STIM1 overexpression in hypoxia microenvironment contributes to pancreatic carcinoma progression
Jian Wang, Junling Shen, Kaili Zhao, Jinmeng Hu, Jiuxing Dong, Jianwei Sun
1Department of Pancreatic Cancer, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, Tianjin's Clinical Research Center for Cancer, Tianjin 300060, China; 2State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Center for Life Sciences,School of Life Sciences, Yunnan University, Kunming 650091, China
ABSTRACT Objective:Stromal interaction molecule 1 (STIM1) overexpression has been reported to play an important role in progression of several cancers. However, the mechanism of STIM1 overexpression and its relationship with hypoxia in pancreatic ductal adenocarcinoma (PDAC) remains unclear.Methods:STIM1 and HIF-1α expression was tested using immunohistochemistry in tissue microarray (TMA) including pancreatic cancer and matched normal pancreatic tissues, and their relationships with clinicopathological parameters were statistically analyzed. q-PCR, Western blot, ChIP, and luciferase assay were employed to 030 analyze transcriptional regulation between HIF-1α and STIM1 in pancreatic cancer PANC-1 cells.Results:Both STIM1 and HIF-1α showed higher positive rates and up-regulated expression in cancer tissues compared to that of normal tissues (P < 0.05). The Kaplan-Meier method revealed that higher HIF-1α and STIM1 expression levels were significantly correlated with decreased disease-free survival (P = 0.025 and P = 0.029, respectively). The expression of HIF-1α showed a significant positive correlation with that of STIM1 in cancer tissues (rs = 0.3343, P = 0.0011) and pancreatic cancer cell lines.Furthermore, ChIP and luciferase assays confirmed that HIF-1α bound to the STIM1 promoter and regulated its expression in PANC-1 cells.Conclusions:In hypoxia microenvironment, up-regulated expression of STIM1 mediated by HIF-1α promotes PDAC progression. HIF-1α and STIM1 are potential prognostic markers and/or therapeutic targets for PDAC treatment.
KEYWORDS Pancreatic cancer; STIM1; HIF-1α
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive and difficult to treat cancers, with a median survival of 6 months and a 5-year overall survival rate of 5%1.Owing to the lack of effective early detection methods,majority of pancreatic cancers are detected at advanced stages when their tumors have progressed locally or metastasized already2. Despite advances in surgical resection and chemotherapy, little progress has been made in the development of effective treatments due to the highly resistant nature of PDAC.
Numerous studies have emphasized the key role played by calcium and calcium-permeable ion channels in the progression of tumor growth, invasiveness, and metastasis3-6.Store-operated calcium channels (SOCs) represent one of the major calcium-entry pathways in non-excitable cells. SOCs and their major components Orai1 and stromal interaction molecule 1 (STIM1)6,7, in particular, have been implicated in a number of physiological processes, including proliferation,development, contraction, and motility3-6,8. Moreover, recent studies have suggested an important role for Orai1 and STIM1 in the carcinogenesis of various malignancies including cancers such as prostate, breast, colorectal and cervical cancer, as well as glioblastoma9-13. Kondratska et al.14demonstrated the role of calcium signaling in growth,metastasis, and drug resistance in pancreatic cancer. In our tissue microarrays (TMAs) of PDAC, STIM1 was also upregulated in PDAC cells and its overexpression was closely associated with poor prognosis. However, its regulatory mechanism remained unclear.
Hypoxia is a common microenvironmental feature within a tumor mass, which contributes to tumor growth and metastasis, especially in PDAC15. Hypoxia-inducible factor-1(HIF-1), consisting of highly regulated HIF-1α and a constitutively expressed HIF-1β, is one of the most important transcription factors, which can mediate many adaptive physiologic responses16. In PDAC, HIF-1α expression level is up-regulated and associated with tumor progression, fibrotic focus, angiogenesis, cell migration, and hepatic metastasis17-22.STIM1 is also involved in cancer cell growth and infiltration as well as angiogenesis in PDAC. However, the association between HIF-1α and STIM1 in PDAC is unclear. Here, we found that the expression of HIF-1α in pancreatic cancer tissues was positively associated with the expression of STIM1. HIF-1α bound to the HER2-3 region of the STIM1 promoter and regulated STIM1 transcription. In this study,STIM1 was characterized as a biomarker of the progression and poor prognosis of PDAC, as well as a functional downstream target of HIF-1α, which contributed to the progression of PDAC. HIF-1α-STIM1 axis is a potential therapeutic target in PDAC.
Material and methods
Patients
Clinicopathological data were collected as described in our previous study23,24. One hundred and twenty-six patients with PDAC treated at Tianjin Medical University Cancer Institute and Hospital between 2010 and 2013 were selected according to previous criteria23,24. Of the 126 patients, 92 cases consisting of 57 males (62.0%) and 35 females (38.0%)were finally included in our analysis. Histological evaluation indicated that 46.2% (30/65) of the tumors were classified as grades 1 and 2, while 53.8% (35/65) of the tumors were classified as grade 3. The patients were followed-up at the outpatient clinic in the same hospital as previously described23,24. The mean time of follow-up was 29.6 months(3 months to 60 months). PDAC and non-neoplastic tissues were collected and stored at -80 °C until analysis. Informed consent was obtained from patients prior to specimen collection. The study protocol was approved by the Ethics Committee of Tianjin Medical University (No. E2014092)and all investigations were conducted according to the Declaration of Helsinki.
