Decreased numbers of circulating endothelial progenitor cells are associated with hyperglycemia in patients with traumatic brain injury
Hui-Jie Wei , Li Liu , Fang-Lian Chen Dong Wang Liang Wang , Zeng-Guang Wang Rong-Cai Jiang Jing-Fei Dong , Jie-Li Chen ,Jian-Ning Zhang
1 Department of Neurosurgery, Tianjin Medical University General Hospital; Tianjin Neurological Institute; Key Laboratory of Post-trauma Neuro-repair and Regeneration in Central Nervous System, Ministry of Education; Tianjin Key Laboratory of Injuries, Variations and Regeneration of Nervous System, Tianjin, China
2 Department of Neurosurgery, Peking University International Hospital, Beijing, China
3 Thrombosis Research Section, Department of Medicine, Baylor College of Medicine, Houston, TX, USA
4 Department of Neurology, Henry Ford Health System, Detroit, MI, USA
Abstract Hyperglycemia reduces the number of circulating endothelial progenitor cells, accelerates their senescence and impairs their function.However, the relationship between blood glucose levels and endothelial progenitor cells in peripheral blood of patients with traumatic brain injury is unclear. In this study, 101 traumatic brain injury patients admitted to the Department of Neurosurgery, Tianjin Medical University General Hospital or the Department of Neurosurgery, Tianjin Huanhu Hospital, China, were enrolled from April 2005 to March 2007. The number of circulating endothelial progenitor cells and blood glucose levels were measured at 1, 4, 7, 14 and 21 days after traumatic brain injury by flow cytometry and automatic biochemical analysis, respectively. The number of circulating endothelial progenitor cells and blood sugar levels in 37 healthy control subjects were also examined. Compared with controls, the number of circulating endothelial progenitor cells in traumatic brain injury patients was decreased at 1 day after injury, and then increased at 4 days after injury,and reached a peak at 7 days after injury. Compared with controls, blood glucose levels in traumatic brain injury patients peaked at 1 day and then decreased until 7 days and then remained stable. At 1, 4, and 7 days after injury, the number of circulating endothelial progenitor cells was negatively correlated with blood sugar levels (r = -0.147, P < 0.05). Our results verify that hyperglycemia in patients with traumatic brain injury is associated with decreased numbers of circulating endothelial progenitor cells. This study was approved by the Ethical Committee of Tianjin Medical University General Hospital, China (approval No. 200501) in January 2015.
Key Words: nerve regeneration; endothelial progenitor cells; vascular repair; vascular remodeling; angiogenesis; neovascularization; blood glucose; hyperglycemia; traumatic brain injury; mobilization; suppression; senescence; alternative therapy; brain damage; neural regeneration
Introduction
Endothelial progenitor cells (EPCs) are bone marrow-derived and lineage-specific cells committed to become mature endothelial cells. They are involved in vascular restoration and neovascularization in tissue damaged by traumatic,in flammatory or ischemic injury. The numbers of circulating EPCs are regulated in a variety of disease conditions;including diabetes, cancer, coronary artery disease and trauma (Madonna et al., 2017; Balistreri et al., 2018; Wang et al., 2018b). EPCs are mobilized from the bone marrow and enter the blood circulation to ultimately “home in” to the site of injury. Increased EPC mobilization and homing in contribute to neovascularization and promote new bone formation in fracture healing (Vafaei et al., 2017). Circulating EPC levels predict outcome in patients with atherosclerotic vascular disease and in patients with congestive heart failure(Chesnut et al., 1993; Rodriguez-Carrio et al., 2017; Elia et al., 2018). The increase in the number of circulating EPCs after acute ischemic stroke is also associated with good functional outcome and reduced infarct growth in the ischemic brain (Chesnut et al., 1993; Chen et al., 2009). However, the role of circulating EPCs remains unknown in patients with traumatic brain injury (TBI).
Many factors regulate EPC mobilization and function.Recent studies have found that hyperglycemia downregulates the number of circulating EPCs, accelerates EPC senescence and impairs EPC function (Kim and Kim, 2018; Stormann et al., 2018; Wang et al., 2018c; Yamashita et al., 2018). There is a growing body of experimental and clinical literature showing a significant association between persistent hyperglycemia and poor outcomes in different acute medical and surgical conditions (Yamashita et al., 2018). Blood glucose variability and hyperglycemia were associated with greater risk of both hospital death and prolonged duration of stay after brain injury (van Bommel et al., 2018). Hyperglycemia has been associated with worse outcome following TBI or cardiac surgery in adults, and is related with mortality in patients with severe TBI (Lemmey et al., 2018; Ng and Bax, 2018; Solymar et al., 2018). A previous investigation in our institute showed that numbers of circulating EPCs in 29 patients with TBI decreased and increased with time. Recently, we showed that CD34+hematopoietic stem cells (EPCs are a subpopulation of CD34+cells) accumulate in injured cerebral tissue and are competent to be incorporated into new vasculature in TBI rats (Rodriguez-Carrio et al., 2017; Zhao et al., 2018). However, it is not known whether the regulation of circulating EPCs after TBI is associated with blood glucose levels or functional outcome, and the relationship between blood glucose and circulating EPCs has not been fully investigated.
In this study, we performed a clinical investigation to measure blood glucose and the number of circulating EPCs in 101 TBI patients from 1 to 21 days after TBI. This large sample enabled us to conduct subgroup analyses to determine whether the number of circulating EPCs and blood glucose levels within 21 days after TBI relate to clinical TBI prognosis. This is the first study to demonstrate the relevance of circulating EPCs and blood glucose levels to clinical TBI prognosis.
Participants and Methods
Design
A retrospective observation study.
Participants
TBI patients admitted to Tianjin Medical University General Hospital and Tianjin Huanhu Hospital, China from April 2005 to March 2007 were enrolled. All patients were treated according to the guidelines for the management of severe TBI, 4thEdition, USA, the latest clinical guidelines for management of traumatic brain injury (Pattuwage et al., 2017).The treatment strategy was made by two independent senior physicians. The indications for craniotomy were judged according to imaging and clinical symptoms. Craniotomy included decompression with extensive bone resection and removal of subdural hematoma. After surgery, hemostatic drugs and dehydration drugs were routinely used. During and after surgery, blood transfusion was given according to the clinical situation and blood parameters (hemoglobin less than 70 g/L). Blood transfusion components included erythrocyte suspension and fresh frozen plasma. This study was approved by the Ethical Committee of Tianjin Medical University General Hospital, China (approval No. 200501) in January 2015. All subjects or guardians provided informed written consent before enrollment.
Inclusion criteria
(1) TBI was diagnosed by plain head computed tomography scan. (2) The ages of patients are 18 to 90 years. (3) The time from injury to admission were less than 3 hours.
Exclusion criteria
Patients with complex trauma involving body trunk and limbs, hematological disorders, and cancer were excluded.A total of 101 TBI patients and 37 age- and sex-matched healthy controls were recruited. Patients who received more than 1800 mL of blood transfusion (17 cases) were excluded from the study after a pilot study showed that the amount of blood transfusion remarkably affected circulating EPC numbers. As a result, data from 84 patients were analyzed.
Blood samples
Fasting venous blood (6 mL, taken in the morning) was collected at 1, 4, 7, 14, and 21 days after TBI using ethylenediaminetetraacetic acid (EDTA, 0.5 mM final concentration)as anti-coagulant. Blood samples were also collected from healthy controls to obtain baseline normal reference values.Blood samples were collected according to the University guidelines for collecting and using human samples. They were processed within 2 hours of collection to measure EPC and blood glucose levels in plasma. Blood glucose was measured with an automatic biochemical analyzer (7600;Hitachi, Tokyo, Japan; reagents from Roche Diagnostics,Mannheim, Germany).
EPCs measured by flow cytometry
Whole blood samples (2 mL) were first subjected to Ficoll gradient centrifugation to isolate mononuclear cells as previously described (Liu et al., 2007). The isolated cells were suspended in phosphate-buffered saline (pH 7.2) containing 2 mM EDTA and incubated with an FITC-conjugated anti-CD34 antibody (BD Pharmingen, San Jose, CA, USA)and a PE-conjugated anti-CD133 antibody (Milerenyi Biotech, Gladbach, Germany) for 10 minutes at room temperature. The two antibodies were employed to ensure specific detection of “early” EPCs, which have great proliferation and differentiation capacities. Cells were analyzed by flow cytometry (Beckman Dickinson FACSCalibur system, BD Bioscience, San Jose, CA, USA). Two isotype controls of PE-and FITC-conjugated mouse immunoglobin Gs (IgGs) were used for background non-specific binding. Cells were first run on forward and side scatter to select mononuclear cells,and to reduce signal noises from cell aggregates, platelets and cellular debris. EPCs were then detected as the gated cells that were stained for FITC-CD34 and PE-CD133 and were quantified as the number of cells per 2 × 106mononuclear cells (Figure 1).
Statistical analysis
All analysis was performed using SPSS 16.0 software (SPSS,Chicago, IL, USA) or the SAS statistical package (Version 8.1; SAS Institute, Cary, NC, USA). The data are expressed as the mean ± SEM for the continuous variables and as percentages for categorical variables. Categorical variables were compared using the Pearson's chi-square test. Comparisons between groups for continuous variables were analyzed using Student's t-test or one-way analysis of variance followed by the Student-Newman-Keuls post hoc test or repeated measures analysis of variance. Correlation between two continuous variables was performed using Pearson's correlation coefficients. In this clinical investigation, circulating EPCs and serum glucose levels were defined as independent variables and dependent variables, respectively. We analyzed the overall correlation between circulating EPCs and serum glucose at 1, 4 and 7 days after injury. A value of P < 0.05 was considered statistically significant.
Results
Patient population
Table 1 shows the overall study population including healthy subjects (n = 37) and TBI patients (n = 84). They were matched for age, sex, pre-medical history, smoking habits, alcohol consumption and medication.
In total, 101 patients with TBI were initially recruited. Seventeen patients who received more than 1800 mL of blood transfusion were excluded, because the amount of transfused blood noticeably affects the levels of circulating EPCs(data not shown). Table 2 shows that the circulating EPCs in the patients receiving less than 1800 mL blood transfusion (n = 27) were not statistically different from non-blood transfused patients (n = 57) at all five time points (repeated measure analysis of variance, blood transfused subjects versus non-blood transfused subjects, transfusion: F = 2.012, P= 0.160; time course: F = 7.659, P < 0.05; the transfusion and time course interaction: F = 1.485, P = 0.206). Therefore, 84 TBI patients were enrolled for analysis.
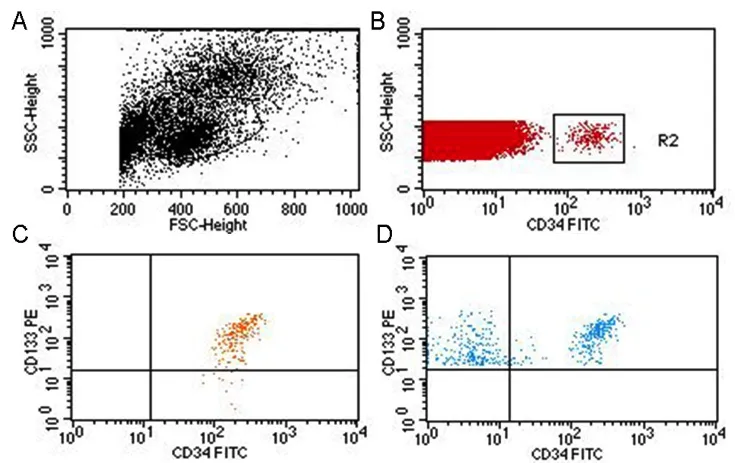
Figure 1 Flow cytometry for detection of CD34- and CD133-positive cells.

Table 1 Clinical characteristics of the study population
General changes in circulating EPC numbers in TBI patients and healthy subjects
To test the changes in circulating EPCs after TBI, EPC numbers in peripheral blood were determined by flow cytometry at 1, 4, 7, 14 and 21 days after TBI. Blood samples were also collected from 37 healthy subjects as controls. The average circulating numbers of EPCs in patients were lower at 1 day after TBI compared with healthy subjects (Figure 2).However, this number rapidly increased thereafter, reaching a peak 7 days post TBI and then gradually declining to the baseline level over the next 14 days (Figure 2). Of 84 TBI patients, 57 underwent surgery to remove hematoma and/or to relieve intracranial hypertension and 27 patients received non-surgical treatments. As shown in Table 2, there was no significant difference between the patients that did or did not undergo craniotomy (57 craniotomy versus 27 non-craniotomy patients, treatment measure: F = 3.124, P = 0.112;time course: F = 8.734, P < 0.01; the treatment measure and time course interaction: F = 1.713, P = 0.186). Therefore, we can exclude the effect of surgery and transfusion on circulating EPC numbers.
Correlation between circulating EPCs numbers and serum glucose levels after TBI
High glucose levels influence EPC homing, migration and function. To test the relationship between blood glucose levels and numbers of circulating EPCs, correlation analysis was performed (Kim et al., 2015). Blood glucose levels and EPC numbers were detected at 1, 4, 7, 14 and 21 days after TBI. As shown in Figure 3A, serum glucose levels were noticeably increased at 1 day after TBI and then steadily decreased over the next 20 days of follow-up. The scatter plot showed that serum glucose level was negatively correlated with the number of circulating EPCs at 1, 4 and 7 days after TBI (Figure 3B; r = -0.147, P = 0.019). To assess if high glucose reduced the number of circulating EPCs, the patients were divided into three subgroups according to the elevation of serum glucose at 1 day after TBI compared with the normal value in healthy subjects: a low glucose group whose increase of glucose was < 0.92 mM; a medium glucose group whose increase of glucose was between 0.92 mM and 4.06 mM; and a higher glucose group whose increase of glucose was > 4.06 mM. These data showed (Figure 4) that the number of circulating EPCs was remarkably decreased in TBI patients with higher glucose compared to TBI patients with lower and medium levels of glucose. These data indicate that blood glucose levels are negatively correlated with circulating EPC numbers. Higher blood glucose correlates with lower numbers of circulating EPCs after TBI.
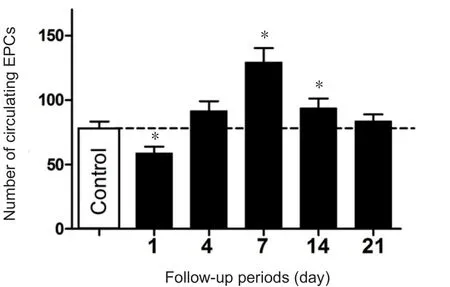
Figure 2 General changes in the number of circulating EPCs after traumatic brain injury.

Figure 3 Correlation between the number of circulating EPCs and serum glucose levels.
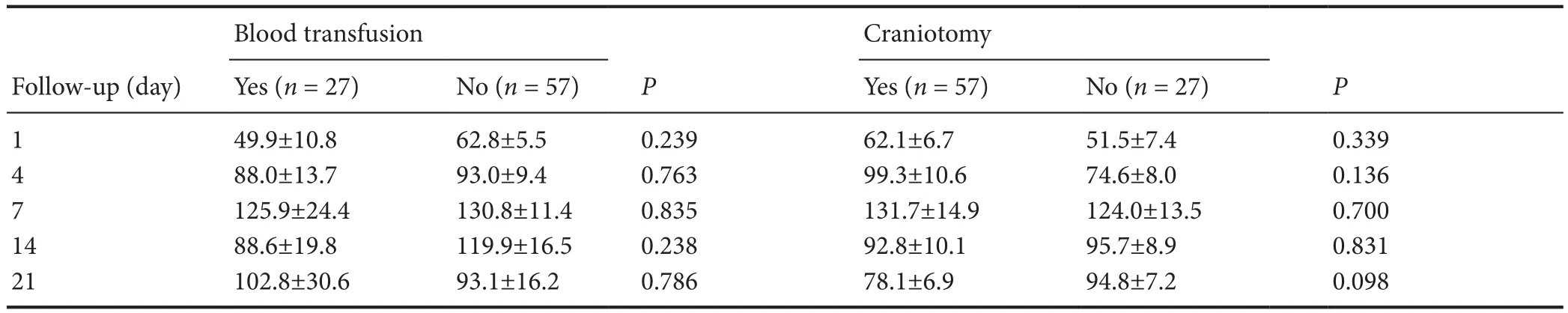
Table 2 Effect of blood transfusion and craniotomy on the numbers (n) of circulating endothelial progenitor cells
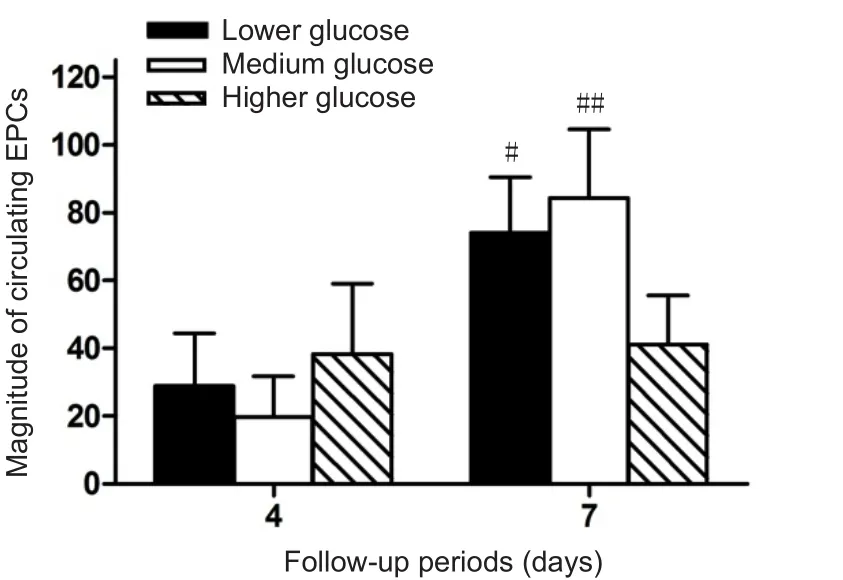
Figure 4 High glucose levels reduce circulating EPCs numbers.
Discussion
This study first investigated the relationship between circulating EPCs and glucose levels after TBI. We found that increased glucose levels were negatively correlated with circulating EPC numbers within 7 days after TBI. EPC numbers were persistently increased in response to decreased blood glucose levels within 7 days after TBI. These data indicate that the decrease in circulating EPC numbers 24 hours after TBI may be caused by high blood glucose levels.
Since initial findings by Asahara et al. (1997), studies have concentrated on characterizing the abundance, phenotype and biochemical properties of circulating EPCs for potential therapeutic and diagnostic purposes. In this clinical investigation, we specifically examined dynamic changes in circulating EPCs in peripheral blood and the clinical significance for TBI. Consistent with our previous study of a smaller cohort of 29 patients (Liu et al., 2007), the number of circulating EPCs was first suppressed to 72.6% of the normal baseline number within the first 24 hours of TBI. This number then increased, reaching 173.1% of the baseline number at approximately 7 days before returning to baseline. These data indicate that circulating EPCs were suppressed after TBI. Initial EPC suppression is also reported in patients with ischemic stroke (Hou et al., 2015; Su et al., 2017) and is likely consumptive in nature as circulating EPCs accumulate at injured vascular beds. The reason for suppression of EPCs in peripheral blood after TBI is not fully understood.Many factors regulate EPC homing, migration and function.Many studies have shown that high glucose levels in fluence EPC homing, migration and function. To test the relationship between blood glucose and circulating EPC levels, we performed correlation analysis (Kim et al., 2015). We found that blood glucose levels were remarkably increased in patients at 24 hours after TBI, which was associated with decreased numbers of circulating EPCs. The dynamic change in circulating EPCs after TBI was negatively correlated with blood glucose levels. Previous studies have found that hyperglycemia induces reactive oxygen species and impairs EPCs (Lee et al., 2018). Hyperglycemia alters the differentiation fate of bone marrow derived EPCs, reducing the potential to generate vascular regenerative cells and favoring the development of proin flammatory cells (Wang et al., 2018a;Zheng et al., 2018). Higher glucose also accelerates the onset of EPC senescence, leading to impairment of proliferative activity, which is associated with the phosphorylation of p38 mitogen-activated protein kinase (Zhong et al., 2019).Therefore, increasing blood glucose levels may play a partial role in regulating circulating EPCs after TBI. Furthermore,compared with controls, EPC numbers were enhanced at 7—14 days after TBI. The blood glucose levels significantly decreased in patients at 7—14 days after TBI accompanied by increased numbers of circulating EPCs. A possible mechanism for this is that the lower blood glucose level attenuates the hyperglycemia-induced reactive oxygen species and the senescence of EPCs (Jacka et al., 2009).
There is a growing body of experimental and clinical evidence showing a significant association between persistent hyperglycemia and poor outcomes in different acute medical and surgical conditions (Solymar et al., 2018). Hyperglycemia is associated with worse outcome following TBI and cardiac surgery in adults (Sriganesh et al., 2012). Our pilot study showed that patients with worse clinical outcome or death had persistently low EPC numbers (data not shown).A key finding of the current study is that circulating EPC numbers are associated with blood glucose levels and clinical outcome of TBI. Therefore, the number of circulating EPCs may predict clinical outcome after TBI. However, the mechanisms of increasing EPCs to promote functional outcome after TBI are not fully understood. Increasing vascular remodeling and angiogenesis by bone marrow stromal cells or recombinant human erythropoietin promotes functional outcome after TBI (Madonna et al., 2017; Balistreri et al.,2018; Wang et al., 2018a). Vascular restoration after TBI-induced brain damage is crucial for reducing secondary ischemic injury after TBI. Circulating EPCs actively participate in tissue repair, largely through vascular repair and angiogenesis to restore blood flow to injured tissue. Circulating EPCs accumulate in the ischemic area to form new vessels and improve regional blood flow (Paynter et al., 2017). The number of circulating EPCs is negatively correlated with the size of ischemic area (Issan et al., 2013) and correlates with the Framingham risk score for ischemic stroke (Oishi et al.,2012). Yip et al. (2008) also showed that patients with severe neurological dysfunction (National Institutes of Health Stroke Scale score ± 12) have a lower number of circulating EPCs 48 hours post ischemic stroke. Increased numbers of circulating EPCs after acute ischemic stroke are associated with good functional outcome and reduced infarct growth.Our previous investigations found that increased numbers of circulating EPCs correlated with CD34 expression and angiogenesis in the brain after TBI (Jacka et al., 2009; Seal et al., 2018). Therefore, EPC regulation of vascular remodeling and angiogenesis might take part in neural repair after TBI.
In conclusion, we have shown a significant association between the numbers of circulating EPCs and blood glucose levels in 84 patients with TBI. The number of circulating EPCs was initially suppressed in the acute phase and then rapidly increased, reaching a peak 7 days after TBI. This pattern of change in circulating EPCs was negatively associated with blood glucose levels. Blood glucose levels were increased at 24 hours and returned back to normal 4 days after TBI. TBI patients with consistently low circulating EPC numbers and higher blood glucose levels were associated with worse clinical outcome or death. The numbers of circulating EPCs and blood glucose levels predicted the outcome after TBI. These results suggest that circulating EPCs may serve as a new clinical prognostic marker after TBI. In addition, increasing EPC mobilization and controlling glucose levels may improve clinical outcome in TBI patients.
A limitation of this study is observational investigation.A prospective research should be done in the future. In addition, the pre-traumatic blood glucose level in each patient was not determined. These factors in fluence the conclusion of this clinical investigation. Animal experiments should be conducted to verify whether hyperglycemia can inhibit the mobilization of EPCs.
Acknowledgments:We acknowledge Professor Shuyuan Yue (General Hospital), Professor Wei Wei (Tianjin Medical University General Hospital), Professor Ying Huang (Tianjin Huanhu Hospital) and all our colleagues in Tianjin Neurological Institution, China for their invaluable assistance and excellent technical support. We also wish to thank all the nurses from Tianjin Medical University General Hospital and Tianjin Huanhu Hospital, China for their kind support in sample collection.
Author contributions:Study design: LL, JFD, and JNZ; manuscript writing: HJW, LL, and ZGW; research and follow-up implementation:HJW, FLC, DW, and LW; data analysis, charts and tables drawing:HJW, LL, JFD, RCJ, and JLC; critical revision of the manuscript for intellectual content: JFD and JLC. All authors approved the final version of the paper.
Conflicts of interest:The authors have no conflicts of interest to declare.
Financial support:This work was supported by the National Natural Science Foundation of China, No. 30772229 (to JNZ), No. 81200907(to HJW); the Natural Science Foundation of Tianjin of China, No.12JCQNJC06800 (to HJW); the Science and Technology Projects in Key Areas of Traditional Chinese Medicine of Tianjin of China, No. 2018001(to ZGW); and the Scientific Research Program Project of Tianjin Education Commission of China, No. 2018ZD03 (to ZGW). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement:This study was approved by the Ethical Committee of Tianjin Medical University General Hospital, China(approval No. 200501) in January 2015. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Declaration of participant consent:The authors certify that they have obtained all appropriate participant consent forms. In the form the participants or patient ‘guardians' have given their consent for participants'images and other clinical information to be reported in the journal. The participants or patient guardians understand that participants' names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement:This study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement.
Biostatistics statement:The statistical methods of this study were reviewed by the biostatistician of Tianjin Medical University General Hospital.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures,and appendices) will be in particular shared. Study protocol form will be available. The data will be available immediately following publication without end date. Anonymized trial data will be available indefinitely at www. figshare.com.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Busting the myth: more good than harm in transgenic cells
- Comparative study of microarray and experimental data on Schwann cells in peripheral nerve degeneration and regeneration: big data analysis
- Lessons from glaucoma: rethinking the fluid-brain barriers in common neurodegenerative disorders
- Characteristics and advantages of adenoassociated virus vector-mediated gene therapy for neurodegenerative diseases
- Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in in flammation, cell death and nociception
- Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke