BRAFV600E vs. TIRADS in predicting papillary thyroid cancers in Bethesda system I, III, and V nodules
Ya Wu, Ting Xu,2, Xingyue Cao, Xin Zhao, Hongyan Deng, Jianxiang Wang, Xiao Li, Qing Yao, Xinhua Ye,Meiping Shen, Xiaohong Wu
1Department of Endocrinology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China;2Department of Endocrinology, Jiangsu Province Official Hospital, Nanjing 210009, China; 3Department of Ultrasound;4Department of Pathology; 5Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University,Nanjing 210029, China
ABSTRACT Objective:Bethesda System for Reporting Thyroid Cytopathology (BSRTC) categories I, III, and V account for a significant proportion of fine needle aspiration cytology (FNAC) diagnoses. This study aimed to compare the diagnostic efficacy of BRAFV600E mutation and the Thyroid Imaging Reporting and Data System (TIRADS) classification in differentiating papillary thyroid cancers(PTCs) from benign lesions among BSRTC I, III, and V nodules.Methods:A total of 472 patients with 479 nodules were enrolled in this prospective study. Ultrasound, BRAFV600E mutation testing, and FNAC were performed in each nodule, followed by surgery or regular ultrasound examination.Results:In the BSRTC I category, BRAFV600E showed similar sensitivity, higher specificity, and lower accuracy when compared with TIRADS. In the BSRTC III/V category, the sensitivity, specificity, and accuracy of BRAFV600E were similar to those of TIRADS. In comparison to BRAFV600E alone, the combination of the two methods significantly improved sensitivity (BSRTC I:93.6% vs. 67.7%, P < 0.01; BSRTC III: 93.8% vs. 75.0%, P < 0.01; BSRTC V: 96.0% vs. 85.3%, P < 0.001). When compared with TIRADS alone, the combination improved sensitivity in BSRTC I nodules (93.6% vs. 74.2%, P < 0.05), increased sensitivity and decreased accuracy in BSRTC III nodules (93.8% vs. 75.0%, P < 0.01, 91.0% vs. 93.6%, P < 0.01), and improved both sensitivity and accuracy in BSRTC V nodules (96.0% vs. 82.0%, P < 0.001; 94.2% vs. 81.3%, P < 0.001).Conclusions:BRAFV600E exhibited higher specificity and lower accuracy compared with TIRADS in BSRTC I nodules, while the two methods showed similar diagnostic value in BSRTC III/V nodules. The combination of the two methods distinctly improved sensitivity in the diagnosis of PTCs in BSRTC I, III, and V nodules.
KEYWORDS Papillary thyroid carcinoma; fine-needle aspiration cytology (FNAC); BRAFV600E; thyroid imaging reporting and data system(TIRADS); Bethesda classification
Introduction
Presently, thyroid carcinoma is the fifth most common cancer in women worldwide1. The most prevalent type is papillary thyroid carcinoma (PTC), which accounts for approximately 85%2. Fine needle aspiration cytology (FNAC)has been widely used in the diagnosis of thyroid carcinoma and can provide reliable preoperative diagnostic results. The accuracy of FNAC has been reported to be 62%-85%3.However, FNAC cannot provide a definitive diagnosis when the Bethesda System for Reporting Thyroid Cytopathology(BSRTC) results are indeterminate for categories, including atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) (i.e., BSRTC III),follicular neoplasm/suspicious for follicular neoplasm(FN/SFN) (i.e., BSRTC IV), and suspicious for malignancy(SMC) (i.e., BSRTC V). In addition, nondiagnostic/unsatisfactory (ND/UNS) (i.e., BSRTC I) may be the result in the case of inadequate FNAC specimens. BSRTC category I,III, IV, and V nodules account for 2%-16%, 2%-18%,2%-25%, and 1%-6% of thyroid lesions, respectively, in clinical practice, with considerable malignancy4,5.Accordingly, a number of patients with nodules in these cytological categories undergo surgery to obtain a definitive diagnosis.
To address this situation, molecular biomarkers have been utilized to detect thyroid carcinoma. Among these biomarkers is the most frequent genetic alteration in PTC,BRAFV600E. BRAF encodes a serine/threonine protein kinase,with the most common mutation at the 1799thnucleotide,resulting in the substitution of valine by glutamate at codon 600. BRAFV600Emutation may lead to constitutive activation of the BRAF kinase, and further aberrantly activate the classical thyroid tumorigenic Ras/Raf/MEK/ERK (MAPK)signaling pathway6. BRAFV600Emutation is a specific biomarker for PTC with a mutation rate of 53.0% to 80.6%,depending on geographical factors and iodine intake7-9.Moreover, trace cellular specimens are sufficient for BRAFV600Eanalysis, which makes the mutation a potential promising biomarker for reclassification of nondiagnostic or indeterminate thyroid nodules.
At present, the value of BRAFV600Emutation in differentiating malignant from benign lesions among indeterminate thyroid nodules associated with the Bethesda system remains controversial. A recent meta-analysis including 32 eligible studies proposed that BRAFV600Emutation played a limited role in the diagnosis of indeterminate nodules owing to its low sensitivity, despite a specificity of nearly 100%10. In contrast, one study from China of 314 thyroid nodules including 52 BSRTC III/IV and 13 BSRTC V nodules demonstrated that BRAFV600Emutation could improve the prediction of malignancy in indeterminate nodules9. All of these studies involved BSRTC IV nodules,which are rarely associated with BRAFV600Emutation11. To date, the value of the BRAFV600Emutation in the differentiation of PTCs in BSRTC category I, III, and V nodules has not been well established.
Ultrasound, the most basic method for screening thyroid nodules, plays a guiding role in therapeutic decisions for the management of BSRTC III nodules12. The Thyroid Imaging Reporting and Data System (TIRADS) classification has been adopted by the vast majority of institutions. Our previous work has demonstrated that BRAFV600Emutation and TIRADS classification could both greatly improve the diagnostic efficacy of the Bethesda system13. In the present study, we aimed to further compare the clinical value of BRAFV600Emutation and TIRADS classification for predicting PTCs in BSRTC I, III, and V nodules.
Materials and methods
Patients
Prospective detection of BRAFV600Emutation in FNAC specimens was initiated at the First Affiliated Hospital with Nanjing Medical University in January 2014. A total of 845 patients diagnosed with 857 nodules between that date and March 2018 were selected as potential study subjects.Inclusion criteria were: 1) nodules in BSRTC category I, III,or V; and 2) nodules with TIRADS classification and BRAFV600Emutation detection. Exclusion criteria were: 1)nodules confirmed to be follicular thyroid carcinoma (FTC),medullary thyroid carcinoma (MTC), or anaplastic thyroid carcinoma (ATC) by postoperative histopathology; 2)BSRTC I or III nodules not removed by surgery with a follow-up period less than 1 year; and 3) BSRTC V nodules or nodules with increased size (≥ 20%) in any one dimension by ultrasound with no surgical histopathology results. A total of 472 patients with 479 nodules were finally enrolled in the present study. Surgery was performed on 288 nodules owing to BRAFV600Emutations, local compression symptoms, or suspected malignancy. Follow-up FNAC or ultrasound examination was performed on the remaining 191 nodules.All patients provided informed consent prior to examination,and the study was performed in accordance with the ethical guidelines of the Helsinki Declaration and approved by the institutional ethics review committee (No. 2012-SR-057).
FNAC, DNA extraction, and BRAFV600E mutation detection
FNAC, DNA extraction, and BRAFV600Emutation detection were performed as described previously13. Briefly, 2 to 3 pass aspirates were rinsed in an alcohol-based preservative liquid for cytological examination and 1 pass was placed in an EP tube containing 180 μL DTL buffer (ADx-FF01, AmoyDx,Xiamen, China) for gene analysis. Based on BSRTC, the cytological diagnostic results of all nodules were classified into 1 of 6 categories: I (ND/UNS), II (benign), III(AUS/FLUS), IV (FN/SFN), V (SMC), and VI (malignant)14.DNA was extracted using a commercial kit (ADx-FF01,AmoyDx). The quality of DNA was detected using a NanoDrop2000 spectrophotometer (Thermo Fisher Scientific, Canoga Park, CA, USA). OD260/OD280values of all samples were 1.8-2.0, and concentrations of all samples were adequate. The BRAFV600Emutation was detected using realtime fluorescence quantitative PCR amplification with a qRT-PCR machine (ABI7900, Applied Biosystems, Inc.,Foster City, CA, USA), and the procedure was conducted following the kit manufacturer's instructions (ADx-BR01,AmoyDx). If the sample CTvalue was less than 28, it was regarded as positive (BRAFV600Emutation); otherwise, it was considered negative (BRAFV600Ewild type).
TIRADS classification
Ultrasound examination was performed using a MyLab Twice Ultrasound unit equipped with an LA523 transducer(The Esaote Group, Genova, Italy). The following characteristics of each nodule were carefully evaluated: size,internal components, echogenicity, margins, calcifications,and shape. Malignant ultrasound features, including solid components, hypoechogenicity or marked hypoechogenicity,microlobulated or irregular margins, microcalcifications, and taller-than-wide shape, were based on those proposed by Kwak et al.15. According to the number of ultrasonic risk features, each thyroid nodule was classified into 1 of 5 grades:TIRADS 3 (no suspicious characteristics), TIRADS 4a (1 suspicious characteristic), TIRADS 4b (2 suspicious characteristics), TIRADS 4c (3 or 4 suspicious characteristics), and TIRADS 5 (5 suspicious characteristics).
Statistical analysis
Statistical analyses were performed using SPSS 23.0 (SPSS Inc., Chicago, IL, USA). Quantitative values were expressed by mean ± standard deviation and analyzed by Student's t test. The Chi-square (χ2) test or Fisher's exact test was applied to evaluate the differences between categorical values.Receiver operating characteristic (ROC) curves sketched by MedCalc 15.2.2 software (MedCalc Software, Ostend,Belgium) were plotted to identify the optimal cutoff for the TIRADS grade according to the Youden index and to compute the sensitivity, specificity, positive predictive value(PPV), negative predictive value (NPV), and accuracy.McNemar's test was used to compare the sensitivity,specificity, and accuracy of different methods. P < 0.05 was considered statistically significant.
Results
Clinical features
A total of 479 nodules from 472 patients with BRAFV600Emutation testing and TIRADS classification were examined in the present study. According to histopathology findings,229 were PTCs (175 classical type PTCs, 52 follicular variant PTCs, and 2 tall cell variant PTCs), and 59 were benign cases(50 nodular goiters, 4 follicular adenomas, and 5 cases of Hashimoto's thyroiditis). The remaining 191 cases were all regarded as benign nodules based on repeated benign FNAC results or no distinct change (< 20%) of nodular size after at least 1 year of ultrasound follow-up.
The clinical features are displayed in Table 1. Malignant nodules displayed significantly lower age and smaller maximal diameters than benign ones (all P < 0.01). Specific information for BSRTC I, III, and V nodules is presented in Table 2. The operative rates, BRAFV600Emutation rates, and malignant rates all increased considerably from BSRTC I to V.
Diagnostic values of TIRADS and BRAFV600E mutation in BSRTC I/III/V nodules
In BSRTC I, III, and V nodules, the ROC curve showed that the optimal cutoff for TIRADS classification was 4c, i.e., cases with TIRADS classification 4c or 5 would be regarded as cancers. The sensitivity, specificity, PPV, NPV, and accuracy of TIRADS classification in BSRTC category I, III, and V nodules were 79.5%, 88.4%, 86.3%, 82.5%, and 84.1%,respectively (Figure 1A).
Among 187 nodules with BRAFV600Emutation, 185 werehistologically validated as PTCs. The remaining two cases proved, after surgery, to be nodular goiter and Hashimoto's thyroiditis. Of 292 nodules without BRAFV600Emutation, 44(15.1%) were diagnosed as PTCs and 57 (19.5%) were diagnosed as benign nodules by histopathology. The sensitivity, specificity, PPV, NPV, and accuracy of BRAFV600Emutation analysis in BSRTC I, III, and V nodules were 80.8%, 99.2%, 98.9%, 84.9%, and 90.4%, respectively(Figure 1A).
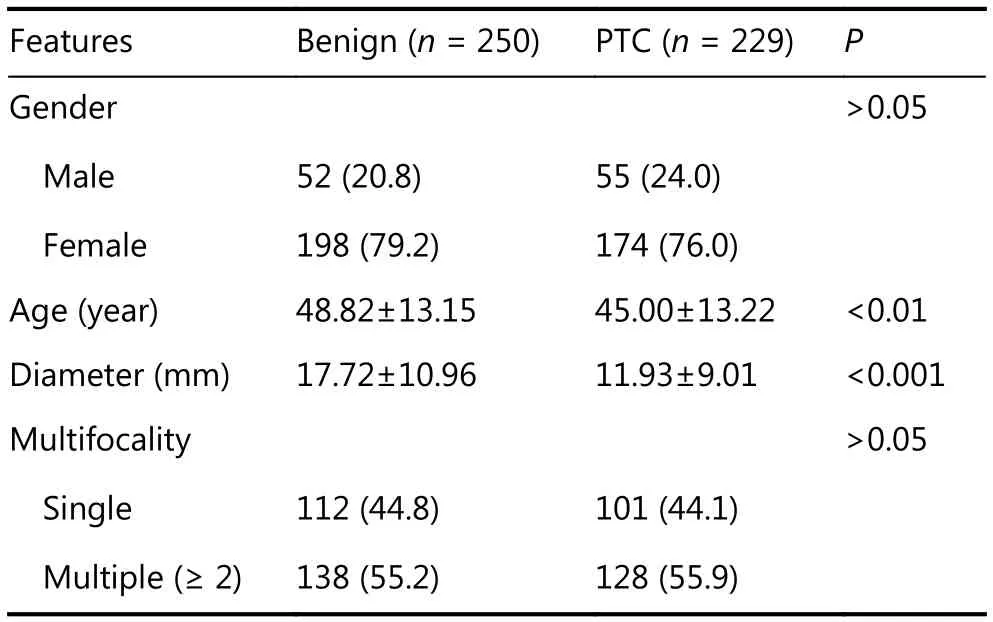
Table 1 Clinical features of the study population and nodules
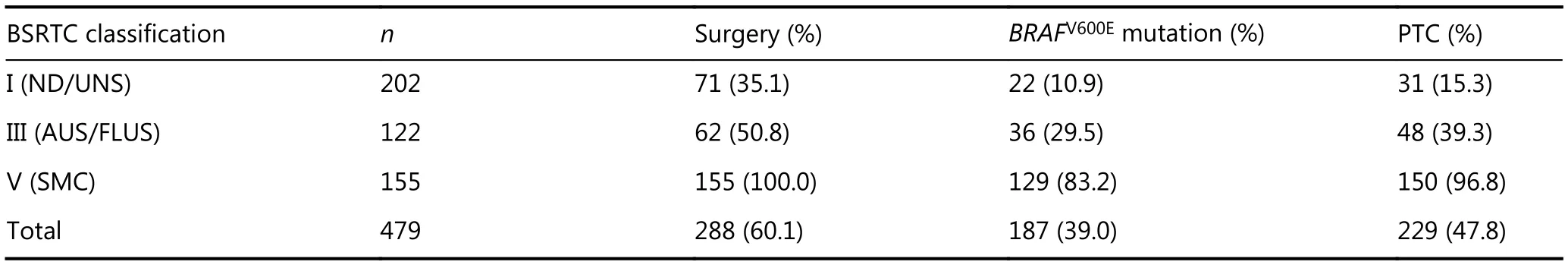
Table 2 Correlation of BSRTC classifications and final diagnosis
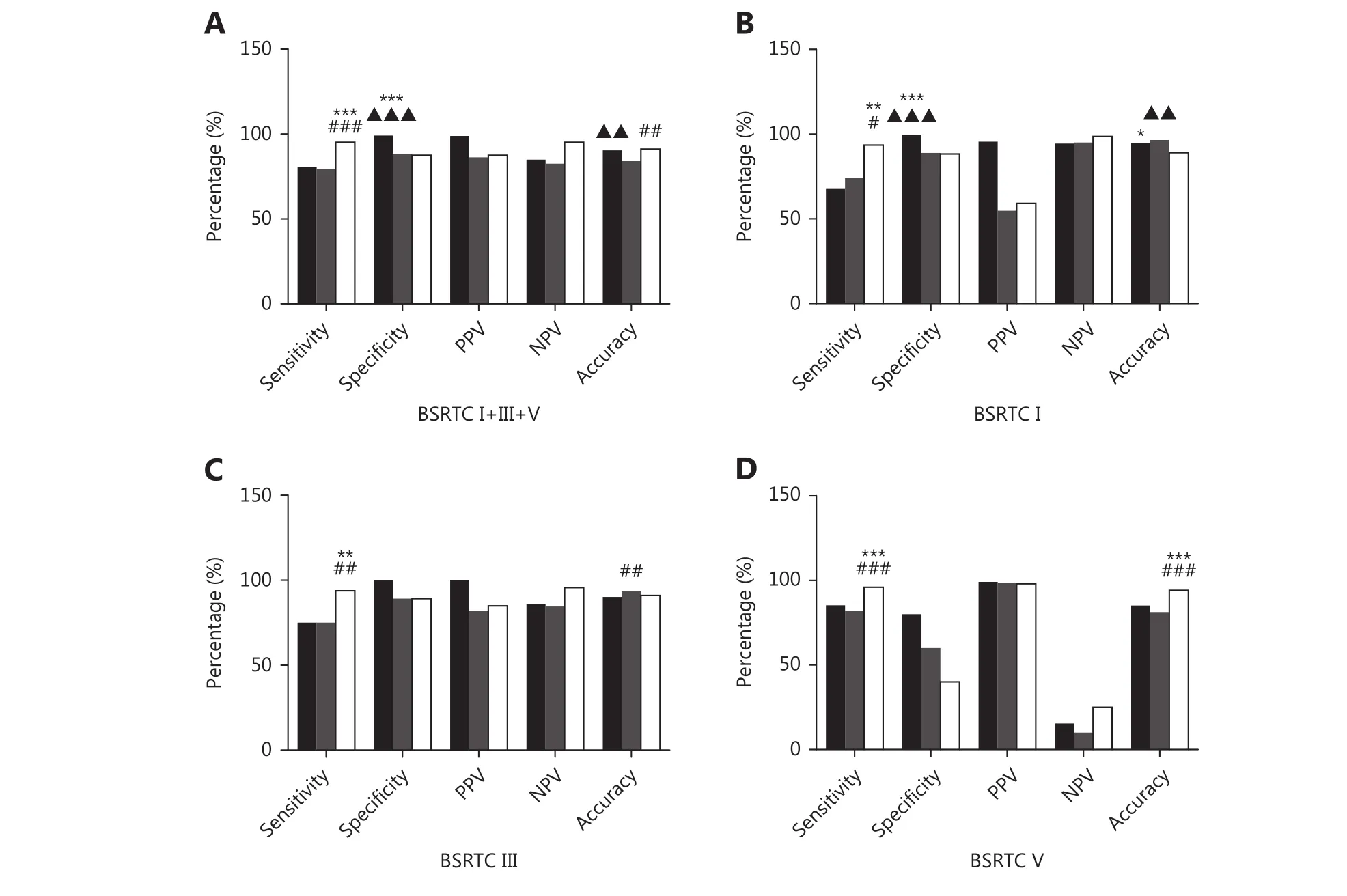
Figure 1 Comparison of diagnostic performance of BRAFV600E, TIRADS, and combination of the two in diagnosing PTCs. (A) Comparison of BRAFV600E and TIRADS in BSRTC I+III+V cases. (B) Comparison of BRAFV600E and TIRADS in BSRTC I cases. (C) Comparison of BRAFV600E and TIRADS in BSRTC III cases. (D) Comparison of BRAFV600E and TIRADS in BSRTC V cases. Black bars, BRAFV600E; grey bars, TIRADS; white bars,combination of BRAFV600E and TIRADS. TIRADS, Thyroid Imaging Reporting and Data System; BSRTC, Bethesda System for Reporting Thyroid Cytopathology; PPV, positive predictive value; NPV, negative predictive value. BRAFV600E mutation vs. TIRADS classification, ▲▲P < 0.01,▲▲▲P < 0.001; BRAFV600E mutation vs. combination of BRAFV600E mutation and TIRADS classification, *P < 0.05, **P < 0.01, ***P < 0.001;TIRADS classification vs. combination of BRAFV600E mutation and TIRADS classification, #P < 0.05, ##P < 0.01, ###P < 0.001.
The diagnostic performances of TIRADS and BRAFV600Emutation in diagnosing PTCs in BSRTC I/III/V nodules are summarized in Figure 1. In BSRTC I nodules, BRAFV600Eshowed similar sensitivity to that obtained with TIRADS.BRAFV600Eexhibited higher specificity and lower accuracy compared to TIRADS (99.4% vs. 88.9%, P < 0.001; 94.6% vs.96.6%, P < 0.01) (Figure 1B). In BSRTC III/V nodules, the sensitivity, specificity, and accuracy of BRAFV600Ewere similar to those of TIRADS (Figure 1C and 1D).
Diagnostic value of the combination of BRAFV600E and TIRADS in BSRTC I/III/V nodules
Regarding the combination of BRAFV600Eand TIRADS, a lesion was predicted to be malignant based on either TIRADS 4c/5 classification or BRAFV600Emutation. When compared with TIRADS alone, the combination showed significantly increased sensitivity (BSRTC I: 93.6% vs. 74.2%, P < 0.05;BSRTC III: 93.8% vs. 75.0%, P < 0.01; BSRTC V: 96.0% vs.82.0%, P < 0.001). The accuracy of the combination decreased when compared with TIRADS in BSRTC III nodules (91.0% vs. 93.6%, P < 0.01), but improved in BSRTC V nodules (94.2% vs. 81.3%, P < 0.001). When compared with BRAFV600Ealone, the combination showed improved sensitivity (93.6% vs. 67.7%, P < 0.01) but decreased specificity and accuracy (88.3% vs. 99.4%, P < 0.001; 89.1%vs. 94.6%, P < 0.05) in BSRTC I nodules, improved sensitivity in BSRTC III nodules (93.8% vs. 75.0%, P < 0.01),and increased sensitivity and accuracy in BSRTC V nodules(96.0% vs. 85.3%, P < 0.001; 94.2% vs. 85.2%, P < 0.001).
Complementary relationship between BRAFV600E mutation analysis and TIRADS classification in BSRTC I/III/V nodules
Although the diagnostic value of BRAFV600Ewas similar to that of TIRADS classification in both BSRTC III and V nodules, the two methods have complementary effects on diagnosis of PTCs (Figure 2). Among 268 benign nodules diagnosed by TIRADS classification, 47 (17.5%) were confirmed to be PTCs by surgery, and 36 (13.4%) harboring BRAFV600Emutation were histopathologically diagnosed as PTCs (Table 3), suggesting that BRAFV600Emutation testing could identify some PTCs that TIRADS classification could not detect. In addition, BRAFV600Ewild type nodules were reclassified as malignant by TIRADS classification in 62(21.2%) cases; 33 of these nodules proved to be PTCs after thyroidectomy (Table 3). Hence, TIRADS classification could also serve as an adjunct to BRAFV600Emutation in the differential diagnosis of PTCs.
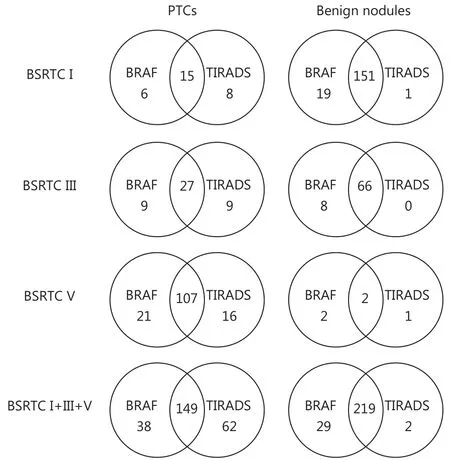
Figure 2 Complementary relationship between BRAFV600E and TIRADS in diagnosing PTCs in BSRTC I, III, and V nodules Intersection areas represent number of nodules correctly diagnosed by both BRAFV600E and TIRADS. Reminders represent number of nodules correctly diagnosed by one method while incorrectly diagnosed by other method. BSRTC, Bethesda System for Reporting Thyroid Cytopathology; TIRADS, Thyroid Imaging Reporting and Data System.
Discussion
The present study findings represent the first comparison of the performance of BRAFV600Emutation analysis and TIRADS classification in the diagnosis of PTCs in BSRTC categories I, III, and V. We discovered that BRAFV600Eexhibited similar diagnostic performance to that of TIRADS in BSRTC III/V nodules, with the exception of higher specificity in BSRTC I nodules. The combination of the two diagnostic approaches significantly enhanced the sensitivity,which facilitated the diagnosis of PTCs in BSRTC I, III, and V nodules.
The rates of malignancy in BSRTC category I, III, and V nodules in the present study were 15.3%, 39.3%, and 96.8%,respectively. These rates were much higher than the estimated malignant risks of these BSRTC classifications14,indicating the overly conservative approaches of pathologists in our institution. Therefore, appropriate auxiliary diagnostic methods were required to help distinguish between malignant and benign lesions among these nodules.Generally, repeated FNAC has been recommended for BSRTC I/III nodules, and surgery has been recommended for BSRTC V nodules by BSRTC14. Currently, ultrasonographic features and molecular markers are used in the attempt to discriminate malignant from benign lesions among cytologically nondiagnostic or indeterminate nodules16-20.
Ultrasound is the most basic method for screening thyroid nodules. According to the guidelines, the risks of malignancy of nodules classified as TIRADS 3, 4a, 4b, 4c, and 5 were <2%, 2%-10%, 10-50%, 50%-95%, and ≥ 95%, respectively15.The malignancy rates corresponding to these TIRADS classifications found in BSRTC I+III+V nodules in our research were 6.3%, 10.5%, 32.0%, 87.1%, and 80.0%,respectively, partially coinciding with the guidelines. Yoo et al.21discovered that taller-than-wide shape, ill-defined margins, and marked hypoechogenicity were malignant predictors in thyroid nodules with AUS/FLUS results. Tallerthan-wide shape and marked hypoechogenicity were also malignant features in the TIRADS scoring system15. Grani et al.22discovered that the sensitivity and specificity of TIRADS in BSRTC III+IV nodules were 53% and 87%, respectively,when selecting 4c as the cutoff point. Our research showed a slightly higher sensitivity and specificity of TIRADS compared to the findings of Grani et al. These results may be attributable to different study populations and types of thyroid cancers, as well as the inclusion of the BSRTC IV category in the study by Grani et al.

Table 3 Correlation of TIRADS classification with BRAFV600E mutation in BSRTC I/III/V categories
BRAFV600Ehas been used extensively to improve the diagnosis of malignancy in thyroid nodules. In our study, this genetic mutation was observed in up to 80.8% of PTCs,consistent with the previously reported rate of 76.5% in a Chinese population9. A comparably high prevalence of BRAFV600Emutation was also reported in a Korean population8. The specificity of BRAFV600Ein indeterminate nodules in the present study was similar to that observed in a previous meta-analysis10, whereas the sensitivity demonstrated an increase from 40.0%, in the meta-analysis,to 80.8%. These discrepancies could mainly be ascribed to different types of thyroid cancers, ethnic variations, and BRAFV600Edetection methods23-25. In addition, the metaanalysis included BSRTC IV nodules, while BRAFV600Eexamination exhibited limited advantages in diagnosing FTCs. It is worth noting that all FNA specimens in the present study, even those from BSRTC I nodules, were adequate for BRAFV600Edetection in the amplification refractory mutation system (ARMS). Samples in DTL buffer can be preserved for at least 2 weeks at -20 degrees Celsius,providing sufficient time to conduct subsequent molecular testing. However, 2 nodules with BRAFV600Emutation were confirmed to be benign nodules by postoperative histopathology in our study, as observed in previously reported false-positive cases26.
In a previous study, we discovered that BRAFV600Eexhibited higher sensitivity and specificity compared with TIRADS in the diagnosis of thyroid cancers13. In the present study, we further compared the diagnostic value of BRAFV600Eand TIRADS in diagnosing PTCs in BSRTC category I, III, and V nodules. Our findings showed that the accuracy of TIRADS was higher than that of BRAFV600Ein BSRTC I nodules. While the specificity of BRAFV600Ewas higher than that of TIRADS, BRAFV600Eexhibited similar diagnostic value when compared to TIRADS in BSRTC III/V nodules. Although both BRAFV600Eand TIRADS demonstrated value in diagnosing PTCs, these malignancies could not be reliably ruled out if BRAFV600Emutation was absent or TIRADS was scored as 3/4a/4b, owing to the relatively low sensitivity of these two methods. The diagnosis of some PTCs may be missed when either of the two is used alone. Thus, we further assessed the value of the methods in combination in the diagnosis of PTCs in BSRTC I/III/V nodules. The sensitivity and accuracy of the combination of BRAFV600Eand TIRADS increased significantly in BSRTC I+III+V nodules. In BSRTC I nodules, the specificity of the combination was slightly decreased to 88.3%, but the sensitivity of the combination was significantly increased to 93.6%, which largely compensated for the low sensitivity(67.7%) of BRAFV600E. The false negative rate of the combination was very low (4.8%) in BSRTC I+III+V nodules, consistent with the risk of malignancy (3.7%) in nodules diagnosed as benign by FNAC5, maximized to avoid diagnostic surgery. Moreover, a recent study reported that BRAFV600Emutation was independently associated with lobulated or irregular margins in solid PTCs, indicating that BRAFV600Eand TIRADS may overlap in diagnosing PTCs to some degree27. Of note, the malignancy rate of up to 96.8%in BSRTC V nodules in our institute rendered the value of BRAFV600Eand TIRADS analysis limited in this category.However, the combination of BRAFV600Eand TIRADS could still result in a markedly increased sensitivity, to 96.0%,which could have potential value in certain cases.
There were several limitations in the present study. First, a high rate of malignancy in nondiagnostic and indeterminate nodules existed in our study, mainly caused by the selection bias resulting from the fact that most patients who underwent FNAC had suspicious ultrasonic features, which may lead to an overestimate of PPV and an underestimate of NPV. Second, the results of a single center study should be verified in multiple centers. Third, the mutation rate of BRAFV600Ewas much higher in classical type PTCs and tall cell variant PTCs than in follicular variant PTCs28. Most of the PTCs in the present study were classical type, which may have an impact on the diagnostic performance of BRAFV600E.In addition, some nodules that did not undergo surgical excisions were evaluated by repeat FNAC or ultrasound follow-up, procedures that may miss the malignancy.
In summary, we found that detection of BRAFV600Emutation and TIRADS classification were reliable ancillary tools in diagnosing PTCs in BSRTC category I, III, and V nodules in a Chinese population. For BSRTC category I and III nodules with BRAFV600Emutation or TIRADS classification 4c/5, surgery should be recommended.Otherwise, regular ultrasound follow-up was found to be appropriate. For BSRTC V nodules, surgery could be considered. BRAFV600Edetection and TIRADS classification might have certain value in some cases. The present study described individual-based therapeutic regimens for patients with BSRTC category I/III/V nodules, according to the combination of BRAFV600Eand TIRADS.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No.81261120566), Jiangsu Province Key Medical Personnel Project (Grant No. RC2011068), 333 Projects in the Fourth Phase of Jiangsu Province (Grant No. BRA2015389), Jiangsu Province "Six First Project" Research Program (Grant No.LGY2016004) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Circular RNAs and human glioma
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