TMA construction
TMAs were constructed using a manual tissue microarray instrument (Beecher Instruments, Sun Prairie, WI, USA)equipped with a 2.0-mm punch needle, as described previously23,24.
Immunohistochemistry
Immunohistochemistry was performed for HIF-1α and STIM1 of TMA using a DAB Substrate Kit (Maxin).Immunoreactivity was semiquantitatively scored according to the estimated percentage of positive tumor cells as previously described25. Staining intensity was scored 0(negative), 1 (low), 2 (medium) and 3 (high). Staining extent was scored 0 (0% stained), 1 (1%-25% stained), 2(26%-50% stained) and 3 (51%-100% stained). The final score was determined by multiplying staining intensity scores by staining extent and ranged from 0 to 9. Final scores(intensity score × percentage score) less than 3 were considered as low staining and 4-9 as high staining. The median intensity value of samples was used as the threshold.The patients were divided into two groups according to HIF-1α and STIM1 expression: (1) below median group expressing low HIF-1α/STIM1 and (2) above median group expressing high HIF-1α/STIM1.
Cell culture and transfection
Human pancreatic cancer cell lines were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM;Invitrogen) supplemented with heat-inactivated 10% fetal bovine serum (FBS; Invitrogen) at 37 °C in a humidified incubator containing 5% CO2. The transfection of shHIF-1α and shSTIM1 was performed according to the protocol described previously23. Expression levels of STIM1 and HIF-1α were quantified 24 h after transfection, and the cells were subjected to Western blot analysis.
Quantitative RT-PCR and Western blot analysis of STIM1 and HIF-1α
RNA extraction and q-PCR were performed as described previously25. Expression of STIM1 and HIF-1α was detected using a TaqMan q-PCR assay system (Applied Biosystems,Foster City, CA, USA). Western blot was performed as previous described25. The antibodies used in this study were:STIM1 (Cell Signaling Technology, 5668), HIF-1α (Abcam,113642), E-cadherin (Cell Signaling Technology, 3195), and vimentin (Cell Signaling Technology, 49636).
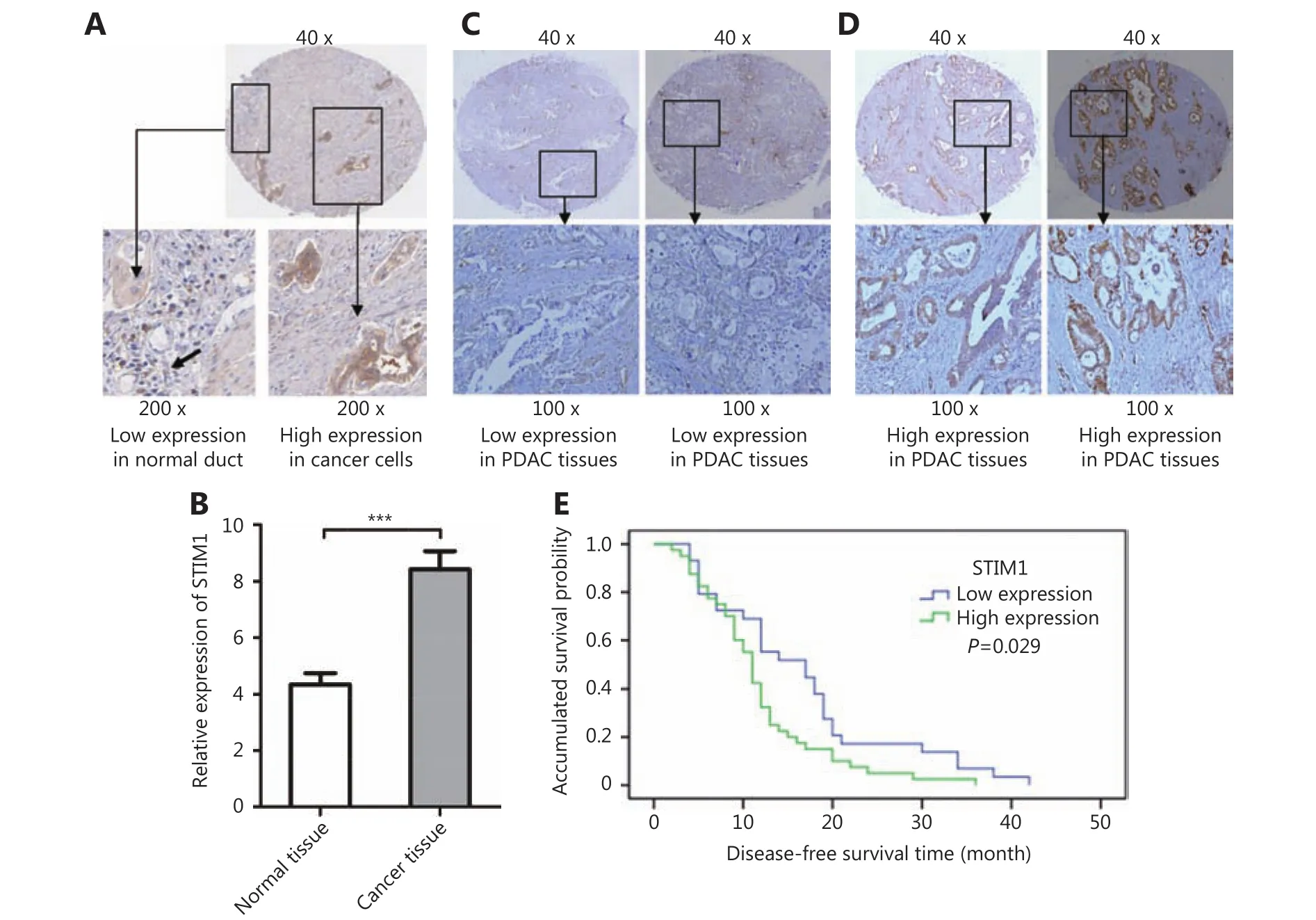
Figure 1 Immunohistochemistry of STIM1 in tumor and adjacent tissues of PDAC patients. (A) Representative images showing low expression of STIM1 in normal ducts and high expression of STIM1 in adjacent tumor ducts. (B) Relative STIM1 expression in cancer samples(8.42±4.72) and matched normal tissues (4.39±2.43) (P<0.01). (C) Representative images showing low STIM1 expression in patient samples.(D) Representative images showing high STIM1 expression in patient samples. (E) Association between tumor STIM1 expression levels and disease-free survival in 69 PDAC patients.
STIM1 promoter luciferase reporter assay
The human STIM1 promoter luciferase reporter was constructed by cloning STIM1 promoter sequence into pGL3 basic vector between KpnI and NheI sites. The STIM1 promoter sequence was amplified by PCR using primer STIM1 promoter forward KpnI: 5'- AATTGGTACCAGA CACATTGAGCTGTATC-3' and primer STIM1 promoter reverse NheI: 5'- AATT GCTAGC CAGGCGGACGCACACC G-3'. Target site deletion of the STIM1 promoter luciferase reporter was generated via site directed mutagenesis with deletion primers. The primers were as follows:
HRE1 forward: 5'-GCGGGCGCGGAGACCGCCCGCCC CG-3'
HRE1 reverse: 5'- CGGGGCGGGCGGTCTCCGCGCCC GC-3'
HRE2-3 forward: 5'- TCAAGATCTGCTGACTCTGTCT TCTTTGGCGCA-3'
HRE2-3 reverse: 5'- TGCGCCAAAGAAGACAGAGTCAG CAGATCTTGA-3'
HRE4 forward: 5'-GAGGTGGCTGAGTTTAGGAAC TTAACGA-3'
HRE4 reverse: 5'- TCGTTAAGTTCCTAAACTCAGCC ACCTC-3'
PANC-1 cells were co-transfected with a HIF-1α plasmid and a wild-type or HIF-1α binding site deletion STIM1 promoter luciferase reporter. Luciferase activities were determined using Dual-Glo luciferase. Data were normalized by dividing firefly luciferase activity by Renilla luciferase activity.
In vitro invasion assays
PANC-1 cell invasion assays were performed using 24-well Matrigel invasion chambers (BD Biosciences, CA, USA). The detailed protocol was performed as previously described23.
Statistical analysis
The correlation between STIM1, HIF-1α, and disease-free survival time was evaluated via the Spearman rank correlation coefficient. Clinicopathological valuables and mean differences were analyzed using one-way ANOVA or Student's t-test. Kaplan-Meier method and the log-rank test were also used for univariate survival analysis. SPSS version 16.0 (IBM) was used to perform statistical analysis. Statistical significance was set at P < 0.05.
Results
STIM1 expression and its association with clinicopathological characteristics
In order to investigate the role of STIM1 in PDAC development, we first evaluated its expression in TMAs containing tumor and matched non-neoplastic tissues from 126 PDAC cases (Figure 1A). STIM1 expression was analyzed in 92 cases on TMA, as the cancer or matched non-neoplastic tissues of the other 34 cases could not be scored due to tissue shedding, insufficient tumor cells or obscured signals.Relative STIM1 expression in cancer samples (8.42±4.72) was significantly higher than that of matched normal tissues(4.39±2.43) (P<0.01) (Figure 1B). These results indicated that STIM1 is frequently upregulated and may contribute to PDAC tumorigenesis.
In order to clarify potential implications of STIM1 expression in PDAC, we analyzed its correlation with some clinicopathological characteristics, including age, gender,tumor size, histological grade, nerve invasion, lymph metastasis, and survival time (Table 1). Patients were divided into two groups based on STIM1 expression as follows: (1)low expression (29 cases) (Figure 1C); and (2) high expression (40 cases) (Figure 1D). More cases of a poor histological grade (P=0.031) were observed in patients with high STIM1 expression. Although the results (P values) were not statistically significant, the patients with higher STIM1 expression exhibited bigger tumor sizes (P=0.081), higher nerve invasion (P=0.083), and lymph metastasis (P=0.097)(Table 1). According to the results of follow-ups, 69 cases were included for statistical assessment. Kaplan-Meier analysis revealed that a high STIM1 expression level was significantly associated with disease-free survival (11.83±1.12 months in patients with high STIM1 expression versus16.55±1.96 months in patients with low STIM1 expression;P=0.029, Figure 1E). These results further indicated the importance of STIM1 in PDAC development.
