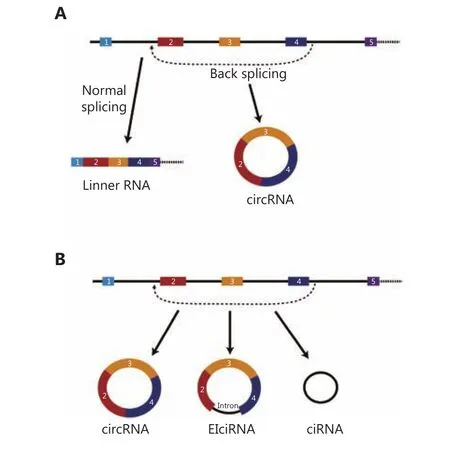
Figure 1 Types of circRNAs. (A) Back-splicing model for circRNAs. (B) Major types of circRNAs.
CircRNAs are a special type of noncoding RNA that directly and covalently bind their 5' and 3' ends together and form a closed single-strand annular structure. The first publication on circRNAs by Sanger et al.10, which appeared in the literature as early as 1976, reported that the plant viroid genome is a single-stranded circRNA. However, for many years, very few reports on circRNAs have been published,generally because most endogenous circRNAs are expressed at a low level and the sequencing technology does not have enough resolution power. Many circRNAs have been identified and characterized after the development of secondgeneration sequencing technology, and have been found in humans and other species. In 2012, Salzman et al.11reported hundreds of back-spliced RNA products that were resistant to exonuclease RNase R, which represent circular RNAs.Since then, many circRNAs have been found in a variety of cells and tissue samples from human and other species.According to the circBase database, more than 140,000 circRNAs have been reported in human cells12. Earlier,circRNAs were widely considered as a “byproduct” of the RNA splicing process and were rarely considered as functional molecules. However, with the development of processes for circRNA screening and identification based on second-generation sequencing, many circRNAs have been observed to exhibit tissue- and disease-specific expression patterns, which suggested that it is necessary to further explore the biological functions of circRNAs13. Functional models of circRNAs have also been developed. The fascinating features of circRNAs, including relative low abundance, high sequence diversity, tissue-/disease-specific expression, and novel functions, make these studies more appealing.
CircRNAs are expressed in tissue-/disease-specific patterns
Main mechanisms of circRNAs biogenesis
The biogenesis of circRNAs is regulated at several levels.CircRNAs can be derived from exons, introns, and intergenic sequences and even via chromosome translocation14,15. Most circRNAs are derived from exons of coding RNAs. These exon-derived circRNAs are formed by back-splicing, that is,the joining of downstream exon with upstream exons14. Most exon-derived circRNAs share the same sequences with corresponding sequence of host genes. However, there are many alternative splicing products corresponding to the same junction point16,17. This reminds us that the mechanism of circRNA biogenesis is high complex and still largely unknown. To our knowledge, the expression of circRNAs can be regulated by both cis-elements and trans-factors. The ciselement is mostly based on the reverse complete sequences in the flanking sequence of upstream and downstream introns.In the human genome, the most apparent reverse complete sequence is the Alu element18. Inverted repetitive Alu elements (IRAlus) are distributed in both sides of a reverse spliced exon-intron joint point and can improve the complete reversion of flanking introns that enhances circRNA production. In addition to IRAlus, short reverted sequences also support the generation of circRNAs19. With the exception of the reverse complete sequence elements,RNA binding proteins (RBPs) can also aid in the biogenesis of circRNAs. As of now, many RBPs such as MBL, QKI,DHX9, NF90/NF110, FUS, HNRNPL, ESRP1, RMB20,ADAR1, and some components of the splicing complex have been reported to be involved in circRNA biogenesis14,20. The mechanisms of tissue- and disease-specific circRNA biogenesis still remain largely unclear.
Cell-/tissue-specific expression pattern of circRNAs
In addition to the wide sequence diversity of circRNAs, they are always expressed in low levels, and some circRNAs are expressed in a tissue-/disease-specific manner. Many studies have explored the tissue-specific expression patterns of circRNAs. Salzman et al.21reported a systematic analysis and identification of circRNAs from online RNA-seq data obtained at the time from several human cell lines as well as Drosophila brain. This study revealed that the expression of circRNAs was cell-type specific. Dang et al.22analyzed the single cell expression pattern of circRNAs in human preimplantation embryos, and found that expression pattern of circRNA undergo a very large dynamic process accompanying the progression of embryo. In another single cell circRNA study, Koh et al.23reported that expression of circRNAs generated from ASXL1 gene exhibited high diversity among cells. Xu et al.24reported the circRNA expression profiles of several human normal tissues. There were 36 samples from adult tissues in this study, including 15 samples from six adult normal tissues (colon, heart, kidney,liver, lung, and stomach), 12 samples from six fetal normal tissues (colon, stomach, liver, heart, lung and kidney), and nine samples from four normal gland tissues (adrenal gland,mammary gland, pancreas, and thyroid gland). The results showed that the expression patterns of circRNAs in different tissues are highly diverse. In another study, Maass et al.25also reported similar results for other types of tissues and cells.Among these tissues, the expression pattern of circRNAs in the brain was highly diverse. Rybak-Wolf et al.26reported that in the mammalian brain, circRNAs are highly abundant,conserved, and dynamically expressed. The authors separated different regions of the brain, primary neurons, and isolated synapses, and found that thousands of circRNAs expressed in these tissues and cells. Some of the circRNAs were conserved between human and mouse, and some were even conserved in Drosophila. The expression of circRNAs in the brain is dynamic, and it was found that ADAR1 negatively regulated the expression of circRNAs26. The expression of circRNAs in different tissues or cells is dynamically regulated by cell-/tissue-specific mechanisms, which are still largely unknown.
CircRNAs in human brain
Compared to other tissues, circRNAs are more diverse in neuronal tissues27. Rybak-Wolf et al.26reported that circRNAs are dynamically expressed at high level in mammalian brains. In this study, authors combined online data from ENCODE project and RNA-seq in separate brain regions, primary neurons, and isolated synapses samples, and found 65, 731 brain-specific circRNAs26. A wide variety of circRNAs have also been found in mouse and Drosophila28,29.Xia et al.30collected the tissue-specific circRNAs in human and mouse tissues, and found that there are 89, 137 brainspecific circRNAs in human fetus. Why circRNAs tend to be enriched in brain tissue? One possible reason is abundant alternative splicing factor and RBP in brain tissues30. Many brain-specific circRNAs are gathered in the synaptic neuropil28. However, the cell type specific expression pattern of circRNAs in neuron and glial cells is still unknown.CircRNAs also play some important roles in brain or central nervous system diseases, such as Alzheimer's disease (AD),Parkinson's disease (PD), and cerebrovascular disease31.
Disease-specific expression pattern of circRNAs
circRNAs are also expressed in disease-specific patterns. The expression of circRNAs is different between diseased and normal tissues, demonstrating a disease-dependent expression. A large number of disease-specific circRNAs has been reported in many types of human diseases, such as cancer, cardiovascular diseases, neurological disorders,diabetes, atherosclerosis, infectious diseases, aging-related diseases, and physiological decline32,33. Guarnerio et al.15reported that the circRNA that generated from a fusion gene(f-circRNA) can directly function as an oncogene in human leukemia. They found that f-circRNA generated from MLL/AF9 fusion gene (F-circM9) can directly influence cell transformation, growth, and drug resistance, as well as promote tumor formation in vivo15. Shan et al.34reported that circHIPK3 is significantly upregulated in diabetic retina.In diabetic retinopathy, circHIPK3 inhibited the function of miR-30a and enhanced endothelial proliferation and vascular dysfunction34. Besides, there are large numbers of reports on association between circRNAs and human diseases. This proves the importance of research about circRNAs in human diseases.
General characteristics of circRNAs in human cancers
The expression of circRNAs in many human cancers shows a high degree of diversity. Many studies have aimed to identify cancer-specific circRNA expression, which may play an important role in cancer diagnosis and treatment. Almost all types of human cancers have been studied for circRNA expression, and some important cancer-specific circRNAs have been reported.
A thorough list of human cancer-specific circRNAs has been generated13,35. The functional models of these circRNAs cover the following four main functions: miRNA sponge,protein sponge, protein translation, and scaffold for protein complex13. Among these circRNAs, some act as oncogenes(e.g., circPVT1, CDR1as, and cirMYLK), while others function as tumor suppressors (e.g., circITCH, circFOXO3,and circMTO1)13. Therefore, the cancer-specific expression status and functional mode of circRNAs may be used in cancer diagnosis and treatment in the future.
Diverse functions of circRNAs
As a newly discovered type of ncRNA, circRNAs share many functions with traditional ncRNAs. At present, five functional models of circRNAs have been reported: (1)circRNAs function as “miRNA sponges”, which competitively bind endogenous miRNAs; (2) circRNAs function as “protein sponges”, which competitively bind endogenous RNA-binding proteins; (3) circRNAs function as“coding RNAs”, which means that they directly encode a micropeptide or protein; (4) circRNAs function as “protein scaffolds”, which means that circRNAs directly bind to proteins and maintain the stability of the protein complex;(5) circRNAs have “prion-like functions”, which indicates that circRNAs exhibit prion-like activity by catalyzing secondary structure changes.
