The breakthrough in primary human hepatocytes in vitro expansion
Mei Feng, Ruirui Kong, Yisheng Pan
1Translational Cancer Research Center; 2Department of General Surgery, Peking University First Hospital, Beijing 100034, China
Introduction
As one of the most frequently diagnosed devastating diseases,liver failure is responsible for approximately two million deaths annually worldwide with poor prognosis1. Although liver transplantation has been developed for the most effective treatment for liver failure, it is far from demands for patients due to the shortage of high-quality donor livers and expensive treatment costs. Currently, with the development of cell therapy, cell transplantations including primary human hepatocytes (PHHs), human hepatocyte-like cells(HLCs) and liver organoids are emerging as great potential tools to alleviate this growing burden. Much work so far has focused on PHHs, since accumulating evidence indicates that HLCs and organoids have very limited engraftment capability and repopulation efficiency. PHHs' virtually clinical application, however, has been markedly restricted by its extreme difficulty to culture and expand in vitro2.
Considerable research efforts have been devoted to tackling these problems in the last few decades. Notably,Khetani and his team3tried to culture and expand the PHHs in a miniaturized, multi-well culture system using elastomeric stencils, as a result, the PHHs can just maintain phenotypic functions for several weeks. In addition, coculture of hepatocytes with endothelial cells in serum-free media under 95% oxygen environment was also established aiming to improve the culture of PHHs. However, the hepatocytes' viability remained rather low in this condition4.Some researchers even attempted to overcome the growth limitations by immortalizing human hepatocytes (HHs) with viral genes such as SV40, E6, and E7 as well as hTERT, but the resorts were stemmed by safety concerns and limited hepatic functions5,6. Then, from a library of 12,480 small molecules, Shan and his group7had been able to identify two groups of small molecules - functional proliferation hit(FPH) for expanding mature human hepatocytes, and functional hit (FH) for inducing human iPS cells to differentiate into mature hepatocytes by high-throughput screening platform. But further tests suggest that there is only a 10-fold increase in the number of hepatocyte expansion.More recently, it was recognized that adding ROCK, TGFβ and GSK3 inhibitors to the medium can achieve the longterm in vitro expansion of mature mouse hepatocytes and turn these cells into bipotent liver progenitor cells with regenerative capacity8. Meanwhile, another laboratory has demonstrated that mouse hepatocytes could be converted to liver progenitor-like cells when cultured in the transition and expansion medium (TEM) with the ability to passage for more than 30 times without apparent morphological changes9. Nevertheless, these methods do not seem to be promising when applied on human hepatocytes. But recently,a research by Hui et al.10brings another player to the table:human hepatocyte medium (HM).
Published in Cell Stem Cell, Hui's study10reported that they may solve the most critical problems for PHHs in vitro expansion by establishing a new human hepatocyte culture system - with the addition of Wnt3a and removal of Rspo1,Noggin and forskolin - as HM in which HHs can achieve a 10,000-fold expansion (Figure 1). Through examining global genes expression profiling and cell function, they find that the proliferating human hepatocytes (ProliHHs) cultured in this system are at a bi-phenotypic “intermediate” status between mature hepatocytes and liver progenitor cells (LPCs)(Figure 1). This bi-phenotypic status closer to primary hepatocytes may enable ProliHHs to function more like PHHs in vitro and in vivo than liver progenitor cells.Moreover, after being cultured in a 3D liver-induced system,ProliHHs showed approximately similar gene expression and cell function to primary hepatocytes. Also, they exhibited the comparable ability to integrate and repopulate in vivo to that of PHHs when being transplanted into the liver of immunodeficient Fah knockout mice (Figure 1). More importantly,the repopulation efficiency of ProliHHs accounts for 64%and remains comparatively higher than before (2%)11,12.Moreover, the transplanted ProliHHs showed similar therapeutic effect in mice compared to primary liver cells in repairing liver damage and improving survival time.
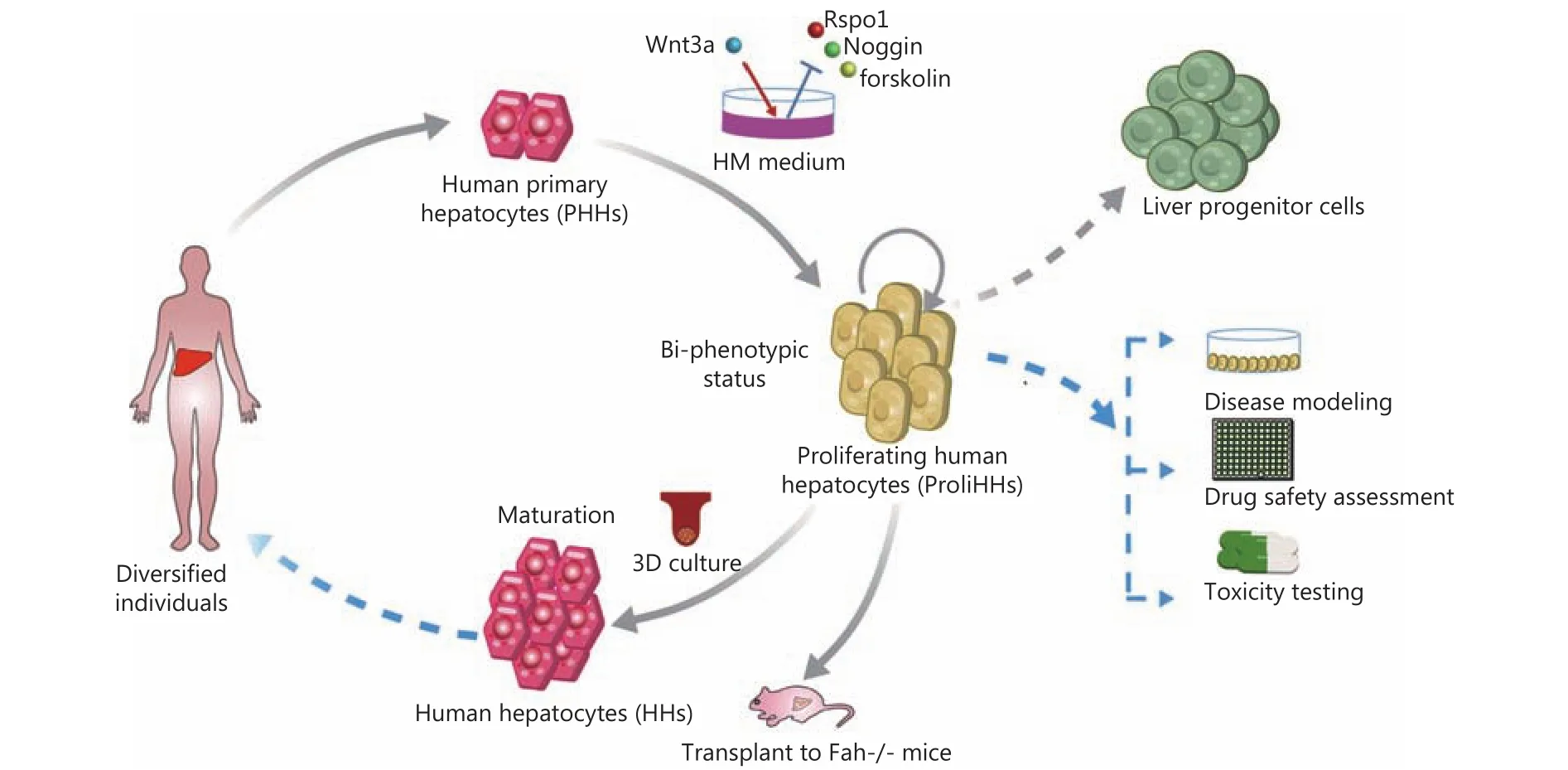
Figure 1 PHHs in vitro expansion. Schematic diagram illustrates the expansion of PHHs from diversified individuals to a large quantity,when cultured in HM established by Hui et al,10 with the addition of Wnt3a and removal of Rspo1, Noggin and forskolin. The ProliHHs maintained a bi-phenotypic status between primary hepatocytes and liver progenitor cells can mature after inducing re-differentiation by 3D culture and repopulate when transplanted into the liver of immuno-deficient Fah knockout mice indicated. Undoubtedly, this will provide a useful model system for disease modeling such as hepatotropic virus infection, as well as for new drug safety assessment and toxicity testing in vitro and paved the way for hepatocyte transplantation therapy to be used on liver disease patients in the future.
Perspective
Key point to this work is technologically that they technologically developed a practical protocol to obtain proliferative, functional human hepatocytes, effectively addressing the problem on lack of human hepatocytes sources. This undoubtedly provides a powerful model system for investigators to study drug metabolism, hepatotropic virus infection, as well as new drug safety assessment, toxicity testing, etc. and lays a solid foundation for hepatocyte transplantation therapy for liver patients (Figure 1).
Another fundamental contribution is that they defined this bi-phenotypic cell status which is between primary hepatocytes and progenitor cells. Previous studies have demonstrated the poor grafting efficiency of PHHs and LPCs whereas this new status cell shows high engraftment capacity in this article. Therefore, this “intermediate” status of cell may open up the possibility for cell therapy in reconstructing the damaged liver and furthermore may also shed some light on other diseases which could be treated with cell transplantations. Since the results of cell-based therapies like using transplants of exogenously derived and in vitro cultured cells or endogenous neural precursor cells on Parkinson's disease have been mostly disappointing13. And several types of cells such as pancreatic progenitors, induced pluripotent stem cells, adult stem cells, multipotent adult progenitor cells, etc. used as renewable sources of insulinproducing beta-cells are not promising as well14. Therefore,the study about a new more suitable “intermediate” status may hold the key to these cell therapies.
Meanwhile, the RNA-sequencing data provided in this article accompanied with additional data in previous studies8,9allow us to characterize PHHs, ProliHHs, HLCs and LPCs. Taken together, these data show us a whole picture of where these cells locate in the transcriptome level respectively, further offering insights into critical molecular mechanisms involved in hepatocytes expansion and repopulation.
Yet, significant impediments still need to be overcome before ProliHHs transplantation become an effective clinical therapy. For one thing, in this article, 11 out of the 14 FRG mice survived more than 4 months after ProliHHs transplantation. Thus, it is imperative to work on prolonging the survival time of mice after ProliHHs transplantation, if they were applied for human in the future. In addition,evidence from that senescence-like phenotype can be induced by the Wnt pathway15make us deeply consider whether adding Wnt3a into culture media may lead to the ProliHHs'senescence.
For the other thing, the GSEA analysis has suggested that the Wnt and YAP pathways played essential roles in inducing ProliHHs and functioned in the process of tumorigenesis, as tumor-associated genes16,17. Hence, it has been a potential risk for tumor growth if the mice survival time was prolonged. Therefore, developing a robust system for early tumor detection after cell transplantation is a priority.Additionally, the possibility of transferring infectious microorganism to the recipient cannot be overlooked as well given that cell transplantation stands a good chance of causing a severe inflammatory response in patients.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (Grant No. 31671452).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- Circular RNAs and human glioma
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
- Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer
