Removal of Methyl Orange from Aqueous Solutions by a Novel Hyper-Cross-Linked Aromatic Triazine Porous Polymer
HE Yan , LI Hao , ZHOU Li , XU Ting , PENG Changjun ,*, LIU Honglai
1Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices, School of Chemistry, Biology and Materials Science, East China University of Technology, Nanchang 330013, P, R. China.
2 Key Laboratory for Advanced Materials, Department of Chemistry, East China University of Science and Technology,Shanghai 200237, P. R. China.
Abstract: Organic dyes, especially the harmful cationic dye methyl orange (MO), are emerging pollutants. The development of new materials for their efficient adsorption and removal is thus of great significance. Porous organic polymers (POPs) such as hyper-cross-linked polymers, covalent organic frameworks,conjugated microporous polymers, and polymers with intrinsic microporosity are a new class of materials constructed from organic molecular building blocks. To design POPs both with good porosity and task-specific functionalization is still a critical challenge. In this study, we have demonstrated a simple one-step method for the synthesis of the hyper-cross-linked aromatic triazine porous polymer (HAPP) via the Friedel-Crafts reaction. The resultant porous polymer was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy, elemental analysis (EA), thermo-gravimetric analysis (TGA), solid-state 13C nuclear magnetic resonance (13C NMR), and nitrogen adsorption-desorption isotherms. The results show that HAPP is a rough,irregular morphology, porous organic polymer that is amorphous in nature. The novel polymer showed high Brunauer-Emmett-Teller surface area (of up to 104.36 m2∙g-1), porosity, and physicochemical stability. Owing to the presence of N heteroatom pore surfaces in the network, the material exhibited a maximum adsorption capacity of 249.3 mg∙g-1 for MO from aqueous solutions at room temperature. This is higher than that of some reported porous materials under the same conditions. To explain this phenomenon more clearly, theoretical quantum calculations were performed via the DFT method using Gaussian 09 software and Multiwfn version 3.4.1. It is performed to analyze the propertiesand electrostatic potential (ESP) of the HAPP monomer and MO. The results indicated that the N heteroatom of HAPP can easily develop strong interactions with MO, supporting the efficient adsorption of MO. The parameters studied include the physical and chemical properties of adsorption, pH, contact time, and initial concentrations. The percentage of MO removal increased as the pH was increased from 2 to 4. The optimum pH required for maximum adsorption was found to be 5.6. Adsorption kinetics data were modeled using the pseudo-first-order and pseudo-second-order models. The results indicate that the second-order model best describes the kinetic adsorption data. The adsorption isotherms revealed a good fit with the Langmuir model. More importantly, the HAPP can be regenerated effectively and recycled at least five times without significant loss of adsorption capacity. Therefore, it is believed that HAPPs with hierarchical porous structures, high surface areas, and physicochemical stability are promising candidates for the purification and treatment of dyes in solution.
Key Words: Hyper-cross-linked porous polymer; One-step; Adsorption; Methyl orange; Regeneration
1 Introduction
Since the invention of the dyestuffs, many dyes have been widely used in textile, leather, papermaking, printing and other industries1. However, dyes can also cause serious environmental pollution problems. Meanwhile, dyes are generally difficult to degrade because they are very stable to light and oxidation2,3. Various methods have been applied for the removal of dyes from wastewater, including physical,chemical and biological oxidation methods2-5. Among them,physico-adsorption is considered one of the most competitive method due to high efficiency, economic feasibility and simplicity of operation3,6-8. The key to remove dyes by physico-adsorption is adsorbents. Therefore, researchers still have been working on developing new efficient adsorption materials to replace the adsorbents used in industrial applications. As for a harmful cationic dye, methyl orange(MO) from aqueous solutions, there are many natural porous adsorbents, such as banana peel9, orange peel9, calcined layered hydroxide10, hyper-cross-linked polymer11and activated carbon12, have been developed for removal of methyl orange from aqueous solution. However, compared with traditional porous materials, porous organic polymers (POPs),new generation porous materials, have not been applied widely in the field of water treatment yet.
Porous organic polymers (POPs) have many advantages such as high specific surface area, low mass density, easy functionality and high stability and so on. In recent years,different kinds of POPs have been developed, such as covalent organic frameworks (COFs)13, hyper-cross-linked polymers(HCPs)14, polymers of intrinsic microporous polymers (PIMs)15,conjugated microporous polymers (CMPs)16and porous aromatic frameworks (PAFs)17and other porous polymers18-20. These materials have been used in gas storage21, separation22, catalytic23, sensor24, and drug delivery25. Recently, the perfluorous CMP materials were synthesized and found its remarkable water treatment ability26.But the expensive monomers, rare metal catalysts used and multiple synthetic steps limit their large-scale applications.Therefore, exploiting easy available and cost-effective adsorbents for water purification is still of significance in fields of porous material.