CircRNAs function as miRNA sponges
Competitive endogenous RNAs (ceRNAs) belong to the general functional model family of ncRNAs. In this model,some ceRNAs can regulate target RNAs by competitively binding other RNAs (usually miRNAs), which prevents the binding of miRNAs with their target RNAs and inhibits the function of these miRNAs. Many reports have considered the miRNA sponge function of circRNAs. A typical example of an miRNA sponge is the antisense transcript of the cerebellar degeneration-related protein 1 (CDR1as) gene36,37. Mature CDR1as, which is located in the cytoplasm, has 74 miR-7 binding sites that can specifically absorb miR-7 molecules. As a result, the miR-7 downstream genes are released from binding to miR-7. In this model, the level of molecular miRNA sponging can subtly regulate the binding panel of target miRNAs. In addition to miR-7 binding sites, binding sites for miR-671 have also been studied. When miR-671 binds to CDR1as, pathways that are mediated by AGO2 RNA interference are activated and CDR1as are degraded. Thus,the absorbed miR-7 is released to regulate downstream genes36,37(Figure 2A).
CircRNAs function as protein sponges
Similar to their role as miRNA sponges, circRNAs also act as protein sponges and can bind to certain RNA-binding proteins and influence their location and function. A typical example of a circRNA protein sponge is the muscleblind(MBL/MBNL1) gene38. circMBL and the flanking introns contain several MBL binding sites. This circRNA can competitively bind to MBL protein and influence the function of MBL38. Another example of a protein sponge is circPABPN139. circPABPN1 can bind HuR protein and influence its binding to PABPN1 mRNA39(Figure 2B).
CircRNAs can be directly translated into peptides or proteins
Similar to linear ncRNA, circRNAs also have coding potential. As of now, five reports have confirmed the translation of circRNAs. Yang et al.40reported that the translation of circRNAs is modified by m6A and a novel capindependent translation mechanism involving eIF4G2 and m6A reader, YTHDF3. Legnini et al.41reported that circ-ZNF609 can be translated by a cap-independent translation mechanism. Using ribosome footprint profiling, Pamudurti et al.42reported that a group of circRNAs is associated with ribosomes, which indicates that these circRNAs can be translated into proteins. Yang. et al.40reported that circFBXW7 has coding potential and that its translated product could be verified by an antibody targeted to related sequences43. Zhang et al.44reported that circSHPRH also has coding abilities and that its translated peptide could inhibit the progression of glioblastoma (GBM) (Figure 2C). With further research, we believe that more examples of circRNAs that can be translated into proteins could be found in the near future.
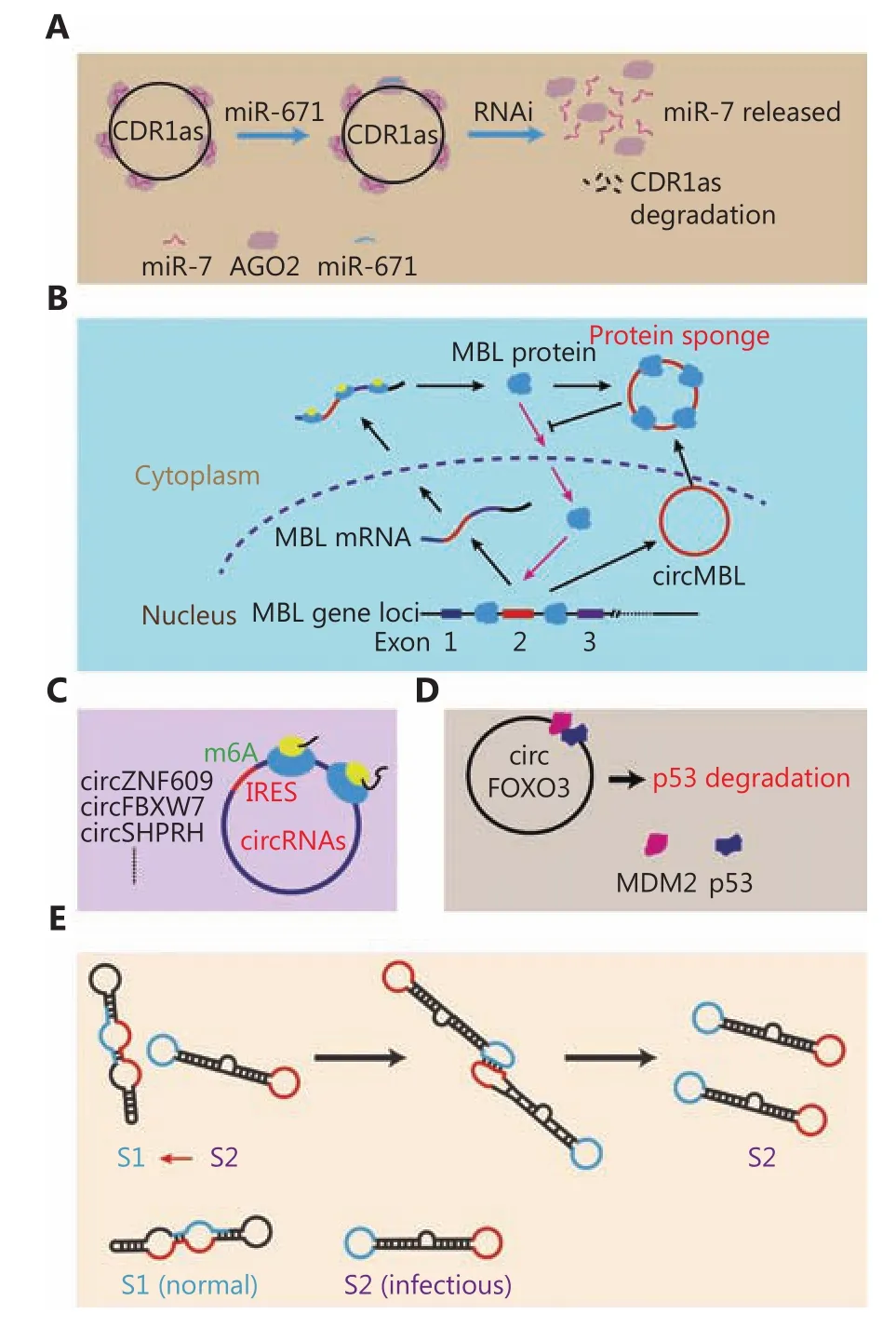
Figure 2 Function model of circRNAs. (A) MiRNA sponge model.(B) Protein sponge model. (C) Ttranslated into peptides. (D)Complex scaffold. (E) Prion-like activity.
CircRNAs function as protein scaffolds
In contrast to the protein sponge model, as scaffolds,circRNAs provide sites for protein interactions and for the formation of larger functional complexes. Li et al.8reported that a type of circRNA in which exons are circularized with introns ‘retained' between exons (EIciRNA) could bind to U1 snRNP, promote the binding of U1 snRNP to RNA polymerase II, and enhance the transcription of host target genes. In this case, EIciRNA acts as a linker of U1 snRNP and the RNA polymerase II complex. Du et al.45reported that circFOXO3 could bind to both p53 and MDM2 and could enhance ubiquitination and degradation of p53. However,circFOXO3 exhibits low binding affinity to the FOXO3 protein, which results in a reduction in the interaction between FOXO3 and MDM2 and prevention of ubiquitination of FOXO3 by MDM2 (Figure 2D). In this model, circRNAs function as linkers between proteins.
CircRNAs with prion-like activity
Ribozyme is a molecule that can catalyze specific biochemical reactions. Whether circRNAs can act as functional molecules,like ribozymes, remains to be determined. Badelt et al.46reported a circRNA molecule that could function similarly to a prion (Figure 2E). In this study, the authors designed a circRNA that could trigger the autocatalytic replication of molecules by interaction. The structures of circRNAs are still poorly understood, and thus, further verification is needed to determine if such a molecule actually exists. However, this study provided some clues that circRNAs may also possess properties consistent with those of prions or ribozymes.
CircRNAs in human glioma
Expression pattern of circRNAs in human glioma
According to the Global Cancer Statistics, 2018, new cases of central nervous system (CNS) tumors comprise 1.6% of all new tumor cases and 2.5% of all cancer-related deaths47.Glioma is a common type of CNS tumor and includes two major subgroups: nondiffuse glioma (glioma cells showing a more limited circumscribed growth pattern) and diffuse glioma (glioma cells exhibiting extensive invasive growth into the surrounding CNS)48,49. Diffuse glioma is the most frequent CNS tumor especially in adults48. Diffuse gliomas mainly include IDH-mutant astrocytoma,oligodendroglioma, and glioblastoma (GBM, both IDH-mutant and wild-type)49. Several studies have focused on the expression pattern of circRNAs in glioma.
Song et al.50conducted a study that included seven oligodendrogliomas, 20 glioblastomas and 19 normal brain specimens to explore the expression level of circRNAs using high-throughput sequencing. The authors developed a computational pipeline termed UROBORUS to analyze the data in this study. They found that the total number of detected circRNAs in GBM was significantly lower than that in normal brain tissue and in oligodendroblastoma samples.However, no difference was observed between normal brain tissue and the oligodendroblastoma samples. In summary,the authors found 572 highly expressed circRNAs (RPM >0.1) in 46 samples. Among them, 476 circRNAs were differentially expressed between GBM and normal brain tissues. Among the 476 circRNAs, 468 exhibited higher expression in normal brain tissues than in GBM samples, and eight exhibited higher expression in GBM than in normal brain tissues50. However, even among normal brain samples from different parts of the brain, different circRNA expression was observed50. This study provided strong evidence of tissue- and disease-specific expression patterns of circRNAs.
Wang et al.51analyzed 33 paired IDH wild-type GBM and para-cancerous tissues by microarray and found 254 upregulated and 361 downregulated circRNAs in GBM compared with para-cancerous tissues (fold-change > 1.5).Wang et al.52also reported circRNA expression in GBM by microarray. In this study, they selected three paired GBM and para-cancerous tissues, and subjected them to a microarray analysis (circRNA, lncRNA, mRNA), and found 548 upregulated circRNAs/lncRNAs in GBM (fold-change > 2, P< 0.05)52. Zhang et al.44compared the circRNA expression level between ten paired glioma and adjacent normal brain tissues by high-throughput sequencing and found upregulation of 2,709 differentially expressed circRNAs (foldchange > 2). Furthermore, the authors also performed a microarray assay to analyze 12 primary GBMs and five normal brain specimens, and they found 709 differentially expressed circRNAs in GBM. In all, 105 differentially expressed circRNAs were found after cross-matching the above two datasets44. Xu et al.53analyzed circRNA expression by downloading RNA-seq data for three paired glioma and normal brain tissues from the Gene Expression Omnibus(GEO) database. Using five different circRNA analysis tools,they found 12 commonly expressed circRNAs. Though many studies have focused on the differential expression of circRNAs in glioma, rare circRNAs have been observed. The expression pattern of circRNAs in glioma may also show a high degree of diversity among individuals. This indicates the necessity of a larger sample size for screening.
In addition to these high-throughput screening studies,several studies have reported that some circRNAs may be potential biomarkers of glioma. Song et al.50reported that the eight highly expressed GBM-specific circRNAs might be good GBM-specific biomarker candidates. However, the authors did not further verify the expression of these circRNAs in a larger number of samples. Barbagallo et al.54detected the expression level of circSMARCA5 in 56 formalin-fixed paraffin-embedded (FFPE) GBM biopsy samples and 7 normal controls. The results showed that circSMARCA5 was significantly downregulated in GBM compared with the control, while the levels of linear mRNA of the host gene SMARCA5 were not significantly different54. Bian et al.55analyzed the expression level of circCFH in 31 glioma tumor samples and paired adjacent normal tissues55, and showed that circCFH was significantly upregulated in both grade I-II and grade III-IV glioma samples55. Xie et al.56reported the expression of hsa-circ-0012129 in 31 paired glioma tumors and adjacent normal tissues, and the results revealed a significantly higher expression of hsa-circ-0012129 in glioma tissues compared with adjacent tissues.
In summary, circRNAs that may be candidate biomarkers of glioma are listed in Table 1, and the sample number of glioma tissues and normal controls is also shown.
Function of circRNAs in human glioma
In addition to the expression analysis of circRNAs in glioma,knowledge of the functions of circRNAs in glioma has also advanced. Here, we summarized the functional models, roles in glioma carcinogenesis, and the values of candidate circRNAs in the treatment of glioma.
Modes of circRNA function in glioma
Along with the development of functional studies of circRNAs, knowledge of the functional models of circRNAs in glioma has also progressed. Several circRNAs function as miRNA sponges that influence many important genes and pathways related to glioma. circFBXW7 and circSHPRH can be directly translated into proteins, and both play a role in the inhibition of GBM progression. circSMARCA5 can bind to SRSF1 and regulate the splicing process. Other functional models of circRNAs have not been reported until now.
Several studies have focused on the miRNA sponge function of circRNAs in glioma. Wang et al.52reported that circNT5E can bind to miR-422a and inhibit its activity. In this study, they selected miR-422a as a candidate miRNA to screen for circRNAs that directly bind to miR-422a. miR-442a is a brain-enriched miRNA that also plays a role in the progression of head and neck squamous cell carcinoma65,squamous cell lung cancer66, and glioma67. Both bioinformatic prediction analysis and a biotin-labeled miR-442a pull-down assay confirmed the binding of miR-442a with circNT5E, which was significantly upregulated in glioma samples52. In this study, using RNA pull-down assay and fluorescence in situ hybridization (FISH) to verify the interaction between miR-442a and circNT5E, the authors showed that circNT5E could bind to miR-442a and inhibit itsfunction. In this case, the expression levels of miR-442a and circNT5E were opposite of each other. This relationship between circRNA and target miRNA is pervasive in many reports considering the role of circRNA as an miRNA sponge. However, in the earliest miRNA sponge model of CDR1as and miR-7, the expression level of CDR1as was not influenced by miR-7, but rather, was regulated by another miRNA, miR-67136,37. Similarly, Xu et al.53also found that circNFIX interacted with miR-34a-5p using RNA immunoprecipitation (RIP). In this case, circNFIX could bind to miR-34a-5p, and NOTCH1 was found to be a target of miR-34a-5p. When circNFIX was inhibited by siRNA, the expression level of miR-34a-5p was increased, and NOTCH1 was inhibited53. The relationship of circRNAs and related miRNAs in this model will be discussed in the next part of this review.
The miR-671/CDR1as/miR-7 axis also plays a role in glioma. Barbagallo et al.68analyzed the expression of miR-671 in 45 GBM samples and five GBM cell lines, and the results showed that the miR-671/CDR1as/miR-7 axis was also present in GBM. Overexpression of miR-671-5p significantly increased migration but had a lower impact on cell proliferation in GBM68. In addition to these cases, many other reports based on the miRNA sponge model in glioma have been published, and these are summarized in Table 2.
In addition to their ability to act as an miRNA sponge,circRNAs that are translated into peptides or proteins have also been reported. Using ribosome footprinting, Yang et al.43found that circ-FBXW7 may be translated. circ-FBXW7 contains a spanning junction open reading frame (ORF),which encodes a 185-aa protein, termed as FBXW7-185aa.The translation of circ-FBXW7 is driven by an upstream internal ribosome entry site (IRES). The translation product,FBXW7-185aa, was verified by an antibody generated inhouse that targets the related sequences; mass spectrometry fingerprint analysis to determine the related peptide sequences also revealed the translation of circ-FBXW7.Similar to circ-FBXW7, the same group found another circRNA that could be translated. Carrying an IRES and an ORF, the translation product of circ-SHPRH could also be verified by an antibody generated in-house and by mass spectrometry fingerprint analysis44. It is noteworthy that the ORF in circ-SHPRH is an overlapping codon, including the start codon “AUG” and the stop codon “UGA” using the same “A” base44. This might be the first finding of an overlapping codon in mammalian coding genes.
In addition, circRNAs that function as scaffolds for protein complexes have also been reported in glioma. Using enhanced UV crosslinking and immunoprecipitation(eCLIP) to screen for proteins that interact with circSMARCA5, Barbagallo et al.54found an interaction between circSMARCA5 and SRSF1. Overexpression of circSMARCA5 resulted in the upregulation of SRSF3, which positively regulated glioma cell proliferation54.
Oncogenic role of circRNAs in glioma
Given the glioma-specific expression pattern of circRNAs,their function in glioma carcinogenesis has also received considerable attention. Functioning in an oncogenic role, a series of circRNAs have been reported to be promoters of the malignant progression of glioma cells (Figure 3A).circNFIX is upregulated in GBM tissues and cell lines53.The inhibition of circNFIX could inhibit cell migration and proliferation by downregulation of the NOTCH1 pathway53,which indicates that circNFIX participates in the improvement of glioma progression. circNT5E can affect the cell proliferation, invasion, and migration abilities of GBMcells by sponging miR-422a52. Zheng et al.69reported that circ-TTBK2, but not the corresponding TTBK2 linear mRNA, was upregulated in human glioma cells and tumor samples. The overexpression of circ-TTBK2 was found to promote cell proliferation, migration, and invasion in GBM,which further indicates that circ-TTBK2 plays a role in glioma progression69. Chen et al.60reported that the inhibition of hsa_circ_0000177 by RNAi could significantly inhibit cell proliferation and invasion. Hsa_circ_0000177 participates in glioma progression by sponging miR-638 and influencing the FZD7/Wnt7 pathway60. In addition to the circRNAs mentioned above, circ-CFH55, hsa-circ-001212956,hsa_circ_004670157, and circHIPK362have also been reported to promote glioma cell proliferation and migration.
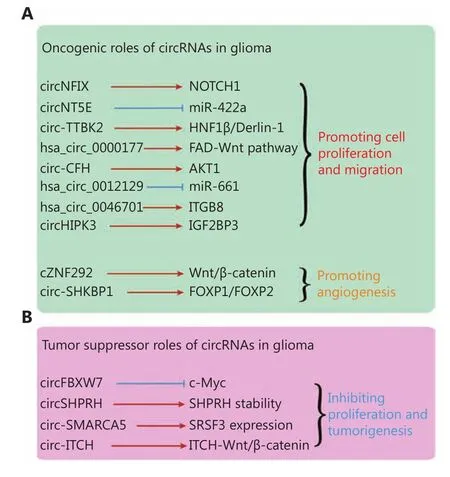
Figure 3 Function of circRNAs in glioma. (A) Oncogenic roles of circRNAs in glioma. (B) Tumor suppressor role of circRNAs in glioma.
In addition to cell proliferation and migration, circRNAs can also promote angiogenesis. He et al.58found that circ-SHKBP1, but not the corresponding SHKBP1 mRNA, was upregulated in U87 glioma-exposed endothelial cells (GECs)compared with astrocyte-exposed endothelial cells (AECs).The knockdown of circ-SHKBP1 could significantly inhibit cell proliferation, migration, and tube formation of GECs.The circ-SHKBP1 influences GECs via the miR-544a/FOXP1 and miR-379/FOXP2 pathways58. In another study, Yang et al.73reported that cZNF292 could participate in tube formation in human glioma. cZNF292 circRNA was also found to inhibit tube formation in glioma cells via the Wnt/β-catenin pathway73.
Tumor suppressor role of circRNAs in glioma
On the contrary, many circRNAs also function as tumor suppressors in glioma (Figure 3B). FBXW7-185aa, encoded by circ-FBXW7, can significantly inhibit cell progression,migration, and tumor formation in vivo43. In one study,FBXW7-185aa reduced the half-life of c-Myc by antagonizing USP28-induced c-Myc stabilization43. An in situ GBM mouse model revealed the tumor suppressing effect of FBXW7-185aa but not of circ-FBXW7 circRNA with an IRES mutation43. In addition, circ-SHPRH also could be translated into a peptide of 146aa, termed SHPRH-146aa44. SHPRH-146aa is downregulated in GBM compared with paracancerous tissues, and in one study, the overexpression of SHPRH-146aa significantly inhibited glioma growth in xenograft mouse models. SHPRH-146aa could also protect full-length SHPRH protein from degradation by the ubiquitin proteasome system44.
In addition to circ-FBXW7 and circ-SHPRH, some other circRNAs have been reported to participate in the inhibition of glioma progression. circSMARCA5 is downregulated in glioma, and the overexpression of circSMARCA5 could increase the expression of SRSF3 and positively regulate the proliferation of glioma cells54. As reported, circ-ITCH59and hsa_circ_000164961also function as tumor suppressors in glioma.
CircRNAs as therapeutic targets or strategies in glioma
CircRNAs have various roles in human glioma. In the future,due to their functional characteristics, circRNAs could be used as therapeutic targets or as components of strategies for the treatment of glioma. According to several reports, the knockdown of these circRNAs could significantly inhibit cell proliferation, migration, or angiogenesis. The use of siRNAs specific to these circRNAs might be a good approach for the treatment of glioma in the future. On the contrary, circRNAs that act as tumor suppressors can be used as part of an overexpression strategy.
Besides these traditional ideas, some new progress has been made in engineering circRNAs, which may lead to novel circRNA-based treatments for glioma. Wesselhoeft et al.74invented a novel method to express proteins by engineering a circRNA vector. Since circRNAs are relatively stable compared with normal linear RNA, protein expression based on circRNAs may improve production efficiency. The results showed that exogenous circRNAs produced exceptionally good protein and exhibited stable production. This suggested that circRNAs might be a good and highly efficient RNA molecule that can be used for protein expression. Meganck et al.75constructed a tissue-specific circRNA expression vector based on recombinant adeno-associated viral (AAV) vectors.The results showed that this vector efficiently expressed circRNA and translated circRNAs in mouse brain and eyes as well as the heart75. Based on these two reports, we could design circRNAs with specific brain targets based on a protein expression system for the treatment of glioma in the near future.
In addition, the miRNA sponge function of circRNAs can also be used in glioma treatment in the future. Recently, Liu et al.76reported a circRNA carrying five miR-21 binding sites,termed scRNA21, which can significantly decrease the expression level of miR-21 and inhibit cell proliferation of gastric cancer cell lines. This work is not related to glioma,but the idea of the design and synthesis of a circRNA that targets a certain miRNA is valuable for glioma treatment.miR-21 and many other miRNAs have some important roles in glioma progression77, which has led to the idea that scR21 can also be used to interfere with these miRNAs in glioma.AAV-based circRNA expression vectors are a powerful choice for this idea.
Discussion
The role of RNA in cellular activities is multilayered and multifaceted. CircRNAs, as the most recently discovered RNA molecule, still follow the basic rules of RNA. The current knowledge of circRNAs supports this view. In the human genome, circRNAs are mostly functional molecules in various tissue and cells, and they are expressed in a dynamic manner and function at several levels. However, further detailed important ideas and assumptions still require further exploration.
Challenges of circRNA research in glioma
Although there are a large number of reports about circRNAs in human glioma as well as other diseases, there are many challenges related to circRNA research. Glioma is a highly heterogeneous disease; its pathological manifestation among patients even at different stage of the same individual is everchanging. In this way, relationship between circRNAs and glioma clinical features is complex and still largely unknown.The mechanism of glioma-specific circRNA expression pattern need to be explored in the future. Furthermore,knock-out model for circRNAs is still a challenge till now.Most circRNAs are generated from coding genes, so accurately knock-out of circRNAs without influencing host gene is still unfeasible for most circRNAs. General low expression level may also hinder the clinical translation for circRNA in glioma and other human diseases.
Nomenclature for circRNAs is still disputed
Not synchronized with the high-speed development of circRNA researches, some important questions have not been solved till now. The nomenclature for circRNAs is a typical question for circRNA. The most used circRNA naming system is based on circBase database ID number12, which consists of a string of numbers and letters. This ID number has no information about the circRNA source and is difficult to remember and orally communicate. The naming rules for circRNAs are urgently needed. A good naming system needs to contain the information of circRNA host gene and should be easy to remember and orally communicate.
Are circRNAs the regulators or targets of miRNAs?
Binding to miRNAs is the most mechanistic model for circRNAs, and experimental evidences have confirmed the binding between circRNAs and miRNAs. The miRNA sponge model is an extension of the ceRNA model, in which RNAs that function as ceRNAs could only bind to target miRNAs,block their free movement, and bind to target molecules. In the case of CDR1as and miR-7, the expression level of CDR1as is not influenced by miR-736,37. MiRNAs function via base-pairing of their seed regions with complementary sequences of their targets78. In the case of mRNAs, miRNAs can target mRNAs via inhibition of protein translation79,regulation of their promoters80, as well as promotion of deadenylation and decay of target mRNAs81,82. In the case of circRNAs, although they can also have translation abilities,few studies have been reported on this topic. The relationship between circRNAs and miRNAs may not simply be due to miRNA sponge activity, which just emphasizes the regulatory role of circRNAs with respect to miRNAs. Conclusive evidence considering circRNAs as the target of miRNAs has already been shown, using miR-671 and CDR1as. Only one site on miR-671 binds to the CDR1as sequence, but this site in miR-671 has near-perfect complementarity and exhibits very little variation across species37. As a result, the expression level of CDR1as is tightly regulated by miR-671 and is based on an RNA interference mechanism. After a review of the relevant reports, in many cases, the expression levels of circRNAs and miRNAs were usually altered in a mutual cause and effect manner. This phenomenon could not be explained solely by the miRNA sponge model.
Are circRNAs the result of alternative splicing?
Alternative splicing is more active in cancer than in normal tissues83. The biogenesis of circRNAs deeply depends on the RNA splicing process14. Many cancer-specific circRNAs are expressed, but the mechanism that underlies their biogenesis has not been fully described. Whether tissue-, cancer-, or disease-specific circRNAs are regulated by alternative splicing remains unknown. The answer to this question may, at least in part, explain the specific expression pattern of circRNAs.Gene fusion by chromosome translocation is also widely found in high grade glioma and secondary GBM (sGBM).Some fusion genes play an oncogenic role in GBM, such as FGFR-TACC, and PTPRZ1-MET84,85. FGFR-TACC fusion gene account for 3% of human glioblastoma cases85; this fusion gene causes a metabolism shift in fusion gene positive cells by activating oxidative phosphorylation and mitochondrial biogenesis pathway, generating sensitivity for inhibitors of oxidative metabolism86. As previously reported,circRNAs derived from fusion gene can also function as oncogenes15. What about circRNAs derived from glioma related fusion gene? Were there any circRNAs that derived from FGFR-TACC, PTPRZ1-MET, and many other gliomarelated fusion genes? What are the roles of these circRNAs in GBM? This is an interesting question.
In summary, circRNAs belong to the most recently discovered group of RNA molecules. CircRNAs can function as miRNA sponges, protein sponges, and as scaffolds for protein complexes, and can also be translated into proteins.In some cases, circRNAs may also possess special properties.The expression of circRNAs is cell- or tissue-specific, and many circRNAs are also disease-specific, some of which play important roles in the progression of human cancers. In human glioma, all the major features of circRNAs are exhibited. CircRNAs may also be promising therapeutic targets or components of therapeutic strategies for glioma treatment as well as treatment of other human diseases.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
- Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer
