Lessons from glaucoma: rethinking the fluid-brain barriers in common neurodegenerative disorders
Francisco Javier Carreras
Department of Surgery, Faculty of Medicine, University of Granada, Granada, Spain
Abstract Glaucoma has been recently characterized as a member of the group of anoikis-related diseases. Anoikis, a form of apoptosis, can be triggered by the unfastening of adherent junctions present in astrocytes. In those areas of the central nervous system in which the soma of the neurons or their axons and dendrites are metabolically dependent on the activity of astrocytes, a derangement of the lactate shuttle caused by a separation between the plasma membranes of neurons and astrocytes would result in metabolic impairment of the neurons themselves. In glaucoma, the triggering event has been attributed to the posterior deviation of aqueous humor towards the astrocyte-rich prelaminar tissue of the optic nerve head. The mean calcium content in the aqueous is able to interfere with calcium-dependent adherent junctions and induce anoikis of the astrocytes. As the cerebrospinal fluid has a similar base calcium concentration, a shunt of cerebrospinal fluid through the cerebral parenchyma would be able to interfere in the astrocytic architecture with dire consequences to the metabolically dependent neurons. Here the similitude between glaucoma,amyotrophic lateral sclerosis and Alzheimer's disease are discussed and the concept of the break in the fluid-brain barrier, as an event separated from the blood-brain barrier, is stressed.
Key Words: fluid-brain barriers; blood-brain barrier; cerebrospinal fluid; aqueous humor; calcium ion;glaucoma; amyotrophic lateral sclerosis; Alzheimer's disease
Introduction
In spite of its clinical and social importance, pathological origins of Alzheimer's disease are still elusive. Rational attempts to establish research objectives should be welcomed and subjected to scrutiny. We had suggested that anoikis, a type of apoptosis, could be at the base of glaucoma (Carreras et al., 2009) and later proposed that it could also imply amyotrophic lateral sclerosis (Carreras, 2016). Here we would like to analyze at the light of an extended concept of the fluid-brain barrier, some shared aspects of glaucoma that could be as well present in Alzheimer's disease, that might situate this disease under the radar in the search for anoikis-related processes.
To situate anoikis in context, we must revise the concept of brain barriers. Since the initial experiments by Ehrlich and the final refinements of the concept by Davson, it is widely accepted that the blood and the neural parenchyma are separated by a well-characterized blood-brain barrier. Breaks in the blood-brain barrier have been lately related to a host of common neurological disorders, including amyotrophic lateral sclerosis and Alzheimer's disease (Zlokovic, 2008).
The cerebrospinal fluid, that envelops the brain and cord and fills its inner cavities, is maintained separated from the parenchyma as the ionic composition of both fluids differ in the relative amounts of critical electrolytes. The dissimilarities are slight in chloride concentration, a little higher in magnesium and significantly divergent in potassium and overall, calcium. Calcium ion concentration in cerebral fluid is roughly half that of plasma (Schain, 1964). Interstitial fluid in the neural parenchyma is similar to that of plasma. To avoid ion imbalance both compartments are kept separated by the arrangement of qualitatively different elements. These include: the same osmolality in cerebral fluid and plasma,preventing flows; the pian and arachnoid membranes in the outside; narrow slits between apposed astrocytic membranes; disseminated membrane attachment molecules containing sialic acid; the presence of tight junctions. Occlusive junctions are present in the luminal aspect of ependymal cells of the cerebral ventricles, but only occasionally present in the remaining neural tube (Lippoldt et al., 2000). As a component of the blood-brain barrier (Jiménez et al., 2014),aquaporin might play a similar role in both, blood-brain barrier and parenchyma/cerebral fluid barrier.
In spite of the presence of occasional tight junctions, the impermeability of the ependymal epithelium is far from complete. Tracers and solutes are able to percolate between the ependyma when forced by perfusion, as happens to the inner limiting membrane of Elschnig in the retina and optic nerve when perfused through the vitreous cavity (Carreras et al., 2010a, b). The positive hydrostatic pressure in the parenchyma, depending upon the blood perfusion pressure,would act as a “vis a tergo”, or opposing force, and must be considered as a part of the barrier.
Rupture of the brain fluid/parenchyma barrier is gaining interest on the part of the researchers. Moreover, its main focus has been oriented to the study of hydrocephalus, neural tube defects and ciliary dyskinesia (Jiménez et al., 2014). Elevated intracranial pressure, comparable to high intraocular pressure, would impinge on the central nervous system in a diffuse way, and its consequences would be dispersed rather than local damage. Reactive astrocytes, which have been described in the prelaminar tissue in glaucoma and in the cerebral parenchyma in hydrocephalus, are interpreted in our model as a response to zonular detachment by low calcium concentration and as a precursor to anoikis. Astrocytes are in our model the direct target of calcium imbalance.
Crucial to the functioning of the signaling complex known as adherent junction is calcium ion concentration. Low calcium concentration incapacitates cadherin molecules to form homotypic unions. The detachment of N-cadherin at adhering junctions would send, through β-catenin phosphorylation, a message to the cell nucleus that, depending on its strength, may trigger apoptosis. This is part of a critical protective mechanism against metastasis, known as anoikis, that impedes the survival of cells that lose their tissue attachments (Grossmann, 2002).
Survival of neurons in certain areas of the central nervous system, as the neuropil, is dependent of the lactate shuttle operated through astrocytes (Pellerin, 2003). The invasion of cerebral fluid into areas of parenchyma would cause a local drop in calcium concentration that would signal through adherent junctions into the astrocyte nucleus to trigger apoptosis. Focal interruptions of the cerebral fluid-brain barrier can be perpetuated once it is initiated provoking deep tissue destabilization, including astrocyte detachment and secondary neuronal starvation.
Main aspects to establish a role to anoikis in one disease are: Presence of lactate-dependent neurons; presence of lactate-feeding astrocytes; presence in the astrocytes of cadherin rich adherent junctions; a fluid in the immediate vicinity with relatively low levels of calcium compared to the interstitial neural medium.
Etiopathogeny of Alzheimer's Disease
In spite of sensitive advances in the characterization of the primary lesions, the pathogenesis, and even the pathology, of Alzheimer's disease remains uncertain. Instead of a single well-defined disease, some authors speak of a host of pathological processes (Ashford et al., 2011). A landmark for the disease, common to all admitted forms of Alzheimer's disease, is the accumulation of amyloid beta peptides,which is a component of the neuritic plates. The other essential pathological features are the neurofibrillary tangles dependent of the hyperphosphorilation of tau protein. On the original cause of Alzheimer's disease two main streams of thought give prominence either to the fibrillar deposits within the neurons or to the amyloid deposits outside the cells (Ashford et al., 2011). A new current of research explores vascular participation, including alteration of the blood-brain barrier (Zhao and Gong, 2015). Other pathological changes, although of variable significance, are being identified. We will mention only the loss of cholinergic neurons in the nucleus basalis of Meynert and the hippocampal sclerosis consistent in pyramidal cell loss and gliosis in the hippocampus. Although the definitive diagnosis of Alzheimer's disease rests in histopathologic evidence, due to the mentioned uncertainty, and also to the risks related to cerebral biopsy, the diagnosis of Alzheimer's disease is mainly clinical. It makes the clinic of this disease heavily dependent of the development of technological advances that allow the identification and inform of the distribution of amyloid plaques, as for instance, positron emission tomography .
The scarcity of early histopathologic material is shared with the other two pathological processes co-studied here,glaucoma and amyotrophic lateral sclerosis. Reactive gliosis and activated astrocytes are a common feature to glaucoma(Schneider and Fuchshofer, 2016) and many other neurological diseases, including Alzheimer's disease (Zhao et al.,2011; Pekny et al., 2014). In glaucoma, the neurons (axons)are basically sound until they are “suddenly”, disrupted(Whitmore et al., 2005). It is the clinical accessibility of the optic nerve to direct and indirect observation that may justify a leading role in the awareness about the astrocytic role in anoikis-related diseases. The absence in both glaucoma and amyotrophic lateral sclerosis of a clear genetic origin is also shared by Alzheimer's disease. This fact, along with the astounding prevalence of this group, points towards a structural-based disease. We seem to be dealing with a problem related to the basic, anatomic and physiological structure of a region.
Similitude to Glaucoma
The pathogeny of glaucoma has been summarized elsewhere(Carreras, 2014). Its essence is that the aqueous humor, poor in calcium ion, interferes with the attachments of astrocytes,settled during embryonic development. Activation of the zonular signal system leads eventually to apoptosis of the astrocyte, and the sequel is the metabolic damage to the ganglion cell axon.
The basic idea is that flow of a calcium-depleted brain fluid can permeate a localized portion of the brain parenchyma.This focal area has astrocytes and neurons coupled by the interchange of metabolites due to close membrane apposition.Critical to the maintenance of narrow intercellular spaces are adherent junctions. The adherent junctions act also as a signaling mechanism mediated by β-catenin phosphorylation that may lead, if sufficiently activated, to anoikis, a form of apoptosis. The death of the astrocyte would lead to the death of the neuron, preceded by a variable period of cellular malfunction. The object of this analysis is to point to areas of the central nervous system that would be susceptible to excessive focal flow of any brain fluid.
Another feature shared by this group of diseases is the chronic course of events. The damage, although progressive,is slow and the full fledge disease may take decades to develop. The tissue has mechanisms to oppose the damage and occasionally even to come to a standstill. In glaucoma, there are cases of spontaneous detention of the progression, probably due to a delimitation of the flow of aqueous through areas of preferred flow.
The neuropil, is an extremely complex and intricate tissue composed of axons, dendrites and glial processes. There is ample evidence of the activity of the lactate shuttle in the neuropil and consequently of the dependence of the neuron on the astrocyte (Pellerin, 2003; Pellerin and Magistretti,2012).
