Memory stem T cells generated by Wnt signaling from blood of human renal clear cell carcinoma patients
Cihui Yan, Jingjing Chang, Xinmiao Song, Fan Yan, Wenwen Yu, Yang An, Feng Wei, Lili Yang, Xiubao Ren
1Department of Immunology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China; 2Department of Electromyogram, 3rd Affiliated Hospital of Hebei Medical University, Shijiazhuang 050051, China
ABSTRACT Objective:Memory stem T cells (Tscm) have attracted attention because of their enhanced self-renewal, multipotent capacity, and anti-tumor capacities. However, little is known about Tscm in patients with renal clear cell carcinoma (RCC) and the role of Wnt signaling in these cells. We evaluated Tscm from RCC patients concerning their activation of Wnt signaling in vitro and explored the mechanism of preferential survival.Methods:Flow cytometry identified surface markers and cytokines produced from accumulated Tscm in the presence of the glycogen synthase kinase beta inhibitor TWS119. Apoptosis was evaluated after induction using tumor necrosis factor-alpha.Immunofluorescence and Western blot analyses were used to investigate the activation of the nuclear factor-kappa B (NF-КB)pathway.Results:RCC patients had a similar percentage of CD4+ and CD8+ Tscm as healthy donors. Activation of Wnt signaling by TWS119 resulted in the accumulation of Tscm in activated T cells, but reversal of differentiated T cells to Tscm was not achieved.Preferential survival of Tscm was associated with increased anti-apoptotic ability mediated downstream of the NF-КB activation pathway.Conclusions:The finding that Tscm can accumulate by Wnt signaling in vitro in blood from RCC patients will help in devising new cancer therapy strategies of Tscm-based adoptive immunotherapy, such as dendritic cell-stimulated Tscm, and T cell receptor or chimeric antigen receptor-engineered Tscm.
KEYWORDS Memory stem T cell; TWS119; Wnt signaling; apoptosis; renal clear cell carcinoma
Introduction
Kidney cancer represents 2% of all cancers worldwide annually. The incidence of this disease has been rising in recent years1,2. Approximately 85% of kidney cancer is renal clear cell carcinoma (RCC), but the patients are always diagnosed in the advanced metastatic stage and show low response rates to chemo- and radiotherapy3-5.
Immunotherapy has shown potential in the treatment of RCC owing to advances in the understanding of immunogenic characteristics of this disease6-8. Cytokines,such as interleukin-2 (IL-2) and interferon-alpha (IFN-ɑ),have been studied, with limited overall patient response reported9,10. New immunotherapy with dendritic cell (DC)-based vaccine11,12and checkpoint blockade, such as anti-CTLA-4 and PD-1/PD-L1 antibody13-15, is a promising therapeutic avenue. The effects of these treatments are mediated by cytotoxic T cells, indicating that T cells and the adaptive immune response are functionally critical in human RCC. CD45RO+memory T cell status offers a significant independent prognostic value in both RCC and squamous non-small cell lung cancer16,17. However, these reports did not analyze subsets of CD45RO+memory T cells, such as central memory T cells (TCM) and effector memory T cells(TEM), which show different characteristics of surface markers and function in immune response. Although more TEMcells exist in situ, it is difficult to recover these cells due to proliferative exhaustion18. As a result, studies of memory lymphocytes with long-term survival and self-renewing capabilities are required to provide potential immune therapeutic strategies in RCC.
Memory stem T cells (Tscm) are a sub-population of TCMwith stem cell-like behavior because of their enhanced proliferation, survival, and anti-tumor capacities, which exceed those of TCMand TEMcells19,20. Tscm cells were first reported in a mouse model of graft-versus-host disease and were shown to have a postmitotic CD44loCD62LhiCD8+T cell phenotype that persisted throughout the course of graftversus-host disease and had the abilities of self-renewal and multipotentiality21. Later studies described that human Tscm isolated from peripheral blood had both the naïve T cell phenotype of CD45RO-CCR7+CD45RA+CD62L+CD27+CD28+IL-7Rα+and memory cell phenotype of CD95+IL-2Rβ+CXCR3+LFA-1+, displayed increased proliferative capacity and multipotency to generate all memory and effector T cell subsets in vitro, more efficiently reconstituted immunodeficient hosts, and could mediate superior antitumor responses in a humanized mouse model19,20. These are properties of T memory cells with a differentiation state prior to TCMand after native T cells. The marked survival of Tscm might be associated with anti-apoptotic capacities with elevated Bcl-2 and lack of detectable caspase-3 activity22.However, Tscm in RCC have been less studied so far.
The Wnt/β-catenin signaling pathway regulates the progression of thymocyte development at different stages by downstream transcription factors, such as T cell factor 1(Tcf1) and lymphoid enhancer-binding factor (Lef), while its role in mature T cells requires further study23-25. It was reported that activation of Wnt/β-catenin signaling arrested healthy donor peripheral blood and cord blood T lymphocytes in the naïve stage and blocked their transition into functional T effector cells26. Additionally, Wnt/β-catenin signaling modulates nuclear factor-kappa B (NF-κB)signaling. NF-κB signaling is involved in the inflammatory response, but little is known of its association with T memory cells. As RCC is an immunogenic tumor, adoptive transfer of Tscm or their modified forms, which are stimulated by DCs or engineered to express T cell receptor or chimeric antigen receptor, might produce better therapeutic outcomes.
The purpose of this study was to identify Tscm in RCC patients and assess the anti-apoptotic ability of Tscm that accumulate following activation of Wnt signaling with glycogen synthase kinase-3β (GSK-3β) inhibitor in vitro. The findings provide new insights concerning Tscm in RCC and will benefit cellular immunotherapy.
Materials and methods
Antibodies and flow cytometry
We obtained human samples from patients diagnosed with RCC and healthy donors at Tianjin Medical University Cancer Institute and Hospital. The Institutional Review Board of Tianjin Medical University Cancer Institute and Hospital approved the study protocol. All experiments were conducted in accordance with the Declaration of Helsinki.Informed consent was obtained from all subjects. All fluorescently conjugated antibodies were purchased from BD Biosciences. Isotype antibody and fluorescence minus one(FMO) staining were used as a control. Surface marker staining for fresh peripheral blood or cultured cells was performed according to the manufacturer's instructions.Flow cytometry was performed on a BD FACSCanto II flow cytometer. Lymphocytes were first gated out according to forward scatter A and side scatter A. CD4+and CD8+T cells were gated out according to the isotype control. The CD45RA+CD62L+population was gated out from CD4+or CD8+T cells according to fluorescence-minus-one (FMO)-CD45RA and FMO-CD62L control. Subsequently, the CD45RO-CD95+population was gated out from CD45RA+CD62L+cells according to FMO-CD45RO and FMO-CD95. Tscm cells were characterized with CD45RA+CD45RO-CD62L+CD95+expression in CD4+and CD8+T cells. Data were analyzed with FlowJo software 7.6.The details of antibodies used are provided in Supplementary Table S1.
In vitro generation of Tscm
To generate the Tscm cells in vitro, Wnt signaling was activated in T cells by GSK-3β blockade with the inhibitor TWS119. Briefly, peripheral blood mononuclear cells(PBMCs) were isolated from peripheral blood of RCC patients by Ficoll density gradient centrifugation. CD8+and naïve CD8+T cells were purified from PBMCs using the EasySepTMhuman CD8+T cell enrichment kit (STEMCELL,19053) and the EasySepTMhuman naïve CD8+T cell enrichment kit (STEMCELL, 19158), respectively, according to the manufacturer's instructions. PBMCs, CD8+, or naïve CD8+T cells were stimulated by ɑ-CD3/CD28 beads(Invitrogen, 11452D) at a bead-to-cell ratio of 1:1 and 300 IU/mL IL-2 (PeproTech, AF-200-02) in the presence of 5 μM TWS119 (Cayman Chemical, 10011251) for 7 days. Culture medium (10% RPMI1640) containing TWS119 and IL-2 were supplemented on day 3 and 5.
Real-time reverse transcription-polymerase chain reaction (PCR)
To investigate the downstream and target genes of Wnt signaling, we collected T cells treated with TWS119 for the indicated time. RNA isolation was performed using the RNeasy Mini Kit (QIAGEN, 74104) according to the manufacturer's instruction. cDNA was generated by reverse transcription (Applied Biosystems). Real-time PCR was performed using commercially available probes and primers for the indicated genes (Applied Biosystems) and the 7500 Real-time PCR System (Applied Biosystems). The levels of gene expression were calculated relative to the β-actin housekeeping gene.
Immunofluorescence microscopy
To identify the translocation of β-catenin from the cytoplasm to the nucleus after T cells were treated with TWS119, cells were fixed in methanol, blocked using 1% bovine serum albumin (BSA) dissolved in 0.1% Triton X-100, and incubated with rabbit monoclonal β-catenin antibody [1:100;Cell Signaling Technology, 8480(CST)] at 4°C overnight. An anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 594 Conjugate, 1:1000; CST, 19158) was used to visualize the binding sites of the primary antibody. Cells were observed by immunofluorescence microscopy using a DMIRE2 microscope (Leica). 4′, 6-Diamidino-2-phenylindole (DAPI)Fluoromount-G (Southern Biotech) was used to stain cell nuclei.
Western blot
To assess the levels of protein expression upon the activation of Wnt signaling, cells were collected and lysed, and proteins were extracted using mammalian protein extraction agent(ThermoFisher Scientific, 78501) plus Halt protease inhibitor cocktail (ThermoFisher Scientific, 78430). Protein concentrations were determined using a bicinchoninic acid assay (ThermoFisher Scientific Pierce). Aliquots of protein lysates were separated on sodium dodecyl sulfatepolyacrylamide gels and transferred to a nitrocellulose membrane, which was blocked with 5% blot grade milk (Bio-Rad, 170640) in 0.1% Tween 20 in phosphate-buffered saline(PBST). The membrane was then hybridized with primary antibodies to human β-catenin (CST, 8480), phospho-IKKɑ/β (Ser176/180; CST, 2697) and RelB (CST, 4922),followed by the corresponding secondary antibodies conjugated with horseradish peroxidase, and finally detected using a chemiluminescence assay (Millipore, WBKLS0500).Membranes were exposed to an X-ray film (Kodak China Investment) to visualize the bands. β-actin was used as the loading control.
Detection of apoptosis
The effect of TWS119-mediated Wnt signaling activation on apoptosis of different subsets of T cells was evaluated by flow cytometry. Briefly, activated CD8+T cells were treated with 5µM TWS119. Tumor necrosis factor-alpha (TNF-ɑ, 1 µg/mL;PeproTech, AF-200-04) was added at day 5. Cells were collected on day 6 and were first stained with the antibodies for surface markers to identify Tscm (CD45RA+CD62L+CD95+), TCM(CD45RA-CD62L+CD95+), TEM(CD45RACD62L-CD95+), and effector memory RA (TEMRA)(CD45RA+CD62L-CD95+). The cells were then washed twice with staining buffer and stained with anti-Annexin V antibody to detecting early apoptosis and 7-aminoactinomycin D (7-AAD; Biolegend, 640922) to detect late apoptosis.
Statistical analysis
Data were presented as the mean ± SD. Differences between groups were examined for significant differences by independent t test, ANOVA LSD or multivariate analysis. P-values < 0.05 were considered statistically significant. All the analyses were done using SPSS Statistics 22.0 software(SPSS 22.0).
Results
Identification of T memory stem cells in RCC
We used the surface marker panel (CD45RA+CD45ROCD62L+CD95+) to identify Tscm in gated CD4+and CD8+T cells of peripheral blood from 16 patients with RCC(Supplementary Table S2). Flow cytometry analysis revealed Tscm in both CD4+and CD8+T cells (Figure 1A)(2.06±1.28% in CD4+T cells and 1.94±1.34% in CD8+T cells). We also identified Tscm from 16 healthy donors. The percentages of Tscm in CD4+and CD8+T cells in healthy donors were 1.83±0.88% and 2.38±1.55%, respectively(Figure 1B). No significant difference in the percentage of Tscm was found between patients and healthy donors (Tscm CD4+, P = 0.546; Tscm CD8+, P = 0.397) (Figure 1C and 1D).
TWS119 activates the canonical Wnt/β-catenin signaling pathway

Figure 1 Identification of Tscm cells in periphery blood from patients with renal clear carcinoma. (A) and (B) Flow cytometry analysis of Tscm from patients (A) and healthy donors (B). Numbers indicated the percentage of cells in the parental gate. (C) Percentage of Tscm in CD4+ T cells. P = 0.546. (D) Percentage of Tscm in CD8+ T cells. P = 0.397. Data were from 16 patients and healthy donors individually and represented as the mean ± SD. P < 0.05 were considered to be statistically significant.
To determine the impact of Wnt/β-catenin signaling pathway on mature T cells, we stimulated CD8+T cells from RCC patients in the presence of TWS119. Western blot results showed that TWS119 promoted the rapid accumulation of βcatenin, which lasted 48 hours after TWS119 treatment(Figure 2A). Using immunofluorescence microscopy, we found increased nuclear localization of β-catenin after 6 hours of treatment (Figure 2B). Real-time PCR showed that Wnt downstream genes, such as Tcf7 and Lef1, and Wnt target genes, including Jun, Frizzled (Fzd7), and Nemo-likekinase (Nlk), also increased significantly (Figure 2C).However, we found that four of these five genes exhibited decreased expression in the stimulated CD8+T cells from healthy donors after TWS119 treatment (Supplementary Figure S1).
Accumulation of Tscm cells by activation of the Wnt signaling pathway
To test the effect of Wnt signaling on T cell differentiation,we first polyclonally activated PBMCs in the presence of TWS119 for 7 days and then analyzed the expression of differentiation markers, including CD45RA, CD45RO, and CD62L, in the population of CD4+and CD8+T cells. In the absence of TWS119, activated CD8+T cells predominantly possessed effector T cell characteristics that included increased expression of CD45RO and low expression of CD45RA and CD62L. Activated CD4+T cells also lost the“younger” phenotype of CD45RA+CD45RO-CD62L+CD95+expression (Supplementary Figure S2). In contrast, in the presence of TWS119, the subset of Tscm with the phenotype of CD45RA+CD45RO-CD62L+CD95+increased significantly in CD8+T cells and much more obviously in CD4+T cells(Figure 3A). As there was still a large population of effector memory RA (EMRA) CD8+T cells (CD45RA+CD62L-)under this culture condition (Figure 3A), we then purified CD8+T cells from PBMCs and activated them in the presence of TWS119. As shown in Figure 3B, the percentage of CD45RA+CD62L+cells in purified CD8+T cells was enhanced sharply after TWS119 treatment compared to the cells without TWS119 treatment. We also gated CD8+T cells further into two populations, according to the different levels of CD8+expression and found that the younger CD8+T cells,which possessed the CD45RA+CD62L+CD95+phenotype,largely occupied the population with high CD8 expression(Figure 3B). Furthermore, we found that the TWS119 treated CD8+T cells could respond to Phorbol myristate acetate/Ionomycin stimulation rapidly, producing cytokines, such as IFN-γ, IL-2 and TNF-α (Figure 3C), and shifted from a younger to a more differentiated status (Supplementary Figure S3).

Figure 2 TWS119 activated the canonical Wnt/β-catenin signaling pathway. (A) Protein expression of β-catenin in activated CD8+ T cells culture with 5 µM TWS119 for 0, 6, 24, 48 and 72 h tested by Western blot (left). Bar graph of changes of β-catenin from data of three independent experiment (right). Compared with 0 h, P < 0.001 at 6, 24 and 48 h; P = 0.547 at 72 h. (B) Subcellular localization of β-catenin in activated CD8+ T cells culture with 5 µM TWS119 for 6 h illustrated by immunofluorescence. FITC, β-catenin. DAPI, nuclear. Scale, 50 µm.(C) Expression of Wnt/β-catenin downstream and targeted genes in activated CD8+ T cells cultured with 5 µM TWS119 or DMSO for 0, 3, 6,12 and 24 h analyzed by quantitative RT-PCR. Upper panel: data from two patients. Lower panel: targeted gene changes from the data from five patients, and the P value is shown below. ★, values of P < 0.05 were considered to be statistically significant.