Recently, Tan and co-workers27successfully developed a low cost synthetic method for HCPs. The aromatic benzene,biphenyl, phenol, triphenyl and other monomers can be polymerized into porous materials through one-step of simple Friedel-Crafts alkylation using formaldehyde dimethyl acetal(FDA) as an external cross-linker. Friedel-Crafts alkylation has many advantages, such as economy, simple operation, no expensive catalyst, especially it is suitable for the reaction of aromatic compounds. It can be seen that the Friedel-Crafts alkylation reaction22,23,28,29is a method widely used to synthesize porous materials.
Herein, we utilized the Friedel-Crafts alkylation polymerization monomer 2,4-diamino-6-phenyl-1,3,5-triazine in the presence of cross-linker FDA and Lewis acid FeCl3catalyst and prepared a hyper-cross-linked aromatic triazine porous polymer (HAPP). The polymer HAPP has high specific surface area and good thermal stability. After the optimizing conditions for MO adsorption, the adsorption kinetics,isotherms and regeneration of the polymer HAPP are studied.Additionally, the mechanism of adsorption of the MO on the polymer HAPP is tentatively illustrated by the experimental results and theoretical quantum calculations. Hence, the fast adsorption kinetics, large adsorption capacity, excellent chemical stability and good reusability make HAPP a highly potential adsorbent for the removal of dyes from the water.
2 Experimental
2.1 Reagents and Materials
2,4-diamino-6-phenyl-1,3,5-triazine (98%), anhydrous ferric chloride (FeCl3, ≥ 98.0%), 1,2-dichloroethane (DCE, 99.8%),formaldehyde dimethyl acetal (FDA, 99.8%) were purchased from Sigma-Aldrich company. Methyl Orange (MO, 98%) was purchased in TCI, America. Methanol (AR, 99.5%) was purchased from Aladdin Chemistry Co. Ltd., which was used without further purification. Ultrapure water (18.2 MΩ·cm) was purified by a Milli-Q plus water purification system.
2.2 The synthesis of hyper-cross-linked aromatic triazine porous polymer HAPP
2,4-diamino-6-phenyl-1,3,5-triazine (A1) were selected as pre-functionalized monomer. Briefly, A1 (0.01 mol,1.87 g) was dissolved in 15 mL anhydrous DCE. To this solution,Formaldehyde dimethyl acetal (FDA, 0.04 mol, 3.53 mL) and anhydrous FeCl3(40 mmol, 6.5 g) were added under nitrogen environment. The resulting mixtures were heated to 45 °C for 5 h, then slowly heated to 80 °C for 19 h. After cooling to room temperature, the crude products were collected by filtration and repeatedly washed with methanol until the filtrate nearly colorless. Finally, the product was dried at 60 °C under vacuum to give a brown solid HAPP.
2.3 Adsorption test
2.3.1 MO adsorption
2.5 mg porous polymer HAPP was added to 5 mL different concentration MO solution under mechanical shaker conditions at room temperature. The initial concentration of MO is varied from the 1 to 1000 mg·g-1. The residual concentration of dyes was determined at the maximum wavelength at 464 nm using a UV-Vis spectrometer. The adsorption capacity and removal percentage of dyes were calculated by using the following equations:

where qeand E denote the adsorption capacity (m2·g-1) and removal percentage of MO, respectively; C0and Cewere the initial and equilibrium concentrations (mg·g-1) of MO in aqueous solution, respectively; V was the volume of adsorption solution (mL); m was the mass of the adsorbent (mg).
2.3.2 Adsorption kinetics of MO on HAPP
2.5 mg porous polymer HAPP was added to 5 mL the initial concentration of 10 mg·L-1MO solution under mechanical shaker condition at room temperature. After adsorption for a predetermined time, the mixture was filtered with a 0.22 μm Millipore hydrophobic PTFE membrane, and the filtrate was measured using UV-Vis spectroscopy.
2.3.3 Reusability of HAPP
The adsorption/desorption cycles were conducted as follows:each adsorption experiment consisted of 25 mg of HAPP with 50 mL of 10 mg·L-1MO solution for 24 h. After the adsorption experiments, the dye-loaded powder in the flask was collected using filtration, washed with ultrapure water and stirred in water/ethanol (V/V = 1 : 1) for 24 h to desorb the dyes. The regeneration adsorbent was then filtered and separated from the solution, and rinsed with sodium hydroxide, ethanol, ultrapure water. Finally, the regeneration adsorbent was dried at 60 °C under vacuum. The desorption efficiency was calculated as the percentage of the mass of the analyte in the dyes desorbed to the elution medium of the total mass of the analyte adsorbed on the adsorbent. The above cycle was repeated 5 times.
2.4 Characterization of HAPP
The polymer morphology was characterized by using a Nova NanoS 450 field emission scanning electron microscope(FE-SEM). Transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM)images were recorded on JEM-2100. The thermal stability was detected using a TGA unit (NETZSCH STA 499 F3) at a heating rate of 10 °C·min-1nitrogen. N2adsorption-desorption isotherms at 77 K were measured by a volumetric adsorption analyzer Micromeritics ASAP 2020. Before adsorption measurements the polymers were degassed at 120 °C for 24 h.The specific surface area was calculated by the Brunauer-Emmett-Teller (BET) method within the relative pressure range 0.01-0.1. The Fourier transform infrared (FTIR)spectra were carried out on a Nicolet iS10 FTIR spectrometer using the KBr pellet technique. The Element Analysis was performed on a vario EL III (Germany). The13C NMR measurements were performed on a 9.4 T Bruker Avance spectrometer at a Larmor frequency of 100.6 MHz.Measurements were made with a 4 mm MAS probe spinning at 15 kHz. Chemical shifts were externally referenced to TMS (δ= 0) using the methyl resonance of hexamethylbenzene (δ =17.5 relative to TMS).
2.5 Quantum calculations
All the molecules were fully optimized by the B3LYP method combined with the 6-311G(d,p) basis set for all the atoms involved. During the calculations, no symmetry constraint was performed. Besides, frequency analysis was calculated at the same theoretical method with the same basis set to insure that the configurations found are all real minima on the potential energy surface. All the calculations were performed by the Gaussian 09 software30together with the Multiwfn program of version 3.4.131.
3 Results and discussion
3.1 Characterization of porous materials HAPP
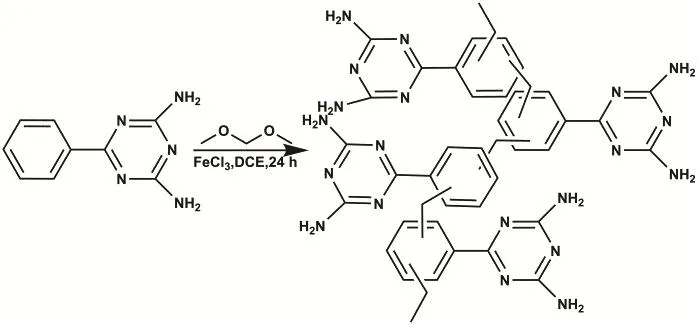
Scheme 1 representation of the synthesis of hyper-cross-linked aromatic triazine porous polymer HAPP.
As shown in Scheme 1, the material HAPP is “knitted” by methylene bond (―CH2―) in the presence of FDA and Lewis acid FeCl3catalyst. Its structure was characterized by FTIR and13C NMR. The FTIR pattern is shown in Fig. 1a, the peaks at 2856 and 2927 cm-1are attributed to the bonds of C―H stretching vibration, which indicated the methylene bond successfully crosslink aromatic ring monomers. The characteristic bands at 1560 cm-1are assigned to the C―N stretching vibrations, indicating the triazine skeleton in the porous network. In13C NMR spectrum (Fig. 1b), the peaks at 165 are ascribed to the carbon atoms in the triazine skeleton.The peaks at 135 and 128 correspond to the carbon atoms in the aromatic systems. In addition, 57.5 corresponds to the carbon in the methoxy (―OCH3) bond, which may be due to the secondary crosslinking of the monomers during the polymerization24. The peak 38 corresponds to the carbon in the methylene bond (―CH2) formed by the Friedel-Crafts alkylation reaction. Thermal gravimetric analysis (TGA) shows that HAPP is stable up to 300 °C (Fig. 2a). The initial small weight loss below 100 °C is most likely caused by the release of entrapped solvent or small gaseous molecules in the micropores. The obtained HAPP is insoluble in dilute solutions of NaOH and HCl, as well as common organic solvents, such as dichloromethane, acetone, methanol, THF and DMF,indicating good chemical stability of the HAPP adsorbent polymer. More importantly, the content of N-doped in the materials was characterized by the element analysis. As shown in the Fig. 2b, except for the C and H, the content of N is the highest, accounting for 14.34% of the total material, indicating that the HAPP material is a rich N-doped porous materials.
The morphologies of HAPP materials were characterized by SEM, TEM and HRTEM. As shown in Fig. 3a, it can be seen that the HAPP material has an irregular cluster of flower morphology with a rough surface with the nanometers size of the particles. The high resolution transmission electron microscopy (HR-TEM) image (Fig. 3b) shows the amorphous microporosity nature of the HAPP material. Furthermore, the porosity of the polymer HAPP was characterized by N2adsorption-desorption isotherms at 77 K. As shown in Fig. 4a,according to the IUPAC classification, HAPP material is a typical Type IV isotherm with the mesoporous properties.According to the de Boer method classification32, the HAPP material displayed the H4 type, which the presence of a hysteresis loop in the pressure region of p/p0= 0.45-1.0 in the N2adsorption-desorption curve of the polymer suggested the mesoporous in the network. In addition, the hysteresis loop at the high pressure of p/p0= 1 indicated the existence of macropores and interparticular voids. Moreover, the pore size distribution (PSD) curve (Fig. 4b) is calculated based on the nonlocal density functional theory (NDFT) further confirms the ca. 32 nm pores are dominant in the polymer, which can also be observed in some other polymer derived from the “knitting”approach. As summarized in Table 1, the specific surface area of HAPP material is 104.4 m2·g-1, and the mesoporous pore volume is 0.24 cm3·g-1, which further indicates that it is mainly mesoporous structure.
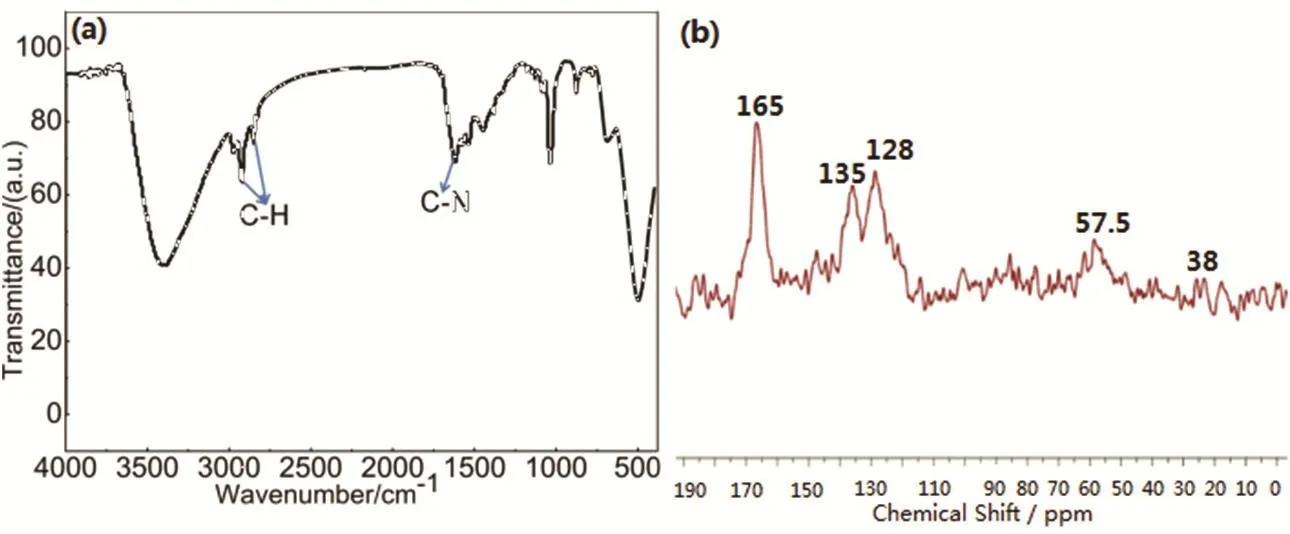
Fig. 1 (a) FTIR and (b) 13C NMR spectra of the polymer HAPP.
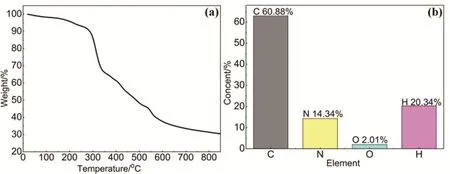
Fig. 2 (a) TGA and (b) element analysis of the polymer HAPP.
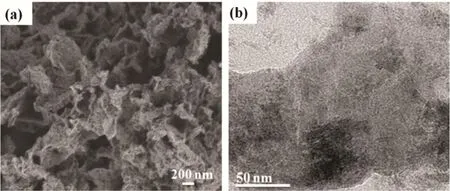
Fig. 3 (a) SEM and (b) HR-TEM image of the polymer HAPP.
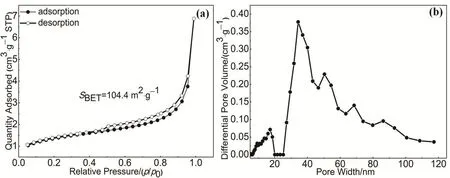
Fig. 4 (a) N2 adsorption-desorption isotherms at 77 K of polymer HAPP; (b) Pore size distribution (PSD) of polymer HAPP.

Table 1 The porosity of the polymer HAPP.
3.2 Effect of pH on the adsorption of MO on HAPP
MO has two chemical structures, whose chromophores are quinone and azo bonds in the structure (pKa= 3.47), depending on the solution pH. Therefore, the solution pH is an important parameter for the adsorption of dyes. As shown in Fig. 5a, at low pH values, low adsorption of MO is observed due to the strong electrostatic repulsion prevents the MO molecules from approaching to the surfaces of the HAPP, resulting in the relatively less adsorption of MO at low pH. With increasing pH values, the electrostatic repulsion force becomes weaker thus leading to an increase of MO adsorption. When pH reached 4,the removal percentage of MO solution is about 97%. As the pH value continues to increase, the removal percentage of MO solution has not changed significantly. This phenomenon is similar to the behavior of the other porous polymers used for MO adsorption33,34. Owing to the nature pH value of MO solution is 5.6, it is very suitable for the pH conditions of the MO adsorption on the polymer HAPP. Therefore, subsequent adsorption experiments of the solution pH is not intended to be deliberately regulated unless otherwise stated.
3.3 Adsorption isotherms of MO on HAPP
To analyze the adsorption behavior of HAPP materials, the adsorption isotherms can well describe the adsorption process at equilibrium. The plot of initial concentration versus adsorption capacity (Fig. 5b) indicated two distinct processes.First, the adsorption was a function of the initial concentration,but this concentration-dependent adsorption was only valid to a certain extent. Next, the adsorption reached the maximum adsorption capacity, becoming a concentration independent process. It was apparent that the adsorption saturation was reached for the range of concentrations examined.
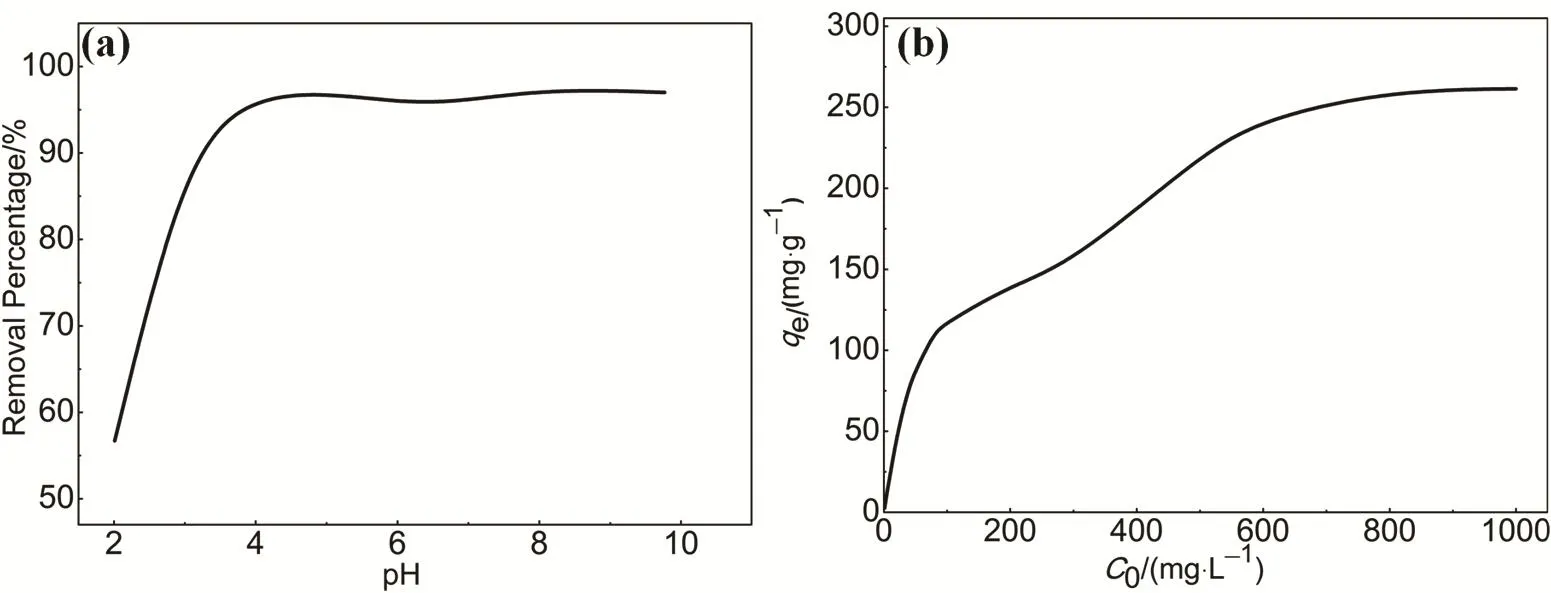
Fig. 5 (a) Effect of pH on the adsorption of the MO by the polymer HAPP; (b) Adsorption isotherm of the MO on the porous HAPP.
The experimental data were fitted with the Langmuir and Freundlich models and the results are shown in Fig. 6. The correlation coefficient R2of the Langmuir model is higher than that of the Freundlich model (Table S1, Supporting Information). This indicates that the experimental data fits better with the Langmuir model, which reflects MO adsorption on HAPP is monolayer adsorption. Furthermore, the maximum adsorption capacity (qmax) evaluated by the Langmuir model is 249.37 mg·g-1. Although the specific surface area of HAPP material is lower than that of our previous work TPP-NH2, the MO adsorption capacity is still higher than that of TPP-NH26.This may be due to the fact that the surface of HAPP have rich N atoms, which improves the MO adsorption capacity owing to its electrostatic interaction between the polymer and the cationic dyes MO.
3.4 Adsorption kinetics of MO on HAPP
Adsorption equilibrium time is one of the important parameters for the design of wastewater treatment system. As shown in Fig. 7a, the time-dependent adsorption of MO on HAPP was investigated at an initial concentration of 10 mg·L-1.The removal percentage increased sharply within 30 min and slowed down thereafter, and, finally, reached equilibrium at 180 min. At the initial stage, there are more active sites on the surface of the HAPP material, so the removal percentage of MO increases rapidly. With the time increased, the active sites available on the surface of the HAPP material are nearly saturated, and the molecules MO needs to diffuse from the adsorbent surface into the pores, thereby causing the adsorption removal percentage to increase slowly and finally to equilibrium. Hence, the adsorption equilibrium time of HAPP to MO is 180 min.
To analyze the adsorption kinetics of HAPP, the adsorption data in Fig. 7a are fitted with pseudo-first-order and pseudo-second-order kinetic models, respectively (Fig. S1,Supporting Information)). An extremely high correlation coefficient (R2> 0.99) is obtained for the pseudo-second-order kinetic model (Table S2, Supporting Information). This suggests that the rate of adsorption of MO on HAPP adsorbent depends on the availability of adsorption sites. In fact, not only the adsorption itself but also the diffusion of MO also plays an important role. According to the intraparticle diffusion model,the dye adsorption capacity (qt) should be linear to the square root time (t1/2). If the curve passes through the origin, the intraparticle diffusion model is the only step to control the rate35.However, in this experiment, as shown in Fig. 7b, the plot of qtversus t1/2is multilinear: the first region describes the external resistance to mass transfer and the second region is attributed to intraparticle diffusion. In the second region, the intraparticle diffusion begins to slow down owing to the decrease of MO concentration in the aqueous phase, as well as the decrease of active sites available for adsorption. The above results show that HAPP adsorption of dyes MO involves more than one process,and intraparticle diffusion is not a rate-controlling step, which is similar to the previous work reported in the literature36-38.
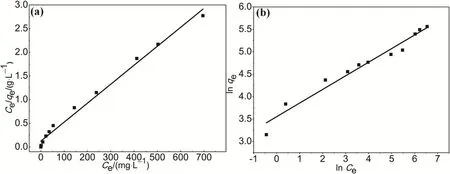
Fig. 6 Adsorption isotherms of MO on HAPP based on (a) Langmuir isotherm model and (b) Freundlich isotherm model.
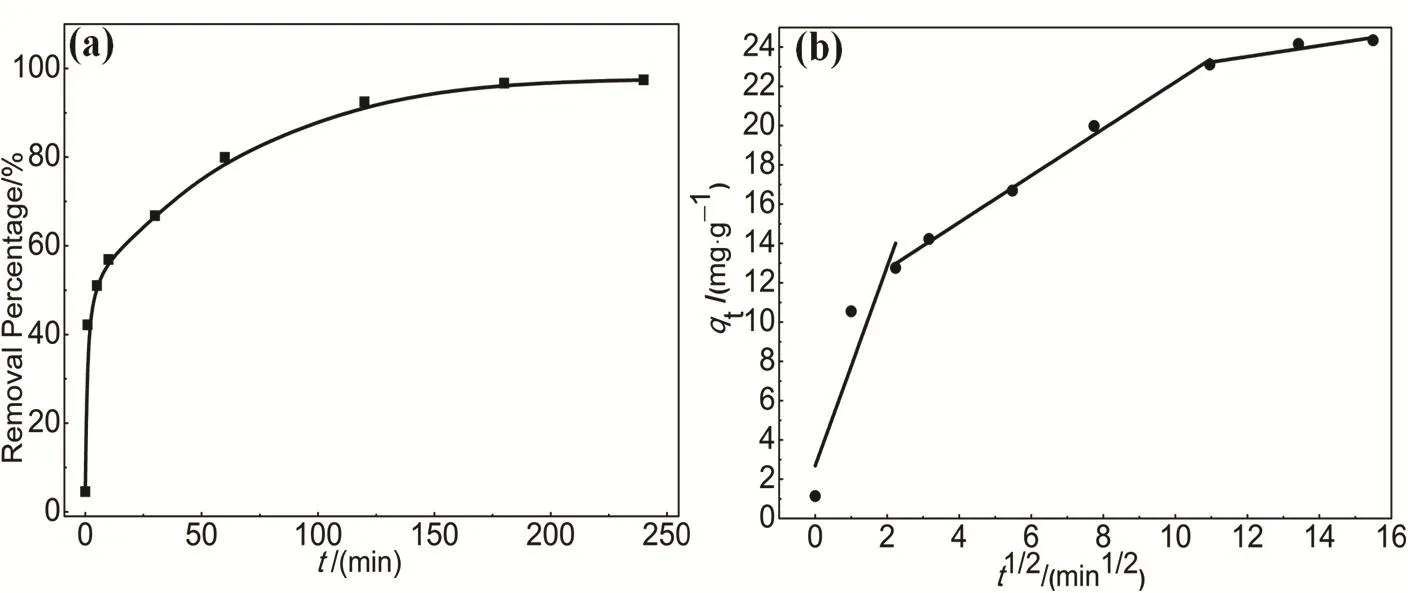
Fig. 7 (a) The effect of adsorption time on MO adsorption by HAPP; (b) Plots of qt versus t1/2 of intraparticle diffusion model for the adsorption MO on HAPP.
3.5 Adsorption mechanism of MO on HAPP
The mechanism of dye adsorption is affected by various factors, such as physical and chemical properties of adsorbent,mass transfer process and so on39,40. The MO molecule is a planar molecule, so it is possible to form a π-π stacking interactions between the aromatic rings of the MO and the aromatics rings of HAPP41. MO has two chemical structures,whose chromophores are quinone and azo bonds in the structure (pKa= 3.47), depending on the solution pH. It is predicted that the azo structure should have a more conjugated π system42than its quinone counterpart. Indeed, the pH studies(Fig. 5) discussed previously suggests that the MO adsorption capacity of HAPP at basic conditions is higher than that at acidic conditions. It can been seen that the π-π stacking interaction may be one of the mechanisms for HAPP material to adsorb MO.
In addition, theoretical quantum calculations are performed to analyze the property and electrostatic potential (ESP) of the monomer of HAPP and MO. The ESP of HAPP and MO are graphically depicted in Fig. S2 (Supporting Information). As seen, the ESP surfaces of dyes MO is red color with a Vs,minof-526.2 kJ·mol-1. Simultaneously, the monomer 2,4-diamino-6-phenyl-1,3,5-triazine is blue color with a Vs,maxof 152.3 kJ·mol-1suggesting that it is favorable for the adsorb MO through electrostatic interaction. Furthermore, the interaction energy of the HAPP-MO complexes has also calculated. As shown in the Scheme 2, one of the possible adsorption models was shown. The computed binding energy of the HAPP-MO complexes is -69.24 kJ·mol-1which indicates that MO can form relatively strong interactions with N heteroatoms of HAPP. Therefore, the electrostatic interaction force may also be one of the mechanisms for HAPP material to adsorb MO.
3.6 Regeneration of HAPP
We also examined the reusability of HAPP materials and in experiments we noticed that the adsorbed-dye porous HAPP material could be substantially completely released in ethanol solution, at the next washed with sodium hydroxide, ethanol,water and dried. As shown in Fig. 8, after 5 adsorption-desorption recycle experiments, the HAPP material still maintains more than 95% removal percentage. The results show that the material has good reusability and can be qualified for efficient removal of dyes from the aqueous solution.
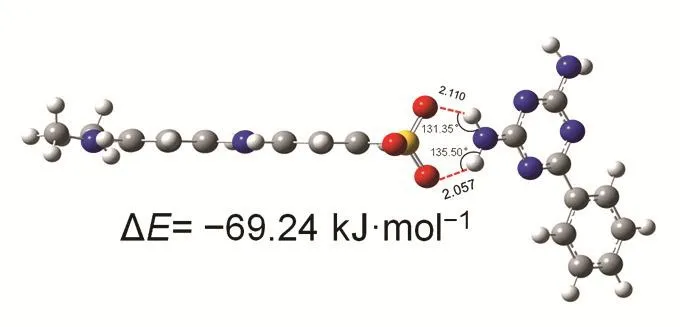
Scheme 2 Optimized geometries and the interactions for the complexes and MO.
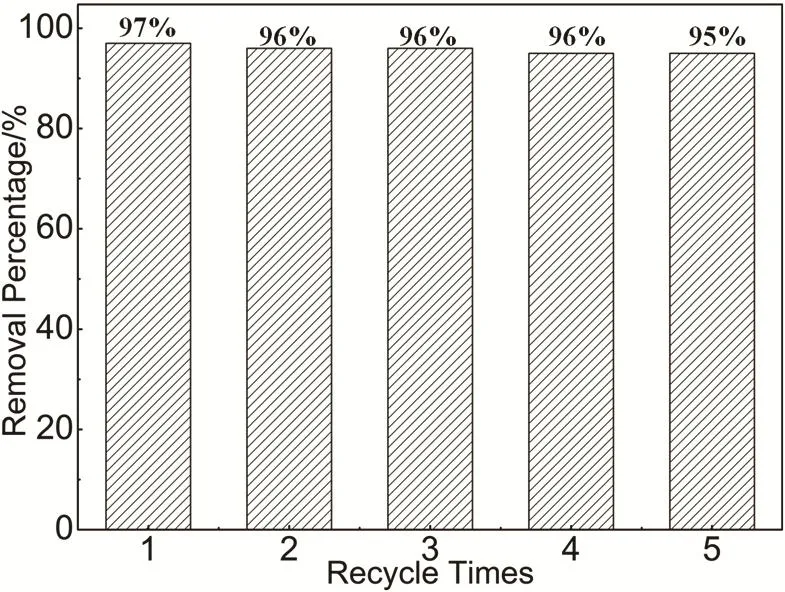
Fig. 8 Reusability of the polymer HAPP of MO.
4 Conclusions
The novel hyper-cross-linked aromatic triazine porous polymer with high surface area, good porosity and high density of functional groups is successfully synthesized by a convenient and efficient Friedel-Crafts reaction. The synthesized HAPP absorbent is successfully used for MO adsorption, reaching a maximum adsorption capacity of 249.3 mg·g-1. The high adsorption capacity is due to the π-π stacking interaction and the strong interactions with N heteroatoms of HAPP on MO. In addition, the HAPP materials can be reused for at least 5 cycles with little loss of the adsorption capacity.Hence, the convention and low cost synthesis, coupled with the excellent MO adsorption properties, make this novel functionalized polymer HAPP an attractive adsorbent for dyes removal.
Supporting Information:available free of charge via the internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Preparation and Sensing Properties of Organic Gel Fluorescence Films Based on ZnS Nanoparticles
- ZnO@ZIF-8 Core-Shell Structure as Host for Highly Selective and Stable Pd/ZnO Catalysts for Hydrogenation of CO2 to Methanol
- Removal of Fluorides from Aqueous Solutions Using Fresh and Regenerated Activated Alumina
- Application of Ag Nanoparticle-Modified Fiber Probe for Plasmonic Catalysis Reaction
- In Situ Study of the Conversion Reaction of CO2 and CO2-H2 Mixtures in Radio Frequency Discharge Plasma
- Effect of Oxygen Partial Pressure on Solid Oxide Electrolysis Cells