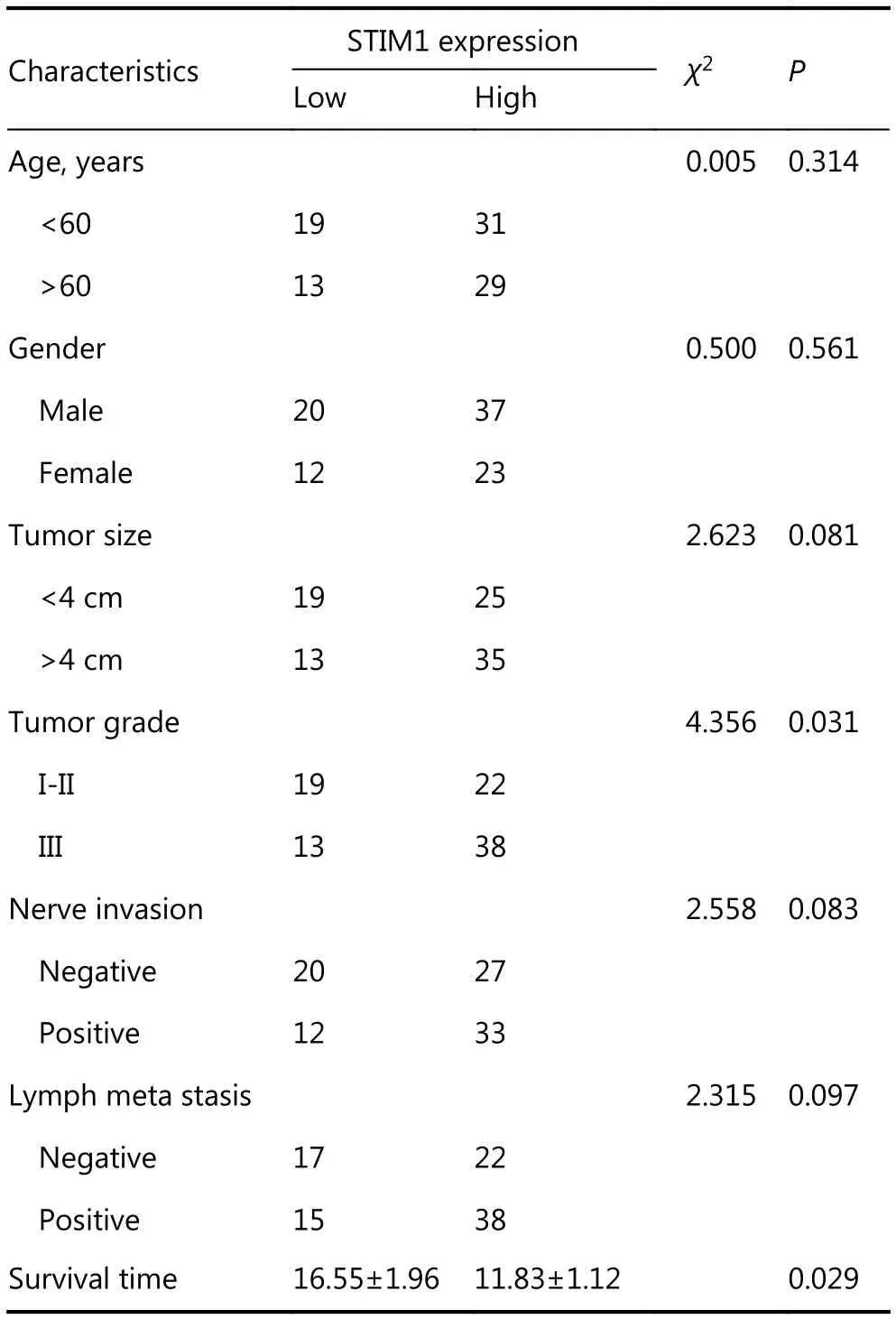
Table 1 Correlation between STIM1 expression and clinicopathologic data in patients with PDAC
STIM1 promotes pancreatic carcinoma cell proliferation, invasion, and anchorage independent growth
Next, we investigated whether STIM1 regulates the PDAC oncogenic phenotype. In vitro soft agar assay was performed using control shRNA and STIM1-shRNA treated PNAC-1 cells. We found that STIM1 knockdown reduced colony formation by more than 50% (Figure 2A and 2B). Moreover,STIM1 shRNA also inhibited PANC-1 cell proliferation(Figure 2C). To evaluate whether STIM1 promoted cancer progression, we employed in vitro invasion assay and observed that the invasiveness was specifically reduced by approximately 49% (P<0.05) following STIM1 knockdown(Figure 2D and 2E ). Furthermore, down-regulated expression of vimentin and up-regulated expression of E-cadherin were detected in STIM1-shRNA PANC-1 cells (Figure 2F). These data indicate that STIM1 may play a pivotal role in PDAC oncogenic transformation, cell growth and invasion as well as epithelial-mesenchymal transition (EMT).
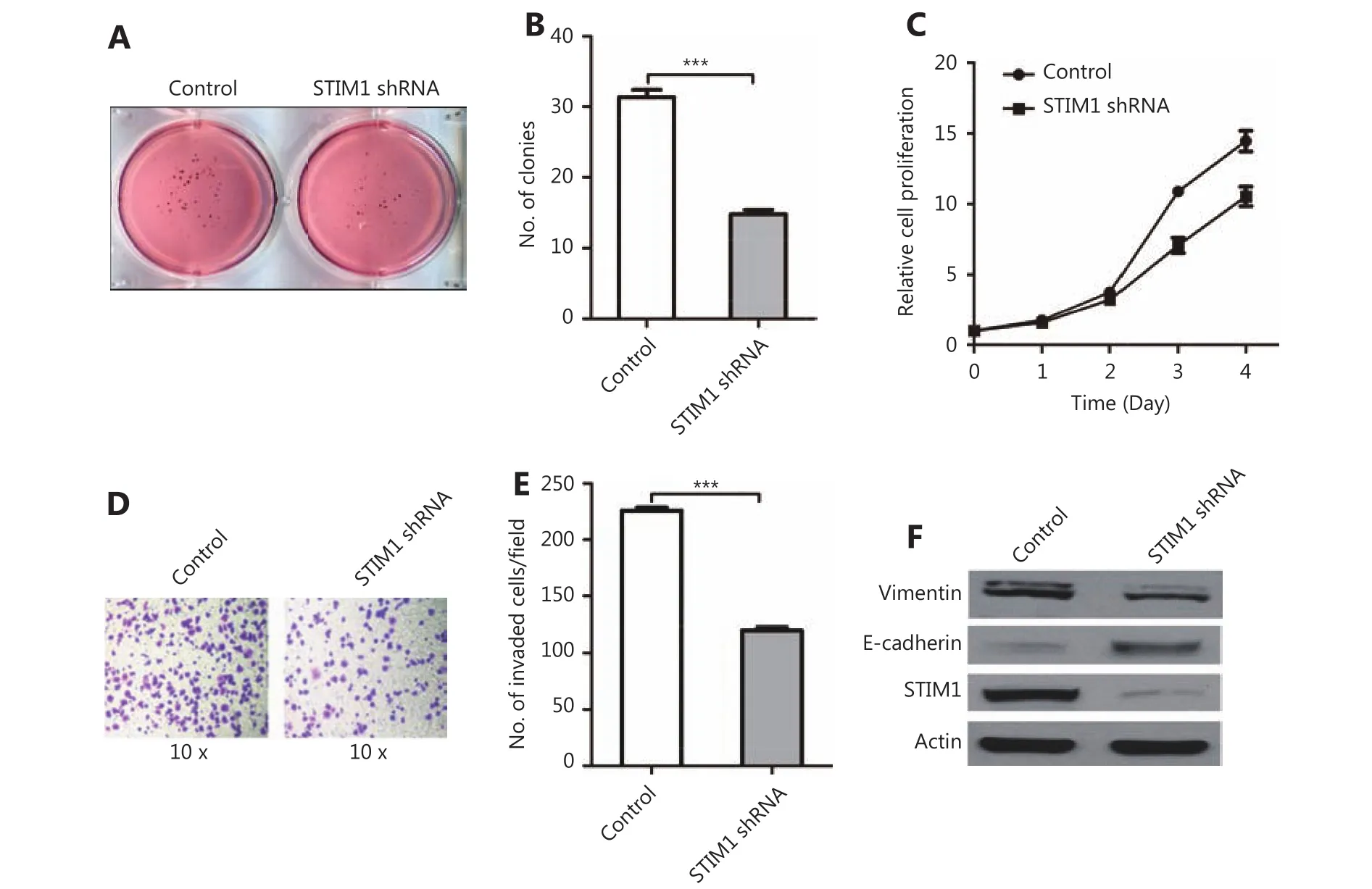
Figure 2 STIM1 is involved in pancreatic cancer progression. (A) Representative images of soft agar colony formation assay in control and STIM1 shRNA PANC-1 cells. (B) Quantified colony numbers in control and STIM1 shRNA PANC-1 cells. (C) Control and STIM1 shRNA PANC-1 cells cultured for 1-5 days. Cell proliferation was performed via MTT assay. Results were expressed as mean±SEM; n=3 independent experiments. (D) Representative images of in vitro invasion assay in control and STIM1 shRNA PANC-1 cells. (E) Quantified invaded cell numbers in control and STIM1 shRNA PANC-1 cells. (F) Western blot of vimentin, E-cadherin, and STIM1 level in control and STIM1 shRNA PANC-1 cells.
HIF-1α and STIM1 were upregulated in PDAC and predicted poor prognosis
HIF-1α, an important transcription factor in cancer,reportedly promotes LASP-1 and Fascin expression in PDAC,and is correlated with poor PDAC prognosis21. Li et al.26reported that HIF-1α regulated STIM1 expression in hepatocellular carcinoma cells. In order to investigate whether HIF-1α regulates STIM1 in PDAC, we examined HIF-1α expression in the same PDAC TMA and observed that the expression patterns of HIF-1α and STIM1 in PDACs were closely related. Representative images showed simultaneous low (Figure 3 A1-A4) and high (Figure 3 B1-B4) expression of HIF-1α and STIM1 in well differentiated PDAC. Moreover, high levels of co-expression of HIF-1α and STIM1 were also detected in poorly differentiated PDAC(Figure 3 C1-C4). Of 126 cases, 86 cases were analyzed statistically. The relative HIF-1α expression in cancer samples(4.34±3.99) was significantly higher than that of matched normal tissue samples (1.52±0.50) (P<0.01, Figure 3D).Those patients with complete clinical-pathological data were divided into two groups (38 cases with low HIF-1α group and 31 cases with high HIF-1α group) according to the HIF-1α expression level. Kaplan-Meier analysis indicated that the group showing a high HIF-1α expression level was significantly associated with disease-free survival (11.16±1.30 months) compared to the group showing a low HIF-1α expression (15.97±1.57 months) (P=0.025, Figure 3E). These results indicated the importance of HIF-1α expression in PDAC development. To examine the correlation between HIF-1α and STIM1, we analyzed the HIF-1α and STIM1 levels in 86 cases and found that the expression of HIF-1α had a significantly positive correlation with that of STIM1(rs=0.3343, P=0.0011).
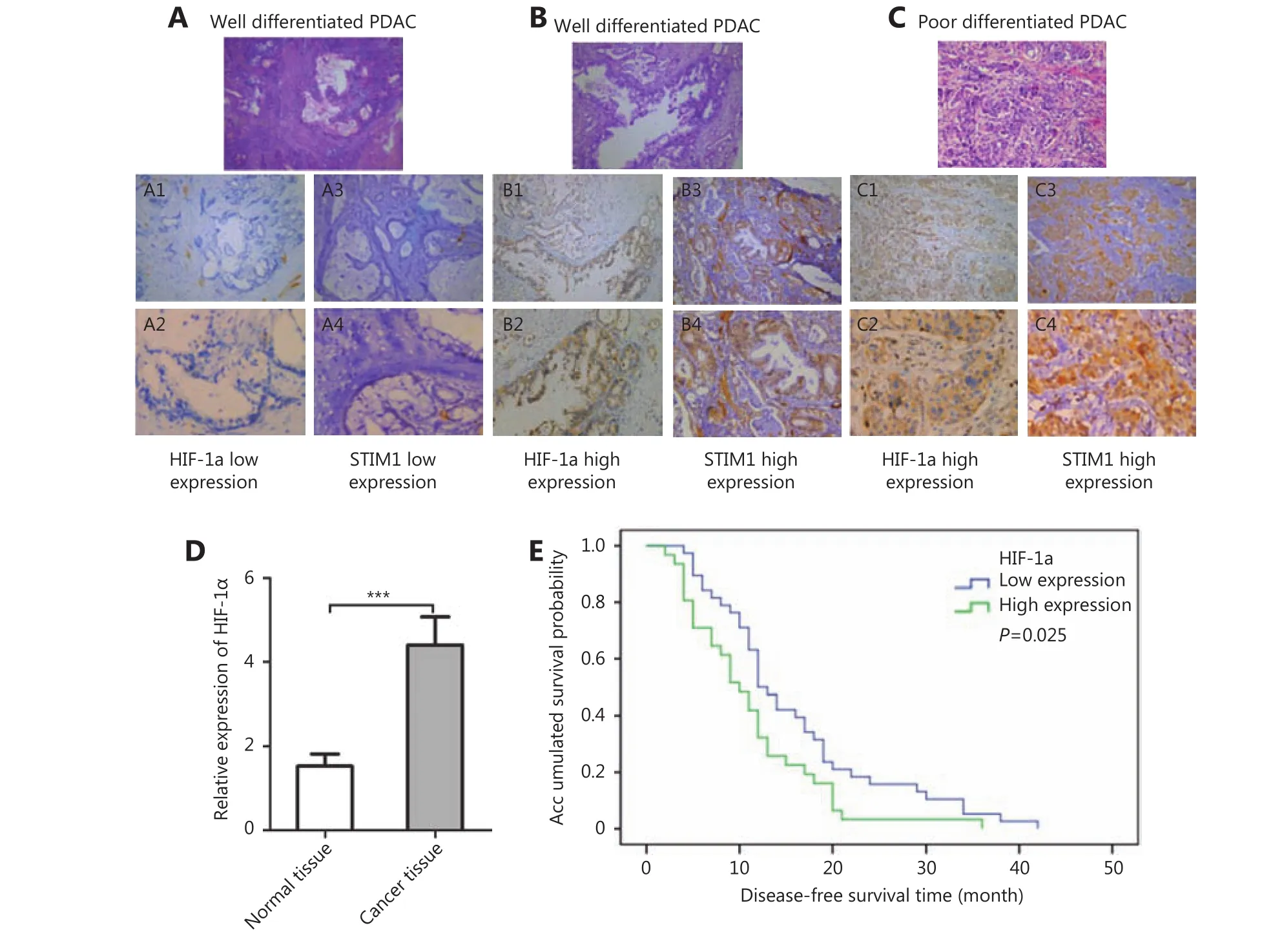
Figure 3 Immunohistochemistry of HIF-1α and STIM1 in tumor and adjacent normal tissues of PDAC patients (100 ×). (A) Representative images showing low expression of STIM1 and HIF-1α in well differentiated PDAC. (B) Representative images showing high expression of STIM1 and HIF-1α in well differentiated PDAC. (C) Representative images showing high expression of STIM1 and HIF-1α in poorly differentiated PDAC. (D) The relative HIF-1α expression in cancer samples and matching normal tissues. (E) Association between tumor HIF-1α expression levels and disease-free survival in 69 PDAC patients.
HIF-1α up regulated STIM1 expression at transcriptional level
The correlation between HIF-1α and STIM1 in PDAC suggested that HIF-1α may regulate STIM1 expression. To test this hypothesis, we tested HIF-1α and STIM1 expression in a panel of PDAC cell lines, PDAC tumor and paired normal tissues with Western blot. A similar expression pattern of HIF-1α and STIM1 was observed in all PDAC cell lines examined (Figure 4A). Moreover, HIF-1α and STIM1 were simultaneously upregulated in PDAC tumor sample 1,sample 3, and sample 4 (Figure 4B). These results suggested that STIM1 may be regulated by HIF-1α.
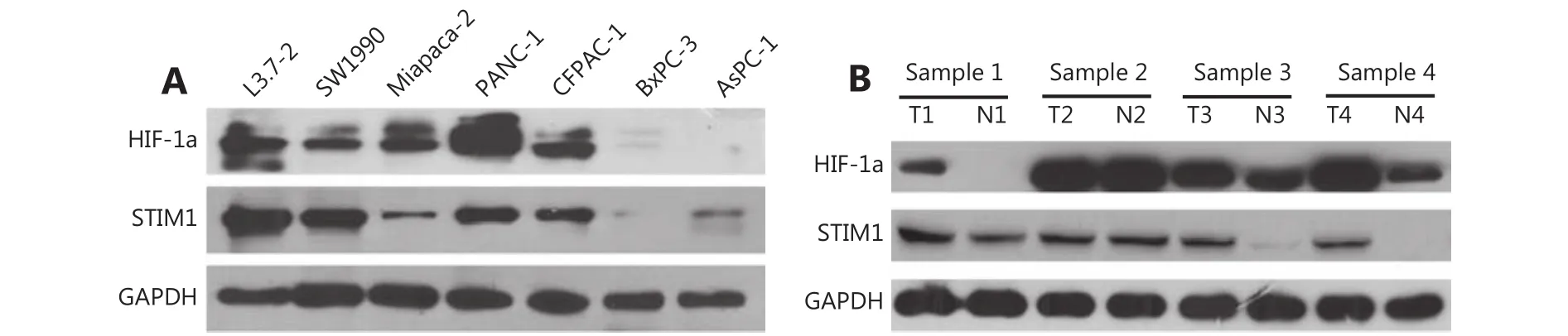
Figure 4 HIF-1α and STIM1 expression in PDAC cell lines, PDAC and paired normal tissues. (A) HIF-1α and STIM1 expression levels in PDAC cell lines. (B) HIF-1α and STIM1 expression levels in PDAC and paired normal tissues. HIF-1α and STIM1 were up-regulated in sample 1, sample 3, and sample 4.
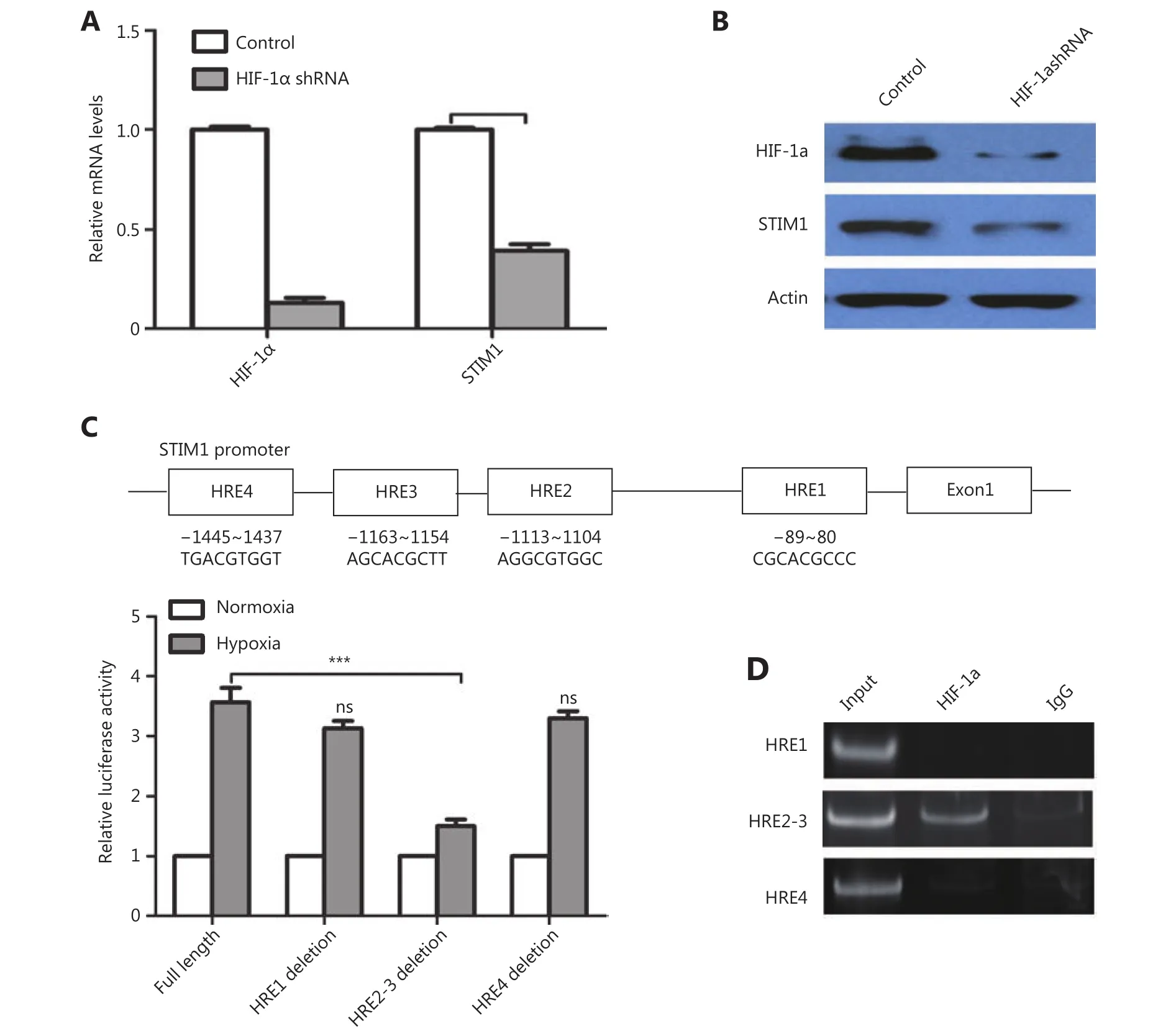
Figure 5 HIF-1α regulates the expression of STIM1 in PDAC. (A) QPCR analysis of HIF-1α and STIM1 mRNA levels in control and HIF-1α knockdown PANC-1 cells. (B) Western blot analysis of HIF-1α and STIM1 protein levels in control and HIF-1α knockdown PANC-1 cells. (C)Predicted binding sites of HIF-1α in the STIM1 promoter including HRE1, HRE2, HRE3, and HRE4 and luciferase assay analysis of HIF-1α binding sites in STIM1 promoter region. (D) ChIP analysis of HIF-1α binding sites in STIM1 promoter region.
To clarify whether STIM1 was regulated by HIF-1α, we knocked down HIF-1α in PANC-1 cells with shRNA (Figure 5A and 5B). A significant decrease in STIM1 mRNA and protein was detected in HIF-1α knockdown PANC-1 cells.These results suggested that HIF-1α may regulate STIM1 at the transcriptional level. Sequence analysis revealed 4 putative hypoxia response elements (HRE), which are HIF-1α binding sites in the STIM1 promoter (Figure 5C). We created the luciferase STIM1 promoter and HRE deletion reporter constructs (Figure 5C). Following co-transfection of PANC1 cells with HIF-1α and STIM1 promoter or its deletion mutant luciferase reporters for 48 h, the cells were cultured under normoxia (N) or hypoxia (H; 1% O2) for 24 hr. A dual-luciferase assay revealed that luciferase activity of full-length STIM 1 promoter was elevated approximately 4-fold under hypoxia conditions. Deletion of HER1 and HRE4 reduced STIM1 promoter activity by 20%. However, HRE2-3 deletion resulted in a 62% reduction of STIM1 promoter activity (Figure 5C). To investigate whether HIF-1α directly regulates STIM1 transcription, ChIP assay was performed. It was determined that HIF-1α bound to HRE2-3 region(Figure 5D). These findings suggested that HIF-1α may regulate STIM1 transcription primarily through binding to HRE2-3 region of the STIM1 promoter.
Considered together, our results indicated that STIM1 overexpression in a hypoxia microenvironment may contribute to PDAC cell invasion and promote PDAC progression.
Discussion
STIM1 has been reported to play an oncogenetic function in breast, colon, prostate, and liver cancer10-13. The current study investigated the role of STIM1 and HIF-1α in PDAC progression. We demonstrated frequent upregulation of STIM1 in PDAC tumor tissues. High STIM1 expression was significantly associated with tumor grade and early recurrence, which are important clinical determinants for the prognosis of patients with PDAC. Hence, STIM1 expression level in PDAC tissues may be used as a novel approach for predicting the prognosis of PDAC patients.
Hypoxia is common in the microenvironment of solid tumors, such as PDAC. As an important transcription factor,HIF-1α mediates many adaptive physiological responses as well as cancer progression17-21. Immunohistochemistry indicated overexpression of HIF-1α in PDAC tissues. Similar to STIM1, patients with high HIF-1α expression showed poor disease-free survival. More interestingly, HIF-1α and STIM1 showed similar expression patterns in hepatocellular carcinoma (HCC)26, PDAC cell lines and tumor samples,which relationship was demonstrated statistically, indicating that STIM1 may be a HIF-1α target gene.
The STIM1 promoter luciferase reporter assay revealed that STIM1 promoter activity was induced by HIF-1α, which directly binds to HREs on the STIM1 promoter, under hypoxia conditions. Importantly, HIF-1α knockdown with shRNA may dramatically reduce STIM1 protein levels in PANC1 cells (Figure 5B).
Previous studies showed that STIM1 was associated with a more aggressive phenotype of prostate cancer and breast cancer9,28. Thus, STIM1 protein signaling pathway may function in cancer cell invasion and metastatic behavior27,28.The cell invasion analysis revealed that STIM1 knockdown inhibited cells invasive ability. More interestingly, E-cadherin was upregulated whereas vimentin was downregulated following STIM1 knockdown, suggesting that STIM1 may participate in EMT progression, which is substantiated by a previous report28.
In conclusion, our study provided evidence that STIM may be a valuable biomarker of PDAC progression and prognosis.We also demonstrated a pivotal role of STIM1 in PDAC cell malignant and aggressiveness phenotypes. Moreover, STIM1 was transcriptionally regulated by HIF-1α under hypoxia conditions. These results indicate that the HIF-1α/STIM1 axis may be a potential therapeutic target in PDAC.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (Grant No. 81472264,31671448 and 81572618 ) and Tianjin Natural Science Foundation Grants (Grant No.13JCYBJC37400).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年1期
Cancer Biology & Medicine2019年1期
- Cancer Biology & Medicine的其它文章
- Application of next-generation sequencing technology to precision medicine in cancer: joint consensus of the Tumor Biomarker Committee of the Chinese Society of Clinical Oncology
- The breakthrough in primary human hepatocytes in vitro expansion
- Circular RNAs and human glioma
- Qidong: a crucible for studies on liver cancer etiology and prevention
- The PI3K/Akt/GSK-3β/ROS/eIF2B pathway promotes breast cancer growth and metastasis via suppression of NK cell cytotoxicity and tumor cell susceptibility
- Estrogen and insulin synergistically promote endometrial cancer progression via crosstalk between their receptor signaling pathways
