In vivo effects of neomycin sulfate on non-specific immunity,oxidative damage and replication of cyprinid herpesvirus 2 in cruciancarp(Carassius auratus gibelio)
Penghui Sun,Fei Yu,Jianfei Lu,Minli Zhang,Hao Wang,Dan Xu,Liqun Lu,∗
aNational Pathogen Collection Center for Aquatic Animals,Shanghai Ocean University,Shanghai 201306,China
bKey Laboratory of Agriculture Ministry for Freshwater Aquatic Genetic Resources,Shanghai Ocean University,Shanghai 201306,China
Keywords:Neomycin sulfate Non-specific immunity Oxidative damage Cruciancarp Cyprinid herpesvirus 2
ABSTRACT Neomycin belongs to the family of 2-deoxystreptamine-containing aminoglycoside antibiotics.It is widely used for bacterial infections,targeting most gram-negative and some gram-positive bacteria.Neomycin has also been reported to show antiviral activity.Here,we evaluated the toxicity of neomycin sulfate,and investigated its effect on non-specific immunity and viral infection in cruciancarp(Carassius auratus gibelio).The safe concentration of neomycin sulfate for cruciancarp was determined to be 102.9 mg/kg in vivo.In oxidative damage assays,neomycin sulfate increased superoxide dismutase and catalase activity and decreased malondialdehyde in the liver of cruciancarp.In non-specific blood immune assays,the white blood cell count and complement 3 content significantly increased after neomycin sulfate treatment,while no significant changes were observed in antibacterial or lysozyme activity.In a challenge test,neomycin sulfate protected the cruciancarp from cyprinid herpesvirus 2(CyHV-2)infection and inhibited CyHV-2 replication.In cytotoxicity assays,low concentrations of neomycin sulfate had no cytotoxicity on cells from the fins of cruciancarp.The results of the present study indicate that oral administration of neomycin sulfate reduced oxidative damage,enhanced immunity and provided protection against CyHV-2 in cruciancarp.
1.Introduction
Neomycin is part of a family of small molecules with one or two hexoses and one furanose attached to a 2-deoxystreptamine core in positions 4 and 5,and amine groups located exclusively on the hexoses(Becker&Cooper,2013).Neomycin sulfate is a widely used aminoglycoside antibiotic with good inhibitory activity against gram-negative and some gram-positive bacterial infections in animals.Neomycin sulfate specifically targetsaconserved sequence of16S ribosomal RNA and interferes with protein synthesis by transporting into the cytoplasm of bacteria(Becker&Cooper,2013;Kaul,Barbieri,&Pilch,2004).
To date,the majority of studies on neomycin sulfate have focused on aquatic or terrestrial animals or on its germicidal activity in vivo and in vitro(Germovsek,Barker,&Sharland,2016;Song,Wang,Wang,Jiang,&Luo,2010;Sun&Liqun,2016).The toxicity of neomycin sulfate and its effect on non-specific immunity in fish,however,has not yet been reported.The specificity,antibacterial potency,effects on the immune system and toxicology of neomycin sulfate should all be considered.
Currently,most of bacterial diseases in aquatic species are treated with antibiotics and herbal medicines(Valladao,Gallani,&Pilarski,2015).In China,neomycin sulfate is widely used in aquaculture to control bacterial diseases affecting the cruciancarp Carassius auratus gibelio(Chen,Ding,Zhu,Kong,&He,2015).In addition to their antimicrobial efficacy,aminoglycosides can also induce ototoxicity and nephrotoxicity.These antibiotics and their metabolites can also have a direct and indirect effect on the immune system(Choi et al.,2014;Murai,Kitahara,Okumura,Kobayashi,&Horio,2016).Mechanosensory hair cells are susceptible to death following treatment with aminoglycoside antibiotics that have ototoxic side effects in mammals(Monzack,May,Roy,Gale,&Cunningham,2015),with similar results reported in bony fish(Chang-Chien,Yen,Li,Hsu,&Yang,2017).Oral neomycin in mice caused an impaired immune response to respiratory influenza virus(Ichinohe et al.,2011).
Cruciancarp haematopoietic necrosis caused by cyprinid herpesvirus 2(CyHV-2)infection has become one of the major diseases threatening the healthy development of carp cultures in Asia.CyHV-2 is an enveloped virus with a large linear dsDNA genome(approximately 275kb),containing about 150 predicted open reading frames(Davison et al.,2013;Zeng,Chen,Deng,Gui,&Zhang,2016).Its genome shares about 80%homology with CyHV-1 and CyHV-3(Xu,Podok,Xie,&Lu,2014).Vaccines or drugs that can effectively prevent and control this viral disease are not available to date.Identifying a broad-spectrum or specific small molecule compound targeting CyHV-2 is a practical approach to inhibit CyHV-2 replication and control cruciancarp haematopoietic necrosis.In addition to its antibacterial properties,neomycin has also been reported to display antiviral activity(Zhang et al.,2009).For example,neomycin-arginine conjugates are multi-target inhibitors of human immunodeficiency virus type-1(Berchanski&Lapidot,2009).Neomycin reduces latent gene expression of the Kaposi's sarcoma-associated herpesvirus and increases lytic gene expression by inhibiting phospholipase Cγ-mediated angiogenin nuclear translocation(Bottero et al.,2013).However,understanding of the antiviral activity of aminoglycosides against herpesviruses is still limited.Therefore,we evaluated the safety and toxicity of neomycin sulfate and investigated its effect on non-specific immunity and viral infection in cruciancarp.
2.Materials and methods
2.1.Animals and virus
Healthy cruciancarp were obtained from Shanghai Jinshan Farm(body weight,150-200g).The fish were cultured temporarily in aerated aquariums at a temperature of 25°C ± 2°C.The fish were fed daily with pelleted dry food but were starved for two days prior to the oral administration of neomycin sulfate(Sigma,Germany).The CyHV-2 was isolated in 2013 and preserved at the National Pathogen Collection Center for Aquatic Animals,Shanghai,China(Xu et al.,2014).
2.2.Acute toxicity testing
Healthy cruciancarp(150-200 g)were randomly divided into six groups of ten individuals each.Neomycin sulfate was administered orally at the following doses:1905,1714,1142,1000,857,and 0mg/kg(the drug solution was delivered into the esophagus of carp using a catheter).The mortality at 96h post administration of neomycin sulfate was recorded for each group(four replicates per group).The 96h median lethal dose(LD50)of neomycin sulfate was calculated and analyzed using SPSS22.0 software and the 96h LC50×0.1 was considered as a safe concentration(YangLihua et al.,2003).
2.3.Sample collection from cruciancarp blood and liver
Based on the established safe concentration,healthy cruciancarp were randomly divided into four groups of twelve individuals and each group received one of the following doses of neomycin sulfate:40 mg/kg,20mg/kg,10mg/kg or 0mg/kg(control group).After oral administration of neomycin sulfate at the specified dose,three fish were randomly selected from each of the triplicate groups.Blood was sampled from the tail vein with a 1ml syringe at 24h,48h,72h,and 96h.Blood samples were mixed with an anticoagulant,2%(v/v)potassium oxalate(Sigma,Germany)and the white blood cell(WBC)count was immediately determined(Zhou,Yang,Xuefen,&Guo,2005).An aliquot of the blood sample was removed prior to the addition of the anticoagulant and placed in a 4°C incubator for 12h and then centrifuged at 5000×g for 10min.The supernatant was placed at-20°C and later used to measure antimicrobial activity and the lysozyme and complement C3 concentrations.
The livers(hepatopancreas)from each group were collected,washed with 0.9%physiological saline and dried with filter paper,after which a 1:9(w/v)solution of physiological saline was added and the tissuewashomogenized.The homogenateswere centrifugedat5000×g for 20min,and the supernatant was stored at-80°C to determine the superoxide dismutase(SOD)activity,catalase(CAT)activity and malondialdehyde(MDA)content(Mao et al.,2013).The total protein concentration of the supernatant was measured using a BCA protein assay kit(Thermo Fisher Scienti fi c,USA).
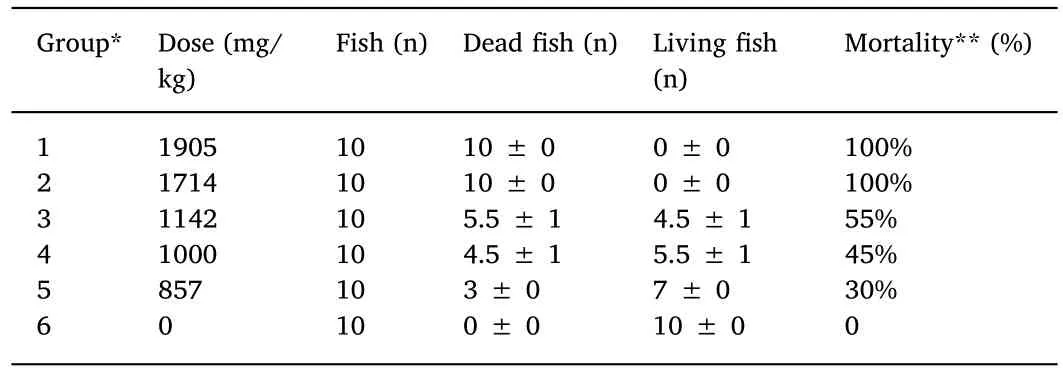
Table 1Acute toxicity of high dose neomycin sulfate in cruciancarp.
2.4.WBC count
Blood samples were mixed with an anticoagulant(2%potassium oxalate,anticoagulant:blood[v/v]=1:4)and the WBC count was immediately determined(Zhou et al.,2005).In brief,anticoagulant(20 μL)was mixed with 380 μL of leukocyte diluent and a 25 × 16 blood cell counting chamber was used to count the WBCs(Olympus,Japan);each sample was counted three times.The number of WBCs was counted in four large corner squares each time.The following formula was used to calculate the number of WBCs per liter of blood:
Number of WBCs/L=(Number of WBCs in four large corner squares/4)×10×25×106
2.5.Serum antibacterial activity
Escherichia coli was inoculated in Luria-Bertani liquid broth and grown at 37°C for 24h.The bacterial pellet was resuspended in phosphate-buffered saline(PBS;pH 6.4,0.1mol/L)to give an absorbance of 0.4(OD570=0.4).The 3ml suspension of bacteria was placed on ice for 5min,mixed with 50μL of the test serum and the absorbance(A0)measured at OD570.The mixture was incubated at 37°C for 30min,placed on ice for 10min and the absorbance(A)measured at OD570.Serum antibacterial activity(Ua)was calculated using the following formula:Ua=[(A0-A)/A]1/2
2.6.Lysozyme content
Micrococcus solution (0.25mg/mL, 4mL, Kanu Biological Technology,China),lysozyme standard solution(2.5 μg/mL,200 μL,Kanu Biological Technology)and test serum(200 μL)were pre-warmed at 37°C for 5min.Two milliliters of the micrococcus solution were mixed with 200 μL of test serum or 200 μL of the lysozyme standard and the transmittance was determined at 530 nm.The transmittance values for the test serum or lysozyme standard were carried out at several time points and designated UT0,ST0at 5s,respectively,and UT2,and ST2at 125s,respectively.
Lysozyme content (μg/mL)=[(UT2- UT0)/ (ST2-ST0)]×2.5×sample dilution ratio.
2.7.Measurements of C3,SOD,CAT,and MDA
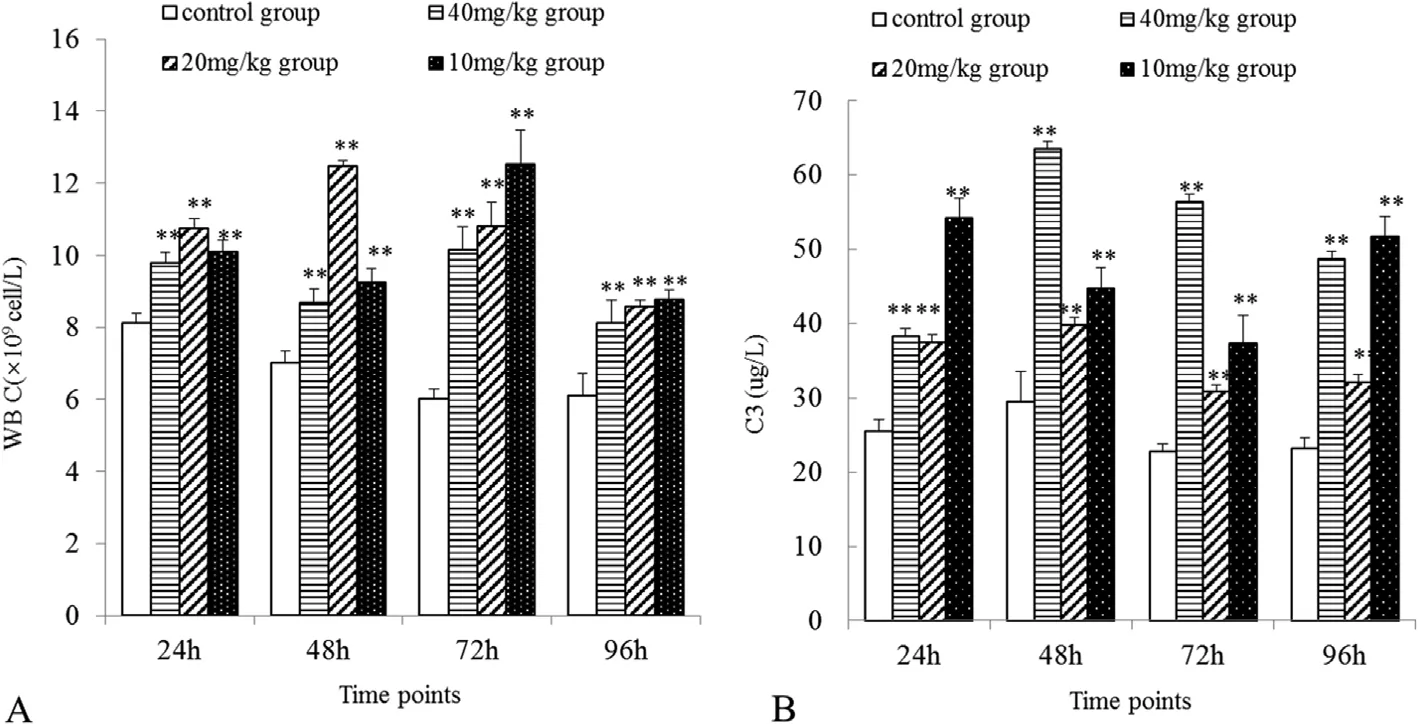
Fig.1.Effect of neomycin sulfate on WBC count(A)and complement C3 content(B)in the blood of cruciancarp following oral administration of neomycin sulfate at designated concentrations.Data represents the mean ± SE.Highly significant differences were considered when **P < 0.01.