Tscm maintenance is required for sustained activation of Wnt signaling
If the activated CD8+T cells from RCC patients were treated only with TWS119 during the first 4 days and then changed into the medium lacking TWS119, the subset of Tscm that were elevated on day 4 subsequently decreased with subsequent culture time (Figure 4A). To assess whether TWS119 could reverse the differentiated T cells back to“younger” cells, naïve CD8+T cells were activated by CD3/CD28 beads for 3 days followed by TWS119 treatment.The CD8+T cells with CD45RA+CD45RO-CD62L+CD95+expression did not accumulate after 7 days of culture (Figure 4A and 4B), suggesting that TWS119 could not reverse CD45RO+to CD45RA+cells. We also found that the decreased percentage of Tscm when activated CD8+T cells from healthy donors were cultured in the TWS119-rescued condition, while the percentage did not change significantly when cells were cultured in the TWS119-suspending condition (Figure 4C).
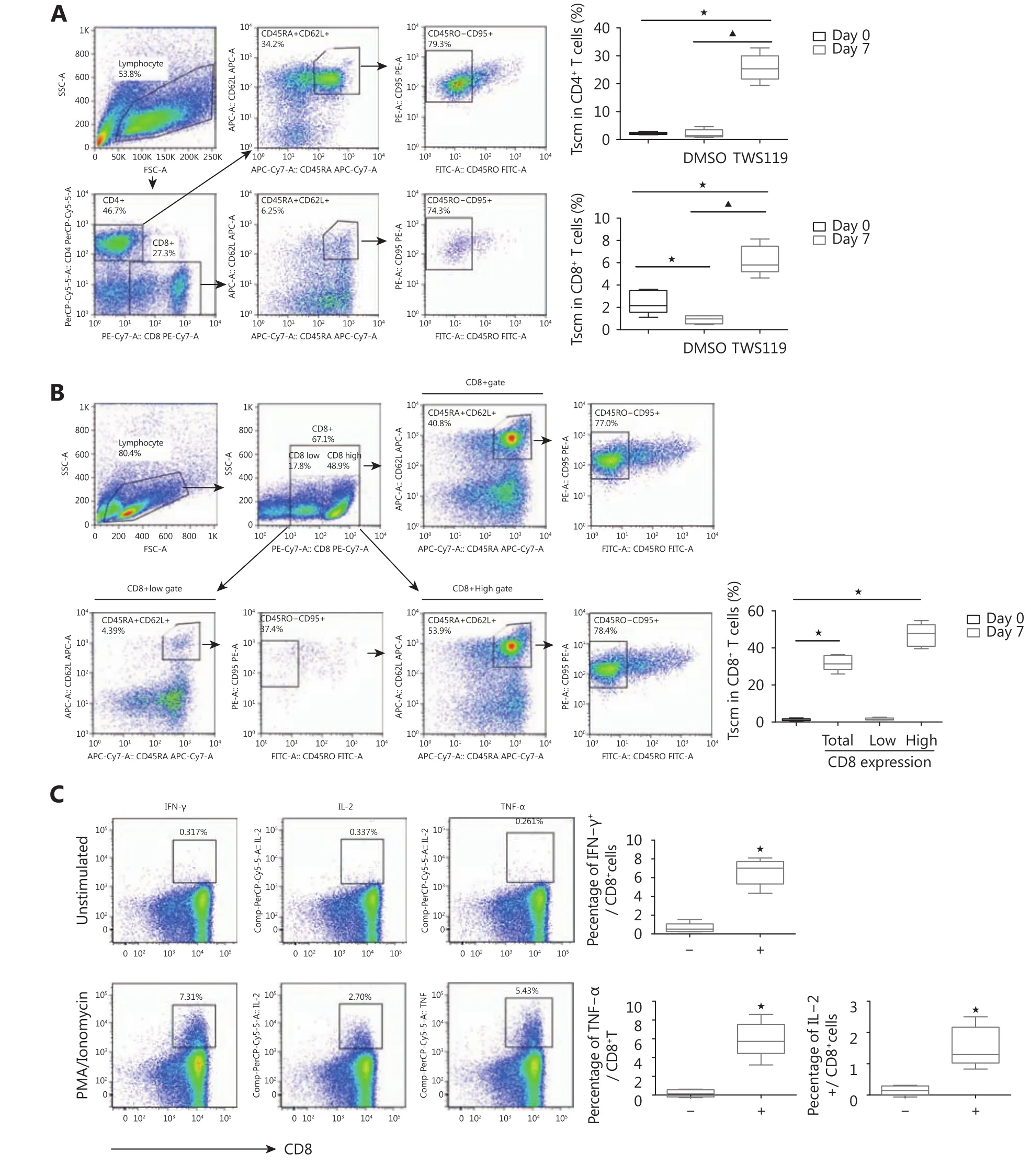
Figure 3 Accumulated Tscm cells by activation of Wnt signaling pathway. (A) Flow cytometry analysis of PBMCs on day 7 after stimulation with ɑ-CD3/CD28 beads and IL-2 in the presence of 5 µM TWS119. Upper bar graph, percentage of Tscm in CD4+T cells; lower bar graph,percentage of Tscm in CD8+T cells. (B) Flow cytometry analysis of sorted CD8+T cells on day 7 after stimulation with ɑ-CD3/CD28 beads and IL-2 in the presence of 5 µM TWS119. Bar graph, percentage of Tscm in CD8+ T cells.★,P < 0.05, compared with day 0. ▲,P < 0.05,compared with DMSO. Bar data, from five patients. (C) Cytokine production in TWS119 treated CD8+T cells after 5 hours of PMA/Ionomycin added (left). Data from five patients.-, no PMA/Ionomycin added (right) +, PMA/Ionomycin added. IFN-γ,P < 0.001. IL-2,P= 0.007. TNF-α,P< 0.001.★, values ofP< 0.05 were considered to be statistically significant.
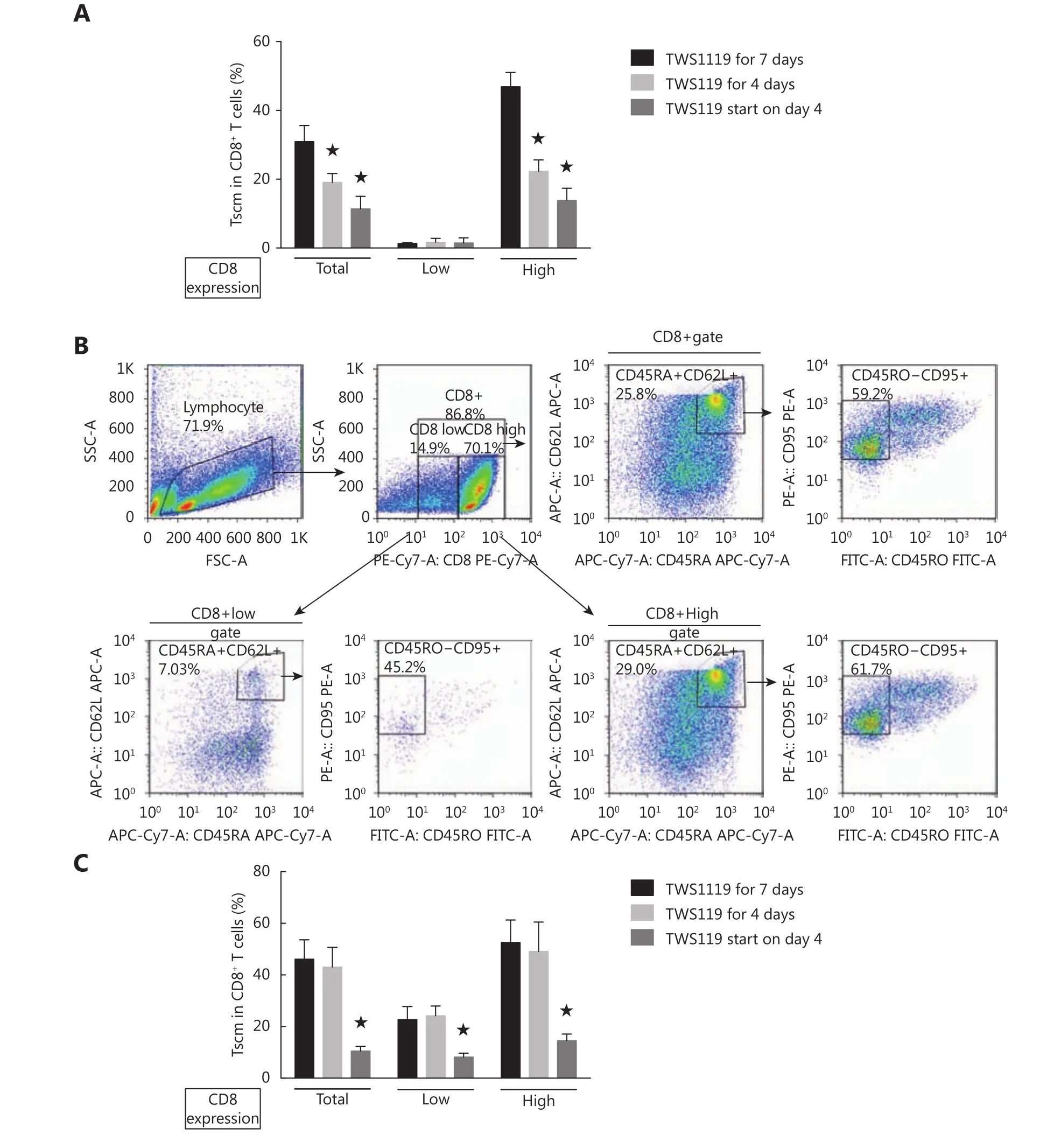
Figure 4 Tscm maintenance required sustained activation of Wnt signaling. (A) Percentage of Tscm in subsets of CD8+ T cells with different levels of CD8 expression. Native CD8+ T cells from RCC patients were stimulated with ɑ-CD3/CD28 beads and IL-2 under different strategies of TWS119 treatment. TWS119 for 7 days, cells were treated with TWS119 from the start of culturing and maintained for 7 days. TWS119 for 4 days, cells were treated with TWS119 during the first 4 days. And then TWS119 was washed out and cells were cultured continually for following 3 days. TWS119 start on day 4, TWS119 was absent in the culture system during the first 3 days and added on day 4. (B) Flow cytometry analysis of naive CD8+ T cells on day 7 after stimulation with ɑ-CD3/CD28 beads and IL-2. TWS119 (5 µM) was absent during the first 3 days and then was rescued in the following culturing period. (C) Percentage of Tscm in subsets of CD8+ T cells with different levels of CD8 expression for healthy donors. ★, P < 0.05, compared with 7 days of continuous treatment of TWS119. The data were from three repeated experiments.
Decreased apoptosis in Tscm by Wnt signaling
To investigate the anti-apoptotic capability of Tscm, we compared the apoptotic percentage among different T cell subsets. Flow cytometry revealed a lower percentage of apoptotic cells in both Tscm and TCMcompared with that in TEMand EMRA by TNF-ɑ induction in the absence of TWS119 (Figure 5A). When cells were treated with TWS119,the apoptotic cells that were induced by TNF-ɑ decreased significantly from 34% to 11% and 44.3% to 7.8% in Tscm and TCMcells, respectively, in contrast to an increase in TEMand EMRA (Figure 5A). Tscm, TCM,TEM, and EMRA cells had different expression levels of TNF-ɑ receptor, TNFR1,and TNFR2. Tscm and TCMcells displayed the highest levels of TNFRs, especially TNFR2, among the four subsets(Supplementary Figure S4). The expression of the antiapoptotic Bcl-2 gene increased after TWS119 treatment, as determined by quantitative real-time PCR analysis (Figure 5B). Western blot showed that the level of IKKɑ/β phosphorylation increased, while RelB expression deceased in the early treatment (Figure 5C), indicating the activation of the classic NF-κB signaling pathway in TWS119-treated cells.
Discussion
In this study, we found that RCC patients had similar percentages of CD4+and CD8+Tscm in peripheral blood as healthy donors. Activation of Wnt signaling by TWS119 could result in the accumulation of Tscm in activated T cells,but was unable to reverse the differentiated T cells back to Tscm. The preferential survival of Tscm was associated with decreased apoptosis mediated downstream of the activation of the NF-КB pathway.
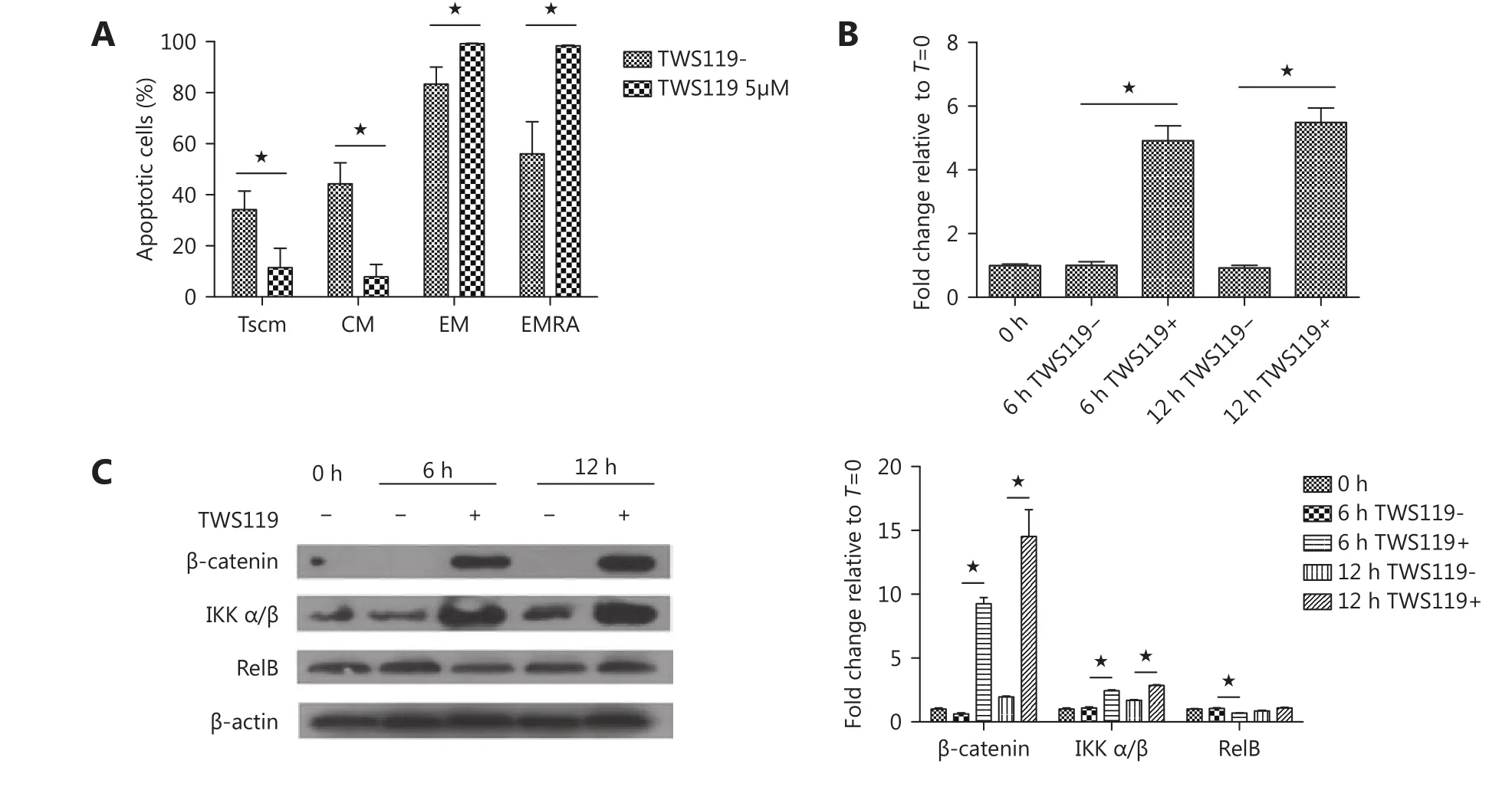
Figure 5 Decreased apoptosis in Tscm by Wnt signaling. (A) Percentage of apoptotic cells in individual subsets of T cells tested by flow cytometry. CD8+ T cells were stimulated with ɑ-CD3/CD28 beads and IL-2 with 5 µM TWS119 or DMSO for six days, and then 1 µg/mL TNF-ɑ was added on day 5. Cells were stained first with fluorescently conjugated antibodies for surface markers and then stained with anti-Annexin V antibody and 7-AAD for apoptosis. (B) and (C) CD8+ T cells were stimulated with ɑ-CD3/CD28 beads and IL-2 in the presence of 5 µM TWS119 or DMSO. Bcl-2 gene expression was analyzed by quantitative RT-PCR (B). Proteins of β-catenin and NF-κB signaling pathway were evaluated by Western blot (C). ★, values of P < 0.05 were considered to be statistically significant.
Understanding the important role of T cells in tumor surveillance has encouraged us to explore multiple strategies of immunotherapy. Chimeric antigen receptor (CAR)T cells engineered to express CAR have exhibited unexpected clinical responses in lymphoma treatment, while high recurrence is still a great obstacle in the clinic. One of the most important limitations of CAR-T cells is their short lifetime in vivo after reinfusion. Tscm cells, which possess multipotent and long-term survival ability, are promising candidates in adaptive or engineered cell immunotherapy.Tscm cells exist as a minimal subset of T cells in peripheral blood, as well as in lymphoid tissues. We originally reported CD4+and CD8+Tscm in RCC patients. We discriminated different subsets of T cells using the molecular panel consisting of naïve T cells (CD45RA+CD45RO-CD62L+CD95-), Tscm (CD45RA+CD45RO-CD62L+CD95+),TCM(CD45RA-CD45RO+CD62L+CD95+), TEM(CD45RACD45RO+CD62L-CD95+), and EMRA (CD45RA+CD45RO-CD62L-CD95+). This panel was slightly different from a prior report in humans20but the same as used in other studies22,26. In the human study, except the surface markers mentioned above, CCR7, CD27, CD28, and IL-17,which presented lymphoid-homing ability and were always expressed on memory cells, were also used in the definition of Tscm20. We found that the population gated by CD45RA+CD62L+in CD4+or CD8+subsets almost merged with that when the subset of CD45RA+CD62L+CD4+/CD8+T cells was gated further by CCR7+(data not shown). In our study, both CD4+and CD8+Tscm were both detected at approximately 2% in comparisons between patient and healthy donors, as well as in the aforementioned human study20. Since Tscm cells have been proven to have enhanced anti-tumor capacity, we speculate that the immune surveillance ability of Tscm cells might be inhibited by some pro-tumor factors in patients, which deserves further study.
Wnt/β-catenin is an evolutionarily conserved pathway that promotes hematopoietic stem cell self-renewal and multipotency by limiting stem cell proliferation and differentiation27,28. We used TWS119, an inhibitor of serine/threonine kinase blocking GSK3β to mimic Wnt signaling, to test the effect of Wnt/β-catenin signaling on T cells. TWS119 efficiently activated Wnt signaling, as evidenced by rapid and sharp accumulation of β-catenin in cell nuclei. β-catenin bound the transcription factors Tcf7 and Lef1, which promoted transcription of targeted genes, as evidenced by the increased gene expression of Jun, Fzd7, and Nlk after TWS119 treatment. Tcf7 and Lef1 are highly expressed by naïve T cells, but their levels decrease following encounter with antigen, as they undergo massive expansion and differentiation into effector T cells19,29,30. The long-lived memory T cells after effector phase express intermediate, but heterogeneous, levels of these Wnt transcription factors30.High levels of Tcf7 and Lef1 expression are found in TCMcells, which express the lymphoid-homing molecules CD62L and CCR7, have long telomeres, high proliferative capacity,and possess stem-like qualities for plasticity and selfrenewal31,32. Conversely, low levels of Tcf7 and Lef1 are found in CD62L low and CCR7 low TEMcells19,29, which have poor replicative potential and have acquired the capacity to produce high amounts of IFN-γ31,32. Tscm cells are between the differentiation stage of naïve and TCMcells, and resemble less differentiated TCMcells, as they share more gene and protein expression compared with naïve T cells20. In our study, the increased expression of Wnt downstream as well as targeted genes indicated that activation of the Wnt signaling pathway by inhibition of GSK-3β maintained less differentiated memory T cells.
We used TWS119 at 5 µM because this concentration retained CD45RA+CD62L+expression without influencing proliferation in T cells during 5 to 7 days of culture. A high concentration of TWS119 (up to 20 µM) produced an obvious cell cycle arrest (data not shown). Owing to the arrested differentiation of T cells by TWS119 in cultured PBMCs, the percentage of CD4+and CD8+Tscm increased significantly. However, the increase in CD4+Tscm was notably higher than the increase in CD8+Tscm, and the majority of CD8+T cells still differentiated to EMRA. There are two possible explanations. First, the CD4+T cells, which comprise more than half of the lymphocytes, might influence the differentiation of CD8+T cells either directly or indirectly resulting from TWS119 treatment. Second, PBMCs were separated from buffy coat after Ficoll density centrifugation.In addition to lymphocytes, other immune cells, such as monocytes, were also included. The effect of TWS119 on monocytes is still unclear. Therefore, it was difficult to exclude the possibility that monocytes treated with TWS119 promote the differentiation of CD8+T cells in this heterogeneous culture system. As a result, the percentage of Tscm increased much more significantly when CD8+T cells were purified from PBMCs and treated with TWS119 compared with the treatment of PBMCs. TWS119 blocked the differentiation of isolated CD8+T cells directly,contributing to the accumulation of Tscm cells. These accumulated cells had the functional capability of producing cytotoxic cytokines and turning into differentiated T cells. In contrast, the differentiation status of T cells was determined by harmonious factors in PBMCs because of the constitution of multiple populations of immune cells.
We found two populations with different levels of CD8+expression in the current study. This difference became remarkable after cells were stimulated in the presence of TWS119. It was interesting that the population with high CD8 expression had a higher percentage of Tscm than that with low CD8 T cells, which had more differentiated EMRA.IFN-γ and IL-4 can reciprocally decrease CD8 expression during primary CD8+T cell activation33. It has been reported that the activation of naïve CD8+T cells in the presence of IL-4 modulates their expression of the CD8 co-receptor and their functional differentiation, resulting in the generation of CD8 low cells that persist in vivo34. In this prior study and in the present study, T cells had different levels of CD8 expression, but both could survive for a long time, which might have resulted from the different culture systems and consequent different progression of differentiation and proliferation in these two studies. IL-4 was used in the activated culture medium in the previous study34. It is possible that the long-lived CD8 low-expression T cells were derived from activated effector cells. However, in our study,TWS119 was used. The CD8 high-expression T cells were probably derived from less differentiated cells because of the blockade of transition of CD45RA-CD45RO+from CD45RA+CD45RO-by TWS119, which then exhibited enhanced anti-apoptotic capability, resulting in longer survival.
When T cells are activated by polyclonal stimulation, they differentiate. Only a few memory T cells remain to respond to the second immune attack and execute immunologic surveillance. Similar to memory T cells, minor Tscm exist in peripheral blood. The activation of Wnt signaling results in the accumulation of Tscm cells and is required to maintain the stem-like characters of T cells. Presently, Tscm cells lost the activation of Wnt signaling and were more likely to differentiate if TWS119 was retrieved during the late period of culture, as evidenced by the decreased subset of Tscm and increased TCMand TEMfractions in the cultured cell population. In addition, although Wnt signaling was capable of blocking the CD45RA-CD45RO+transition, it could not reverse CD45RO-CD45RA+transition when TWS119 was absent in the early culture period.
Additionally, we found Tscm cells were more resistant to TNF-ɑ-induced apoptosis, which was associated with enhanced gene expression of Tcf7 and Lef1 (both regulating self-renewal) and Bcl2 (anti-apoptotic). These results were consistent with a previous study that reported that Tscm had higher levels of Lef1, Bcl2, and Mcl (anti-apoptotic) gene expression, which devoted to preferential survival in simian immunodeficiency virus-derived Gag CM9 peptide specific Tscm after infection in healthy non-human primates35.Furthermore, we found an increased level of phosphorylation of IKKɑ/β when the Wnt signaling pathway was activated,with no effect on RelB, which indicated that the activation of the NF-КB signaling pathway induced by Wnt signaling might be associated with anti-apoptotic gene expression,resulting in prolonged survival of Tscm. Interestingly, we originally found that Tscm cells had the highest expression of TNFRs, especially TNFR2. The convergence of TNFR1 and TNFR2 signaling, and most importantly TNFR2-mediated signaling, contribute to the anti-apoptotic effect. Likely,TNFRs as well as CD95 were expressed at high levels on Tscm cells that were involved in non-apoptotic signaling pathways devoted to proliferation and long-term survival, which might be mediated by downstream activation of the canonical NFКB signaling pathway.
Although Tscm cells could accumulate in the blood from RCC patients and healthy donors, there were some differences between these two different derived cell subsets.In contrast to the increased expression of Nlk, Lef1, TCF7,Jun, and Fzd7 in patient-derived T cells, decreased expression was found in healthy donor-derived T cells after 24 hours of TWS119 treatment. Additional divergence included the relatively lower expression of TNFR1 and higher expression of TNFR2 on patient-derived Tscm cells compared with donor-derived Tscm cells. Furthermore, we found that the accumulated Tscm CD8+T cells from healthy donors exhibited much more resistance to TWS119-suspended treatment. The characteristics of the immunity condition are quite different between tumor patients and healthy donors.As there are multiple gene mutations and tumor antigens in RCC, it is possible that T cells in these patients have more opportunities to experience antigen presentation than cells in healthy donors. The immune system is always synchronized to a status of anti-tumor disability in RCC patients. The antitumor ability of T cells is always inhibited in vivo in RCC patients. Once these cells were isolated from tumor patients and separated from the immune inhibitory environment,they could rapidly respond to stimulation. However,although Tscm were identified using the same panel of surface markers in our study, T cells derived from patients or healthy donors are influenced by different immune environments, leading to different responses to the changes of signaling pathways. The precise regulatory mechanism of Tscm and the differences among RCC patients and healthy donors, as well as multiple tumor types, deserves further study.
In summary, our data show that RCC patients have a similar level of Tscm as healthy donors. The Wnt signaling pathway plays an important role in the accumulation of Tscm, but cannot reverse the differentiated T cells to Tscm cells. Notably, the long-term capability of Tscm is associated with the increased expression of anti-apoptotic genes and activation of the NF-КB signaling pathway. Our results will help in the formulation of new strategies for cellular immunotherapy.
Acknowledgements
This work was supported by grants from the National Science and Technology Support Project (Grant No. 2015BAI12B12),the National Natural Science Foundation of China (Grant No. 81401887), the National Natural Science Foundation of China (Grant No. 81470293), and the Tianjin Natural Science Foundation (Grant No. 14JCQNJC11500). The authors thank Dr. Xin Yao in the Urogenital Department,Tianjin Medical University Cancer Institute and Hospital, for kindly providing periphery blood from patients with renal clear carcinoma.
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Circular RNAs and human glioma
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
