In Situ Study of the Conversion Reaction of CO2 and CO2-H2 Mixtures in Radio Frequency Discharge Plasma
YANG Ruilong , ZHANG Diyu , ZHU Kangwei , ZHOU Huanlin , YE Xiaoqiu , KLEYN Aart W. ,HU Yin , HUANG Qiang ,*
1Science and Technology on Surface Physics and Chemistry Laboratory, Mianyang 621900, Sichuan Province, P. R. China.
2 China Academy of Engineering Physics, Mianyang 621900, Sichuan Province, P. R. China.
3 Center of Interface Dynamics for Sustainability, Institute of Materials, China Academy of Engineering Physics, Chengdu 610200,P. R. China.
Abstract: Currently, worldwide attention is focused on controlling the continually increasing emissions of greenhouse gases, especially carbon dioxide. To this end, a number of investigations have been carried out to convert the carbon dioxide molecules into value-added chemicals. As carbon dioxide is thermodynamically stable, it is necessary to develop an efficient carbon dioxide utilization method for future scaled-up applications. Recently, several approaches, such as electrocatalysis, thermolysis, and non-thermal plasma, have been utilized to achieve carbon dioxide conversion. Among them, nonthermal plasma, which contains chemically active species such as high-energy electrons, ions, atoms, and excited gas molecules, has the potential to achieve high energy efficiency without catalysts near room temperature. Here, we used radio-frequency (RF) discharge plasma, which exhibits the non-thermal feature, to explore the decomposition behavior of carbon dioxide in non-thermal plasma. We studied the ionization and decomposition behaviors of CO2 and CO2-H2 mixtures in plasma at low gas pressure. The non-thermal plasma was realized by our custom-made inductively coupled RF plasma research system. The reaction products were analyzed by on-line quadrupole mass spectrometry (differentially pumped),while the plasma status was monitored using an in situ real-time optical emission spectrometer. Plasma parameters (such as the electron temperature and ion density), which can be tuned by utilizing different discharge conditions, played significant roles in the carbon dioxide dissociation process in non-thermal plasma. In this study, the conversion ratio and energy efficiency of pure carbon dioxide plasma were investigated at different values of power supply and gas flow.Subsequently, the effect of H2 on CO2 decomposition was studied with varying H2 contents. Results showed that the carbon dioxide molecules were rapidly ionized and partially decomposed into CO and oxygen in the RF field. With increasing RF power, the conversion ratio of carbon dioxide increased, while the energy efficiency decreased. A maximum conversion ratio of 77.6% was achieved. It was found that the addition of hydrogen could substantially reduce the time required to attain the equilibrium of the carbon dioxide decomposition reaction. With increasing H2 content, the conversion ratio of CO2 decreased initially and then increased. The ionization state of H2 and the consumption of oxygen owing to CO2 decomposition were the main reasons for the V-shape plot of the CO2 conversion ratio. In summary, this study investigates the influence of power supply, feed gas flow, and added hydrogen gas content, on the carbon dioxide decomposition behavior in non-thermal RF discharge plasma.
Key Words: Radio frequency discharge plasma; Carbon dioxide; Hydrogen; Decomposition ratio; Energy efficiency; Reaction equilibrium
1 Introduction
Carbon dioxide is the major component of the greenhouse gases emitted from the fossil fuel combustions. Climate anomalies caused by the greenhouse effect have had a serious impact on human life. The international agreement on energy saving and emission reduction may be helpful to curb the emission of CO2from the source. Recently, the study of converting CO2into value-added chemicals or fuels has attracted more and more attention. However, as the carbon dioxide is thermodynamically stable, it is very difficult to be converted directly1. A variety of catalytic conversion approaches have been investigated to improve the reaction efficiency or reduce the reaction temperature, such as photo-catalysis2,electrochemical catalysis3, plasma discharge4-6and so on. In fact, the transformation or utilization modes of CO2include:direct decomposition4,5; hydrogenation for preparing formaldehyde, methanol and other small organic molecules7;preparation of CO and H2by reforming reaction with methane8,9,and direct synthesis of organic acids, lipids, and liquid fuels as raw materials10,11. In most cases, the decomposition of CO2into CO is the key process to achieve CO2conversion, as a result of the thermodynamic stability of CO2. Among all the approaches,non-thermal plasma is a promising way for CO2conversion. In non-thermal plasmas, the electron temperature can reach tens of thousands of degrees, while the heavy particles are still near the room temperature. Non-thermal plasma can effectively transfer energy into the reaction system, activate and decompose CO2molecules into CO and O2near room temperature, achieving high decomposition efficiency even without the catalyst. Via this approach, the electrical energy from renewable energy sources can be stored in CO molecules in the form of chemical energy.Meanwhile, the CO2molecules activated in non-thermal plasma can also promote corresponding reactions with other gases at the same time. So, it is necessary to study the CO2decomposition process systematically in non-thermal plasma before future scale-up applications.
Recently, a series of studies about the decomposition of CO2in non-thermal plasma have been carried out. Buser and Sullivan12studied the initial CO2dissociation processes in glow discharge plasma, and the dissociation of CO2was mainly attributed to the attachment dissociation, and collision dissociation of vibrational excited CO2molecules. Xie et al.13investigated the effect of gas residence time on CO2degradation in a corona discharge capacitor and found that there was an optimum reactor size for a particular reactor. Laura et al. studied the CO2decomposition through radio frequency (RF) discharge and obtained a 90%decomposition ratio, while the highest energy transformation efficiency was 3%14. Previously, we have studied the CO2decomposition reaction in both inductively coupled radio frequency discharge and microwave discharge plasma, and energy efficiency of 59.3% and decomposition ratio up to 80.6%was achieved respectively15.
The CO2-H2conversion is also an important research direction for CO2utilization. Zhu et al. studied the activation and conversion of CO2at room temperature through pulsed corona discharge plasma, and they found that the introduction of H2significantly enhanced the CO2decomposition rate, and with the further increase of H2content, the CO2decomposition rate gradually increased16-18. Dobrea achieved a 90% decomposition ratio of CO2in microwave plasma, but the addition of H2led to reduction of CO2decomposition ratio19. Conversion behavior of CO2-H2mixture under radio frequency discharge was studied by Tan and Yang20. Under this experimental configuration, it was found that the higher the content of H2, the higher the decomposition ratio of CO2, but the selectivity of CO decreased.In general, CO2decomposition in plasma can be achieved with a variety of discharge mode, but the current understanding of the decomposition process and corresponding energy utilization is not deep enough. Moreover, there are also some disagreements about the effect of H2on CO2decomposition rate.
In this article, we systematically studied the dissociation of CO2and CO2-H2mixture activated by radio frequency glow discharge, while the reaction processes were in situ monitored by differentially pumped quadrupole mass spectrometry and emission spectroscopy. The conversion ratio of CO2, energy utilization efficiency and the influence of hydrogen on the decomposition of CO2was analyzed, which was helpful for the understanding of the CO2decomposition process in non-thermal plasma.
2 Experiment
The diagram of the experimental device is shown in Fig. 1. The gas flow and composition ratio of raw materials were controlled by gas mass flowmeter. The flowmeter model was d07-7 (Beijing Sevenstar Electronics Co., Ltd., China). The size of the homemade quartz tube was 80 mm × 400 mm and the frequency of RF power source was 27.12 MHz, and the reaction exhaust gas was pumped out by the pump system. The analysis of the reaction state was carried out by spectral and mass spectra.The spectral analysis was carried out by the LSR-UV-NIR fiber optic spectrometer (StellarNet Inc., USA) with spectral range from 200 nm to 1000 nm. The gas component analysis was carried out by HPR20 quadrupole mass spectrometer (Hiden Analytical Ltd., UK). The conversion ratio of CO2is defined by the following formula:

n0CO2is the CO2concentration before the reaction, and nCO2is the CO2concentration after the reaction. The energy efficiency of discharge is defined as the energy proportion effectively utilized for the decomposition of CO215.
The investigation of the decomposition behavior of pure CO2(99.999%) was carried out at different gas flow and different power conditions. Spectral analysis was used to obtain the state information of species in the plasma. The quadrupole mass spectra were used to analyze the gas components after reaction.Gas flows were selected as 30, 60, 90, and 200 mL·min-1, and the RF power supply was selected at 30, 60, 150, and 300 W respectively. While the CO2gas entered into the quartz tube continuously, corresponding reactions happened under the activation of radio-frequency electric field.
The decomposition behavior of CO2and H2(99.999%) mixed gas was studied under confined condition. The pump system was not used during the reaction while vacuum valve was closed, and we mainly concerned about the decomposition rate of CO2. The volume fraction of H2in the mixed gas was 0%, 9%, 25%, 50%,75% and 90%, respectively, and the initial total gas pressure in the tube was 200 Pa. In order to clearly reflect the influence of gas composition on the CO2decomposition, the RF power supply was selected at 100 W which was enough to generate stable plasma. Under these closed conditions, the gas composition in the tube could gradually reach the equilibrium state.
3 Results and discussion
3.1 The discharge of pure CO2
Previous studies have shown that a variety of reactions can occur in CO2plasma, such as decomposition, composite or charge transfer21-26. The most important reactions for CO2decomposition are:

The CO2molecules collide with the accelerated electron,decomposing into CO and producing a free oxygen atom. The oxygen atoms are very active and may react with another oxygen atom to form oxygen molecules or take oxygen from CO2molecules to promote the decomposition of CO2.
The power dependences of CO2conversion ratio with variant gas flow are shown in Fig. 2a. As can be seen from the figure,the conversion ratios of CO2increase gradually with the increasing of power. With the increase of gas flow, the conversion ratios of CO2in corresponding power decreases gradually. This is because the increase of power promoted the further ionization of CO2, while the increase of gas flow requires higher energy to achieve the same proportion of dissociation.With the increase of power, the conversion ratio of CO2gas gradually increases, but the rate of increase has leveled off at low gas flow. However, the conversion ratio of CO2increases linearly with the increase of power in the case of 90 and 200 mL·min-1. Under the condition of 30 mL·min-1and 300 W, the conversion ratio of CO2is 78%.
The power dependences of energy efficiency with variant gas flow are shown in Fig. 2b. It can be seen that the energy efficiencies of CO2decomposition decrease gradually with the increase of power in all cases, but the decrease proportion vary with the flow of gas. In particularly, the energy efficiencies of 90 mL·min-1gas flow are much more stable with an approximate linear alteration.
The conversion ratios and energy conversion efficiencies of CO2ionization decomposition under different flow and power conditions are summarized in Table 1. Under the condition of 30 mL·min-1, the conversion ratio of CO2is the highest, but the energy efficiency is the lowest. And its energy efficiencydecreases by 59% from 30 to 300 W. This can be attributed to the increase of the concentration of CO and O2in the mixture, as well as the temperature, both of which are beneficial to the reactivity of CO and O2to regenerate CO2. With less gas flow,the mixture is more likely to be heated and get warmer. As a result, a part of the energy absorbed by the gas is wasted on the cyclic decomposition and regeneration of CO2. On the other hand, the reaction products themselves could absorb the energy to be ionized, which would do nothing help for the decomposition of CO2, thus the increase of the product proportion could also increase the energy waste in a certain condition. Therefore, the overall energy efficiency decreases with the increase of CO2conversion ratio. In contrast, the CO2conversion ratio of the condition of 200 mL·min-1is low, but the energy efficiency is the highest. The energy efficiency reaches the maximum, 5.78% at 30 W, and decreases to 3.95% by 32%at 300 W. The variance of energy efficiency of CO2decomposition corresponding to 90 mL·min-1discharge conditions is minimum. It only decreases by 12.7% from 3.87%at 30 W to 3.38% at 300 W. Therefore, it is suggested that the gas flow and discharge power need to be adjusted to obtain the best conversion ratio and energy efficiency.
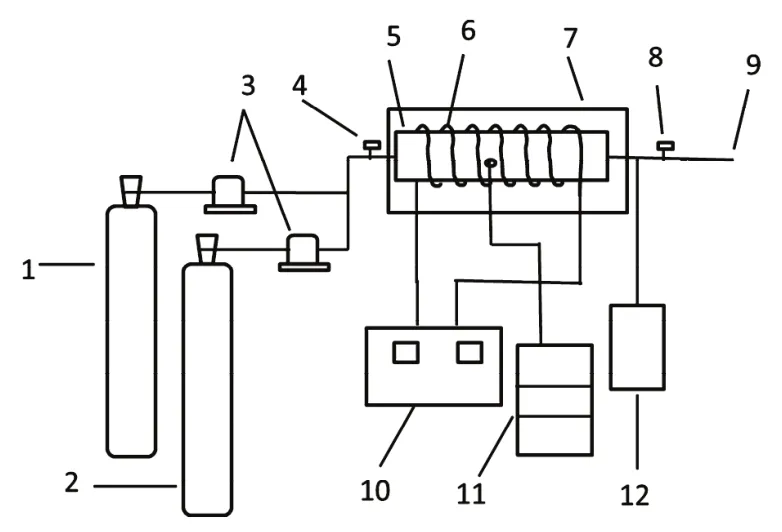
Fig. 1 diagram of RF discharge plasma reactor.
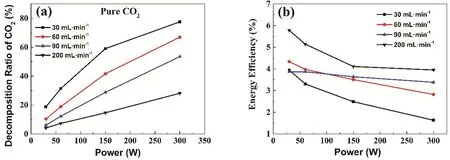
Fig. 2 The power dependences of CO2 conversion ratios (a) and the power dependence of energy efficiencies (b) with variant gas flow.
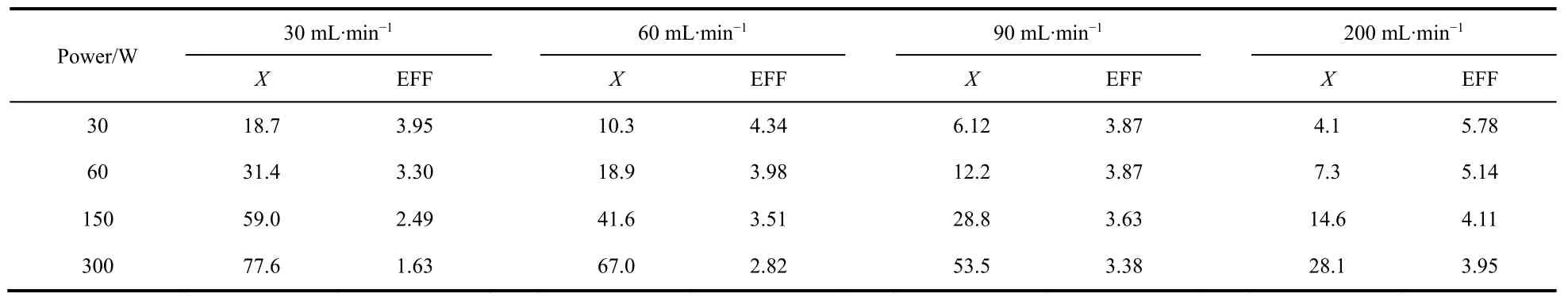
Table 1 CO2 decomposition ratio(X) and energy efficiency (EFF) under different flow/power conditions.
In order to further understand the influences of power on ionization states, the CO2plasma under different power conditions were monitored by optical emission spectra. The results are shown in Fig. 3. It can be seen from the figure that,with the increase of power, the emission peaks of spectra increase gradually, indicating that the ionization ratio of the gas increases, and the new peak position gradually emerges, which can be attributed to the changes in gas composition and the corresponding ionized species as the power increase. When the power is as low as 30 W, the emission spectrum is dominated by the weak glow discharge of the gas. According to Table 1, at this condition the conversion ratio of CO2is only 18.7%, thus the main component of the gas is still CO2and the emission from CO is not visible. In addition, slight emission peaks of oxygen atom at 777 nm (3p5P→3s5S)and 844 nm (3p3P→3s3S)emerge at 60 W, the intensity of which increase with power. The peak intensity at 777 nm is higher than that of 844 nm, which is different from the emission spectra of high frequency microwave conditions reported by Dobrea et al.19. This is due to the different electron energy distributions in these two plasmas,which could affect the optical emission intensively. Furthermore,the conversion ratio of CO2reaches 59% as the RF power increases to 150 W, and new spectra emitting from CO at 266 nm (b3Σ→a3Π) and 451 nm (B1Σ→A1Π) appears and gets much stronger at 300 W27.
The asynchronous luminescence of oxygen atoms and CO molecules indicates that the excitation light of oxygen atoms canbe derived from the free oxygen atoms produced by CO2decomposition at 60 W. At 150 W power, the excitation of CO and O2molecules is enhanced, and the corresponding emission peaks are enhanced significantly. The significant enhancement of O atom emission at 150 W may be due to two reasons. One is the decomposition of CO2under high power conditions, which leads to more free O atoms. And the other reason is the decomposition of O2molecules under high power conditions,resulting in the formation of more free oxygen atoms.

Fig. 3 Emission spectra of pure CO2 plasma under different discharge power with 30 mL·min-1 flow.
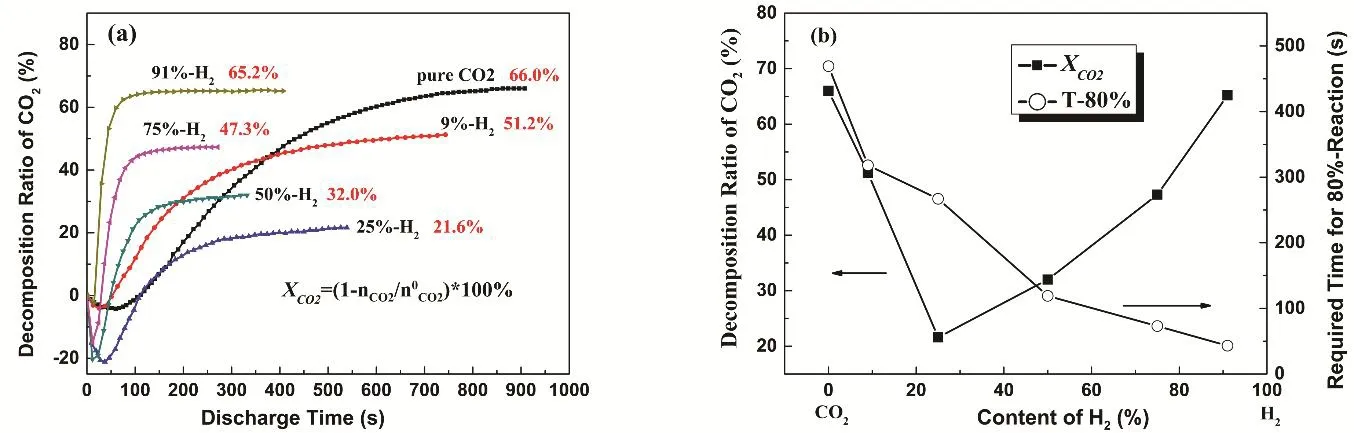
Fig. 4 Reaction behavior of CO2-H2 mixtures in RF plasma: (a) decomposition ratio of CO2 versus discharge time;(b) decomposition ratio of CO2 and equilibrium time versus H2 content.
Also, according to reaction (2), the free O atoms may react with CO2molecules to promote the decomposition, but the reaction constant of direct decomposition (1) is at least three orders higher than that of reaction (2), and the overall promotion of CO2decomposition is negligible. Therefore, under high power conditions the ionization or decomposition of O2and CO further results in the reduction of the overall energy efficiency.
3.2 The discharge of CO2 and H2 mixtures
Catalytic conversion of CO2and H2in to hydrocarbon fuel is an important direction of CO2utilization. Fig. 4a shows the curves of CO2conversion ratio versus discharge time under closed condition. Since the reaction is gradually approaching equilibrium, the reaction rate of each component gas takes the time required for the decomposition ratio increased to 80% of XCO2at equilibrium state (Fig. 4b). It can be seen from the diagram that the addition of H2results in the change of CO2conversion ratio and reaction rate. When a small amount of H2is introduced into CO2gas, the conversion ratio of CO2is decreased. When the content of H2is 25%, the conversion ratio of CO2is only 21.6%. With the further increase of H2content,the conversion ratio of CO2begins to rise. When the H2content is up to 91%, the conversion ratio of CO2is close to that of pure CO2. At the same time, the time needed for reaching equilibrium decreases with the addition of H2, and the higher of hydrogen content, the faster to reach equilibrium. Considering the opposite results from Tan20and Dobrea19, the introduction of H2in our study causes the conversion ratio of CO2firstly decreases and then increases. This may indicate that the addition of H2has two opposite effects on the plasma reaction process.
According to the reaction formula (1), the decomposition of CO2will produce CO and free oxygen atoms. A free oxygen atom may combine with another free oxygen atom to form the O2molecule or react with the CO molecule again to form CO2.Under the same discharge condition, the more the reaction of O and CO occurs, the less effective decomposition of CO2is, so the slower the process is. In the presence of H2molecules, O atoms or O2molecules also react with H2molecules to form H2O.The reaction of H2with oxygen atom or molecule reduces the probability of the reaction of oxygen atom or oxygen molecule with CO and accelerates the reaction to the right direction in formula (1). This makes the decomposition of CO2rapidly reach equilibrium and is beneficial to the increase of CO2decomposition rate.
The glow discharge spectra of the mixer with different H2content at the reaction equilibrium stage are shown in Fig. 5. The main difference in spectrum comes from oxygen atom and hydrogen atom luminescence. It can be seen from the diagram that the emission peaks of O atoms (777 and 844 nm) are obvious in the spectra of pure CO2and mixture with 9% hydrogen discharge, and the decomposition ratios of CO2are high at these conditions. The luminescence of mixture with 9% hydrogen fails to show obvious hydrogen atom emission peak at 656 nm (3d2D→2p2P0). When the content of H2increases to 25%, the weak emission peak of hydrogen atom begins to appear, while the O atom completely disappears and the decomposition ratio of CO2decreases to 21.6% as shown in Fig. 4. We know that the collision cross section of H2is smaller than that of CO2, and the number of electrons is less. It is more difficult for H2to be ionized than CO2under the same applied electric field. At low power supply, the addition of hydrogen dilutes the CO2gas,reducing the collisional excitation of electrons in the mixed gas.Therefore, the decomposition rate of CO2gradually decreases with the addition of a small amount of hydrogen. When the hydrogen content reaches 25%, a weak H atomic emission peak appears, and the ionization decomposition of H2molecule is still weak. With the incorporation of H2and the reduced decomposition of CO2, the content of O2and free oxygen atoms in mixture reduces, which might be account for the disappearance of the emission peaks from O atoms. Referring to Fig. 4, the decomposition of CO2is inhibited by the addition of hydrogen. With further increase of the hydrogen content, the ionization of H2molecules is enhanced, and the hydrogen atom excitation peaks are higher in the spectra. The rapid reaction of hydrogen with oxygen promotes the reaction of formula (1), so the decomposition ratio of CO2begins to increase gradually, and the rate of equilibrium is further accelerated. However, when the external power is relatively high, the composition of the mixed gas will no longer affect the ionization rate of the gas. The oxygen consumption of hydrogen has become the main factor affecting the decomposition rate of CO2. This may explain the different effect of H2on CO2decomposition process.

Fig. 5 Emission spectra of CO2-H2 plasma with different hydrogen content.
4 Conclusions
The decomposition behavior of pure CO2and mixture with H2under RF discharge conditions were investigated, and the effects of power and gas flow on the conversion ratio and energy efficiency were discussed. A low power and broad H2content were chosen to show the effects of H2on the decomposition behaviors of CO2in non-thermal plasma. The results can be summarized as follows.
Under low pressure, the decomposition reaction of CO2can be achieved by RF discharge non-thermal plasma. The decomposition products are CO and O2. The power increase can improve the conversion ratio of CO2, but the composite reaction of CO and O (or O2) is also intensified, so the energy efficiency is reduced.
In RF discharge of CO2and H2mixture, the O atoms and O2products would be consumed by H2, which could suppress the reverse reaction of CO2decomposition and reduce the time required for CO2decomposition process to reach equilibrium.
The conversion ratio of CO2first decreases and then increases gradually with the addition of hydrogen. Under low power conditions, the addition of H2reduces the interaction between CO2molecule and electrons, resulting in the decrease of conversion ratio. However, with the increase of H2content, the oxygen consumption of H2increases, the promoting effect on CO2decomposition increases gradually, and the conversion ratio of CO2gradually rises.
- 物理化学学报的其它文章
- Preparation and Sensing Properties of Organic Gel Fluorescence Films Based on ZnS Nanoparticles
- ZnO@ZIF-8 Core-Shell Structure as Host for Highly Selective and Stable Pd/ZnO Catalysts for Hydrogenation of CO2 to Methanol
- Removal of Fluorides from Aqueous Solutions Using Fresh and Regenerated Activated Alumina
- Application of Ag Nanoparticle-Modified Fiber Probe for Plasmonic Catalysis Reaction
- Removal of Methyl Orange from Aqueous Solutions by a Novel Hyper-Cross-Linked Aromatic Triazine Porous Polymer
- Effect of Oxygen Partial Pressure on Solid Oxide Electrolysis Cells