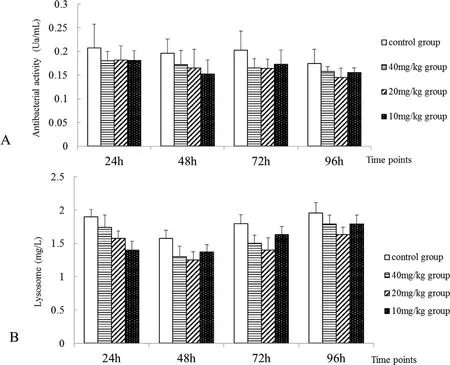
Fig.2.Effect of neomycin sulfate on antibacterial activity(A)and lysozyme content(B)in the blood of cruciancarp.After oral administration of neomycin sulfate,changes of bacterial activity and lysozyme content were determined.Data was presented as the mean±SE.
Complement C3 was measured using a commercial ELISA Kit(Kanu Biological Technology,China).The C3 content(μg/L)was determined by measuring the absorbance at 450nm and calculated using a standard curve(R2=0.999,Figure S1)and following the manufacturer's instruction.Kits were used to measure the total superoxide dismutase(SOD,hydroxylamine method),catalase(CAT)and malondialdehyde(MDA)(TBA method,Nanjing Jiancheng Bioengineering Institute,China)by following the manufacturer's instructions and previously established protocols(Li et al.,2014).The SOD activity was measured at 550 nm,and the data was calculated as units of SOD activity per milligram of total liver protein.The CAT activity was measured at 405 nm using the H2O2hydrolysis method and calculated as U/mg of total liver protein.The MDA content(nmol per milligram of total protein in liver)was determined using thiobarbituric acid at 95°C for 80min and absorbance measured at 532nm.All the experiments were performed in triplicates.
2.8.Cytotoxicity of neomycin sulfate
Cruciancarp cells(CCF)were prepared from the fin and cultured as previously described(Siyao et al.,2016).The cells(1×105cells/mL)were cultured in 48-well cell culture plates,with 200 μL of M199 medium containing 10%(v/v)fetal bovine serum(Gibco,USA).After 6h in culture,the cells were washed three times with PBS and divided into four groups of 12 wells each.Each of the groups was treated with neomycin sulfate at one of the following concentrations:1000,500,100,and 0 μmol/L.The cells were incubated at 30 °C and at 12,24,36,and 48 h,cells from each group were digested with 20μL of 0.25%trypsin for 1min then mixed with 100μL M199 medium.One hundred microliters of the cell suspension from each group was mixed with 100μL of Muse Count Viability Reagent(Millipore,Germany),and following a 5min incubation in the dark,the number of viable cells was counted using a flow cytometer,Muse Cell Analyser(Millipore,Germany)(Wang,Liu,Yu,&Lu,2016).
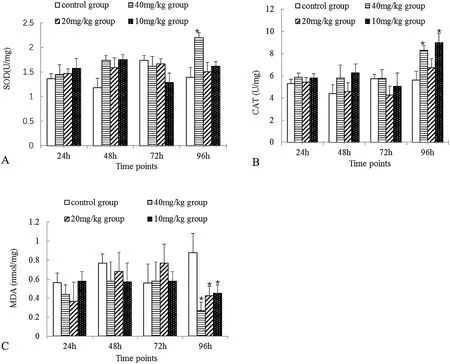
Fig.3.Effect of neomycin sulfate on the SOD activity,CAT activity and MDA content in the liver of cruciancarp.Livers were sampled in triplicate from three fish,and fold changes are presented as the mean ± SE(n=3).Significant differences were considered at*P < 0.05.
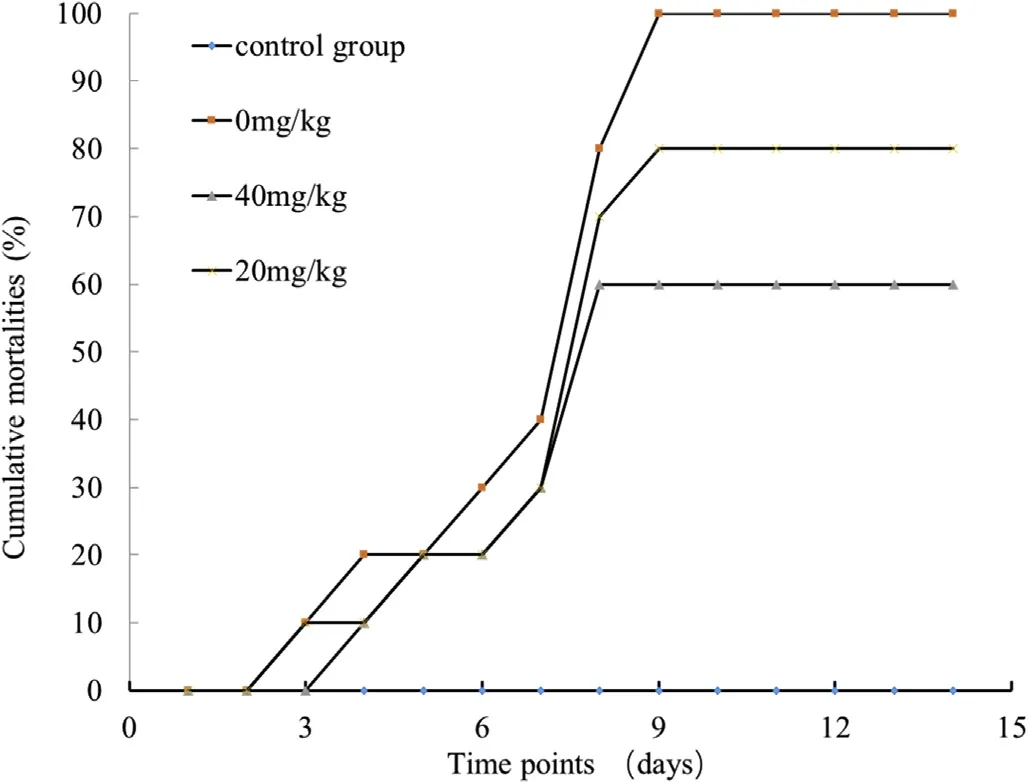
Fig.4.Effect of neomycin sulfate on the mortality rate of cruciancarp after artificial infection with CyHV-2.Infected fish were administrated with 0,20,or 40 mg/kg neomycin sulfate.Fish administrated with 40mg/kg neomycin sulfate without CyHV-2 infection served as the control group.The mortality rate of each group was calculated after two weeks.
2.9.Artificial infection with CyHV-2
Liver and kidney from cruciancarp infected with CyHV-2 were collected and homogenized in PBS(pH,7.0)and the viral DNA was detected by PCR as previously reported(Xu et al.,2014).The tissue homogenates were centrifuged at 5000×g and the resulting supernatant was centrifuged at 12000×g for 20min followed by ultracentrifugation to obtain purified CyHV-2 particles.The titer of CyHV-2 particles was determined by real-time quantitative PCR(Xu et al.,2014).
Not too long after that my grandfather went to a nursing home, and during the next summer he died while I was away at camp. My father turned the attic into a storage room. Now it s filled with dusty boxes of old clothes and shoes and old furniture.
Four groups of 10 fish each(in triplicates)were used for the viral infection experiments:control group,40 mg/kg neomycin sulfate,20mg/kg,and 0mg/kg(untreated).With the exception of the control group,the fish in the remaining three groups were intraperitoneally injected with 0.1 ml virus solution(1×106copies)at 24h following the neomycin sulfate administration.The number of deaths in each group was recorded over a two-week period.
2.10.Titer determination of CyHV-2 in cruciancarp blood
After infection with CyHV-2(1×106copies),tail-vein blood from the cruciancarp was collected at 24,48,72,96,120,144,168,and 192h from the control group and the 40mg/kg neomycin sulfate group.The DNA was extracted from 5μL blood using a DNA kit(Tiangen,China),and the viral titer in the blood was determined by real-time quantitative PCR as indicated above.
2.11.Statistical analysis
The significance level was set at P < 0.05,and differences were considered to be significant at P<0.01.Statistical analyses were conducted using SPSS22.0,and the results are presented as mean±standard error(SE).
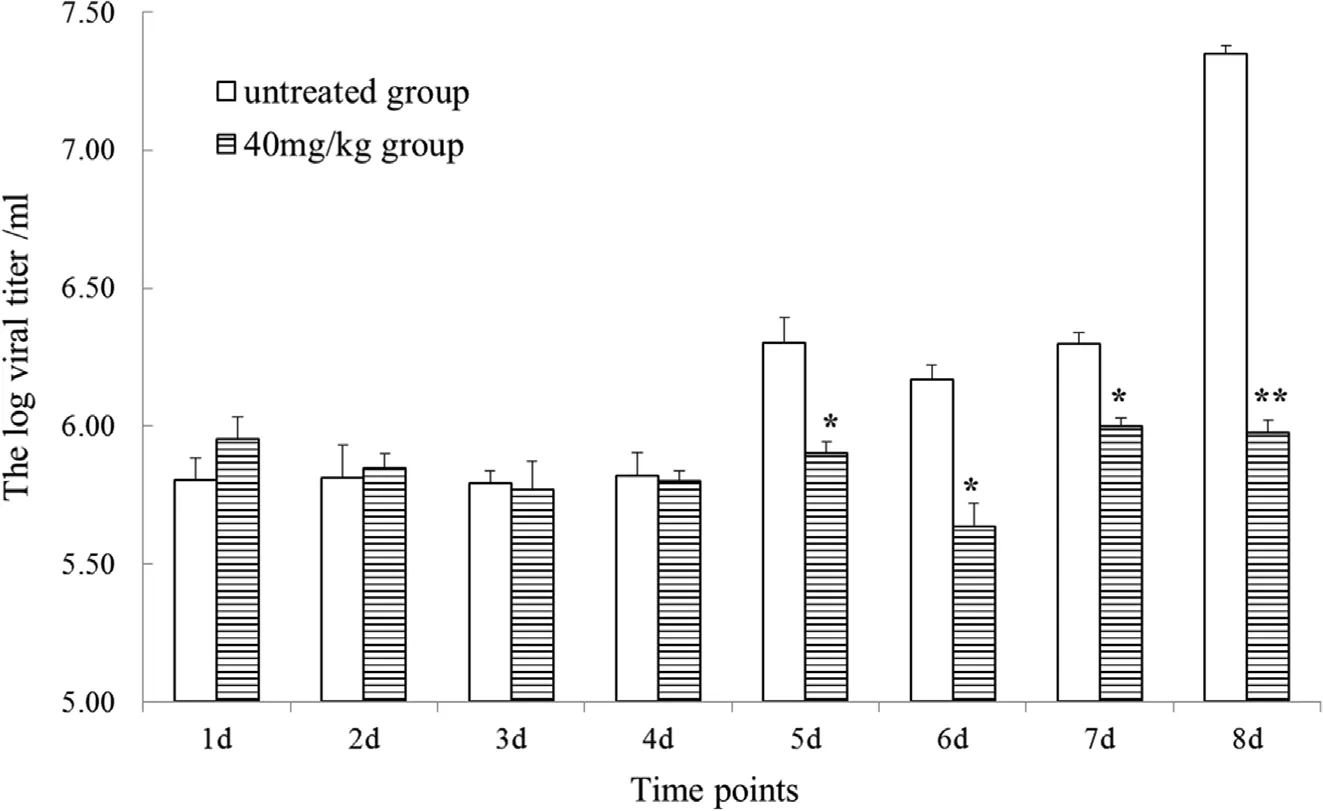
Fig.5.Effect of neomycin sulfate on the viral titer of CyHV-2 in infected cruciancarp.Changes in the viral titer of the group without neomycin sulfate and the 40 mg/kg group are shown at different time points.Significant differences were considered at*P < 0.05 and highly significant differences at**P < 0.01.
3.Results
3.1.Acute toxicity of high-dose neomycin sulfate in cruciancarp
The mortality rate at 96 h post treatment of high dose neomycin sulfate was recorded in each group(Table 1).The 96 h LD50 of neomycin sulfate was 1029.307 mg/kg,with a 95%con fi dence interval of 889.13-1171.58 mg/kg.Based on this result,the safe concentration for administration of oral neomycin sulfate in cruciancarp was calculated to be 102.9 mg/kg.
3.2.Effect of neomycin sulfate on non-specific immunity in cruciancarp
Several immune indexes were also examined at specific time points after neomycin treatment in cruciancarp.The changes in non-specific blood immune parameters after administration of different concentrations of neomycin sulfate in each group are shown in Fig.1.Compared with the control group,the WBC count(Fig.1A)and the C3 content(Fig.1B)significantly increased in the treatment groups(P<0.01),suggesting that administration of neomycin sulfate promotes non-specific immunity in cruciancarp.
Antibacterial activity(Fig.2A)and lysozyme content(Fig.2B)were lower in the treatment groups.However,the differences were not significant,suggesting that inhibition of bacterial infection by neomycin sulfate does not result from the enhancement of antibacterial and lysozyme activity of the host.
3.3.Effect of neomycin sulfate on oxidative damage in cruciancarp
The oxidative damage indexes(Gutiérrez-Salinas et al.,2013;Li et al.,2014)in the liver after administration of different concentrations of neomycin sulfate to each group are shown in Fig.3.In the 40mg/kg group(Fig.3A),the SOD activity increased significantly by 96h(P<0.05)and the CAT activity(Fig.3B)significantly increased by 96 h in both the 10 and 40mg/kg groups(P<0.05).MDA content(Fig.3C)significantly decreased after 96h(P<0.05).Taken together,these results indicate that neomycin sulfate provided protection against oxidative damage in cruciancarp.
3.4.Neomycin sulfate protects cruciancarp from CyHV-2 infection
The viral titers in the blood of infected Cruciancarp treated with neomycin sulfate(0 and 40 mg/kg)were determined by real-time PCR(Fig.5).The results showed that there were no significant differences between the groups at the early stages of viral infection.From the 5th to 7th days,the viral titer in the neomycin sulfate untreated group was significantly higher than those of the treated groups(P<0.05)and on day 8,the differences were even more significant(P < 0.01).Taken together,these results indicate that neomycin sulfate protects cruciancarp from CyHV-2 infection and inhibits replication of the viral genome.
3.5.Cytotoxic effects of neomycin sulfate on CCF cells
It has been experimentally demonstrated that the in vitro cytotoxicity of drugs is a good predictor of their safety in animals(Elisha,Botha,Mcgaw,&Eloff,2017;Thews,Riemann,Nowak,&Gekle,2014).Therefore,to examine the cytotoxicity of neomycin sulfate in vitro,CCF cells were cultured in medium containing different concentrations of neomycin sulfate and the number of viable cells was measured(Fig.6).In comparison with the control group,the number of viable cells in the neomycin sulfate treatment groups first declined and then increased;however,differences were not significant(P > 0.05).The results indicate that neomycin sulfate,at doses from 100 to 1000 μmol/L,does not affect the activity of CCF cells,indicating that there are no cytotoxic effects of low dose neomycin sulfate on CCF cells.
4.Discussion
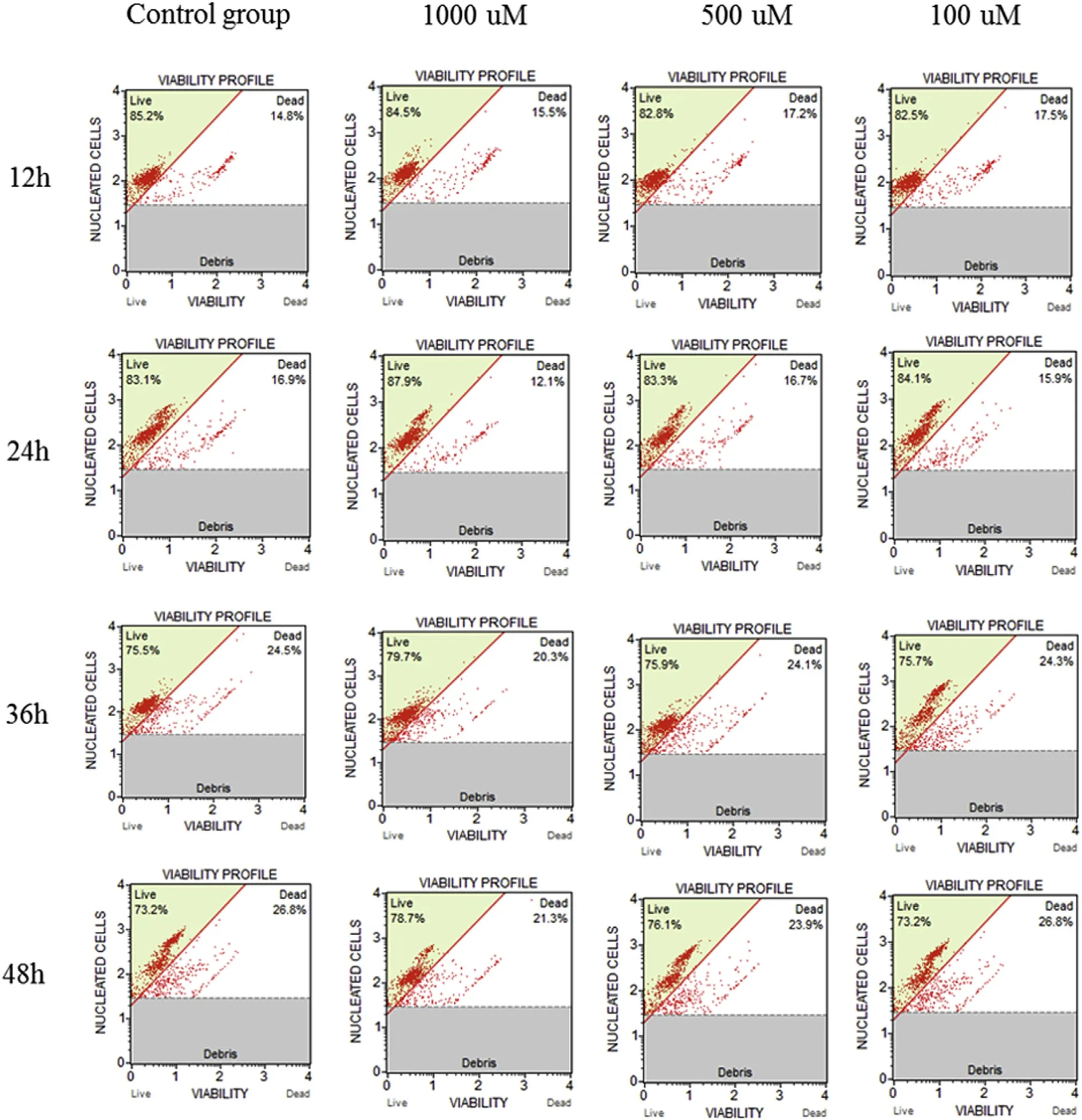
Fig.6.In vitro cytotoxicity of neomycin sulfate in the cruciancarp.Cruciancarp fin cells were cultured with 1000,500,100,or 0μM of neomycin sulfate,and the number of viable cells was measured at 12h,24h,36 h,and 48h.
Over the past decades,the antibacterial activity of aminoglycosides has been fully explored in animals.The effects of neomycin sulfate on cellular activity,oxidative damage,and in flammatory and immune responses in aquatic organisms remain to be defined.In the present study,we evaluated the acute toxicity of high dose neomycin sulfate to identify the safe concentration for administration in cruciancarp.We found that low dose neomycin sulfate protected against oxidative damage and displayed no cytotoxicity in CCF cells,indicating the suitability of neomycin sulfate for aquaculture.Oral neomycin in chicken can lead to a change in immune response to antigens(Murai et al.,2016).In a similar way,neomycin sulfate induced increased levels of leukocytes and complement 3 in cruciancarp,which might be a novel antibacterial and antiviral mechanism.We also provide evidence that neomycin sulfate protects cruciancarp from CyHV-2 infection and effectively inhibits viral replication in blood(in vivo),suggesting that aminoglycosides are a potential antiviral compound against CyHV-2 in carp.This is in agreement with reports that aminoglycosides inhibit replication of DNA and RNA viruses in several species(Bottero et al.,2013;Cabrera et al.,2000;Zhang et al.,2009).
In a previous study,CyHV-2 infection was shown to reduce total erythrocyte count,total leukocyte count,hemoglobin concentration,and thrombocyte count in cruciancarp(Lu,Lu,&Cao,2016).The complement system recognizes viruses and virus-infected cells,and can trigger effector pathways to neutralize viruses and kill infected cells(Agrawal,Nawadkar,Ojha,Kumar,&Sahu,2017).Complement is involved in the acute phase response to CyHV-3 infection in common carp with an organ-and time-dependent response(Pionnier et al.,2014).Total erythrocyte count and complement 3 increases observed during neomycin treatment in the present study might result in inhibition of CyHV-2.Oral administration of the appropriate dose of neomycin sulfate can significantly improve erythrocyte count and complement C3,and to a certain extent,might reduce the effects of the virus on neutrophils,thereby improving non-specific immunity.
CyHV-2 aggregated in the liver of challenged cruciancarp has been observed by electron microscopy and genomic DNA can only be amplified from the liver,spleen,pancreas,intestine and kidney(Zhu et al.,2015),which suggests that the liver is the main target of CyHV-2.In mice,generalized infection of Herpes simplex virus can lead to acute liver failure,one of HSV-induced liver pathologies(Parker,Pasieka,Parker,&Leib,2016).Infection with another herpesvirus(murine cytomegalovirus),impaired accumulation of Treg cells in the liver and led to liver damage(Popovic et al.,2017).Furthermore,a significant increase was observed in SOD and CAT activities,along with a slight decrease in the MDA content after neomycin sulfate treatment.Therefore,neomycin sulfate may protect the liver from oxidative damage and this may explain the inhibition of CyHV-2 replication by neomycin sulfate in the cruciancarp.To our knowledge,we are the first to show the antiviral activity of neomycin sulfate in bony fish.However,this observation should be confirmed and expanded in future studies,and the specific mechanism of action still require further investigation.
Overall,the present findings show that neomycin reduced mortality and inhibited viral proliferation in infected cruciancarp.Furthermore,the mechanism of action of neomycin involves the non-specific immune response and oxidative damage.Furthermore,our cytotoxicity test showed that this drug is safe for use in cruciancarp at the doses tested in this study.Therefore,neomycin sulfate shows potential as a preventive drug against viral and bacterial infections in carp.It is possible that neomycin can inhibit other herpesvirus in fish or animals,presenting an area for future study.The results of this study lay a solid foundation for the application of neomycin as an antibacterial or antiviral agent in fish.
Acknowledgments
This work was supported by the Earmarked Fund for China Agriculture Research System(grant number CARS-45-19).The authors report no conflicts of interest in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aaf.2018.09.003.
 Aquaculture and Fisheries2019年2期
Aquaculture and Fisheries2019年2期
- Aquaculture and Fisheries的其它文章
- Estimationof genetic parameters for juvenile growth performance traits in olive flounder(Paralichthys olivaceus)
- Full-length mRNA sequencing in Saccharina japonica and identification of carbonic anhydrase genes
- Effects of dietary lysolecithin(LPC)on growth,apparent digestibility of nutrient and lipid metabolism in juvenile turbot Scophthalmus maximus L.
- Effectiveness of stocking Sparus macrocephalus fry in situ in the East China Sea
- Current status of research on aquaculture genetics and genomicsinformation from ISGA 2018