In glaucoma, there is a correlation of forces that favors aqueous humor flow almost exclusively through the structures of the anterior segment. When the pressure balance changes the flow is deviated towards the optic nerve which is permeabilised by aqueous humor, chiefly due to the absence of a tight-junction barrier. The colloid osmotic barrier of the vitreous, mainly due to its hyaluronic acid content,is the single obstacle to the posterior flow of aqueous, if the pressure gradient is favorable. A similar situation is present in the ependymal barrier as small quantities of fluid and even macromolecules are allowed free passage (Jiménez et al., 2014). In the retina, a web of spaces formed by perivascular glia has been described that would be able to cope with excess of fluid and solutes in normal conditions (Carreras et al., 2010b).
In the case of Alzheimer's disease, the anatomic features of the hippocampus could be the critical factor. The hippocampus, confidently related to memory processing, bulges along the lateral ventricle and is intimately related to the choroidal plexus. Location of the allocortex between the lateral ventricle and the Cisterna Ambiens allows for a gradient of pressure to favor the cerebral fluid to break through the ependyma and neural tissue and setting off anoikis in the astrocytes. Neuronal degeneration in the neuropil would ensue.
A Note on the Circulation of Cerebrospinal Fluid
It must be recalled here that modern view of the cerebral fluid circulation underscores the rhythmic increase and decrease in pressure in the cerebral ventricles and a multilateral absorption rather than a neat flow of fluid. The immediacy of the choroidal plexus can make local pressure peaks of pressure a risk for the water tightness of the ependymal cells.In short, N-cadherin mediated adherent junctions are an important component of the allo- and iso-cortex both during development as in mature tissue. Proper functioning of N-Cadherin in anchoring and signaling functionality implies the presence of calcium ion modulation for attachment and separation dynamics. Both in other regions of the cortex, but specially in the neuropil, astrocytes, through their hyper abundant laminae, control the lactate supply to the neurons (Pellerin, 2003).
Why Glaucoma Has Disclosed the Field
Awareness that the cerebral fluids are capable of causing ionic stress and interfere with cellular adhesion and neuronal metabolism, and subsistence has been possible due to two main factors: one is the relative simplicity of the histological structure of the optic nerve head compared to the neuropil in the cerebral cortex; the other is the clinical certainty of the disappearance of all the tissue elements in the optic nerve head that manifests as an enlarging optic excavation.
Apparently, the pathology of glaucoma and Alzheimer's disease diverges widely. While glaucoma is characterized by outright destruction, without in flammation, of all structural components of the prelaminar optic nerve, in Alzheimer's disease, cellular death is preceded by long term degeneration of neurons and glia overall characterized by persistent deposits of cumulative materials.
This difference in the level of complexity implies that the response to aggression must depend on the tissue structure at the location of the insult. As only axons surrounded by astrocytes build the prelaminar region of the optic nerve head, an interruption of the metabolic activity in a segment of the axon due to the detachment of the astrocytic membrane is enough to rupture the axon and to cut the communication with the brain. In the neuropil, neuronal somas with myriads of protuberances are in close contact with a similar number of fine protoplasmic prolongations from the astrocytes rich in adherent junctions mediated by N-cadherin. A number of cellular detachments may be enough to imbalance the metabolism of the neuron before the resulting anoikis of the astrocyte and final neuronal death takes place.Sub lethal damage to the neurons of the neuropil is possible through a partial detachment of astrocytes carried away by a local drop in calcium ion levels. The separation of the membrane, widening the intercellular gap, would impinge on the lactate shuttle with an impact on the neuron metabolism. Impairment of the lactate metabolism and its arguable sequels, neurofibril entanglement and amyloid deposition,would merit a thoroughly focused research. Ultimately, the structural simplicity of the prelaminar optic nerve exposes the ingrained susceptibility of the metabolic shuttle between astrocytes and neurons to the ionic stress provoked by the eventual inspissation of the aqueous humor into the nerve substantia. Both the retina and the prelaminar region of the optic disc are part of the brain parenchyma as the myelinated optic nerve, white matter, starts just behind the lamina cribrosa sclerae. The spaces of the lamina are also occupied by astrocytes, not by oligodendrocytes.
Figure 1 summarizes the common features shared by the astrocyte/neuron relationship to be found in separated areas of the central nervous system as the neuropil in the archicortex, the prelaminar tissue in the optic nerve head, and the gray matter of the spinal cord. A drop in calcium concentration will loosen the homotypic binding of N-cadherin with two significant consequences. Firstly, the widening of the intercellular cleft will interfere with the shuttle of lactate and other important metabolic molecules. Secondly, if the signal of the adherent junction's detachment is strong enough,phospho-β-catenin will accumulate at and near the cell nucleus and will trigger anoikis. The detachment of the astrocyte from the tissue leads to the cell auto-destruction. Both processes would impinge on the neuronal prolongations,axons and/or dendrites, depending on the tissue involved,triggering a process of compartmentalized auto-destruction. Consequently, the soma of the neuron would undergo chronic neurodegenerative changes that might eventually lead to apoptosis.
Diseases that for such a long time have been shrouded in mystery regarding their etiopathogeny could be related to a break of the fluid/parenchyma barrier as depicted in Figure 2. The common feature shared by these, until now, unconnected diseases could be their intimate contact with the cerebral fluids and the anodyne fact that both fluids, cerebral fluid and aqueous humor, are poor in calcium ion.
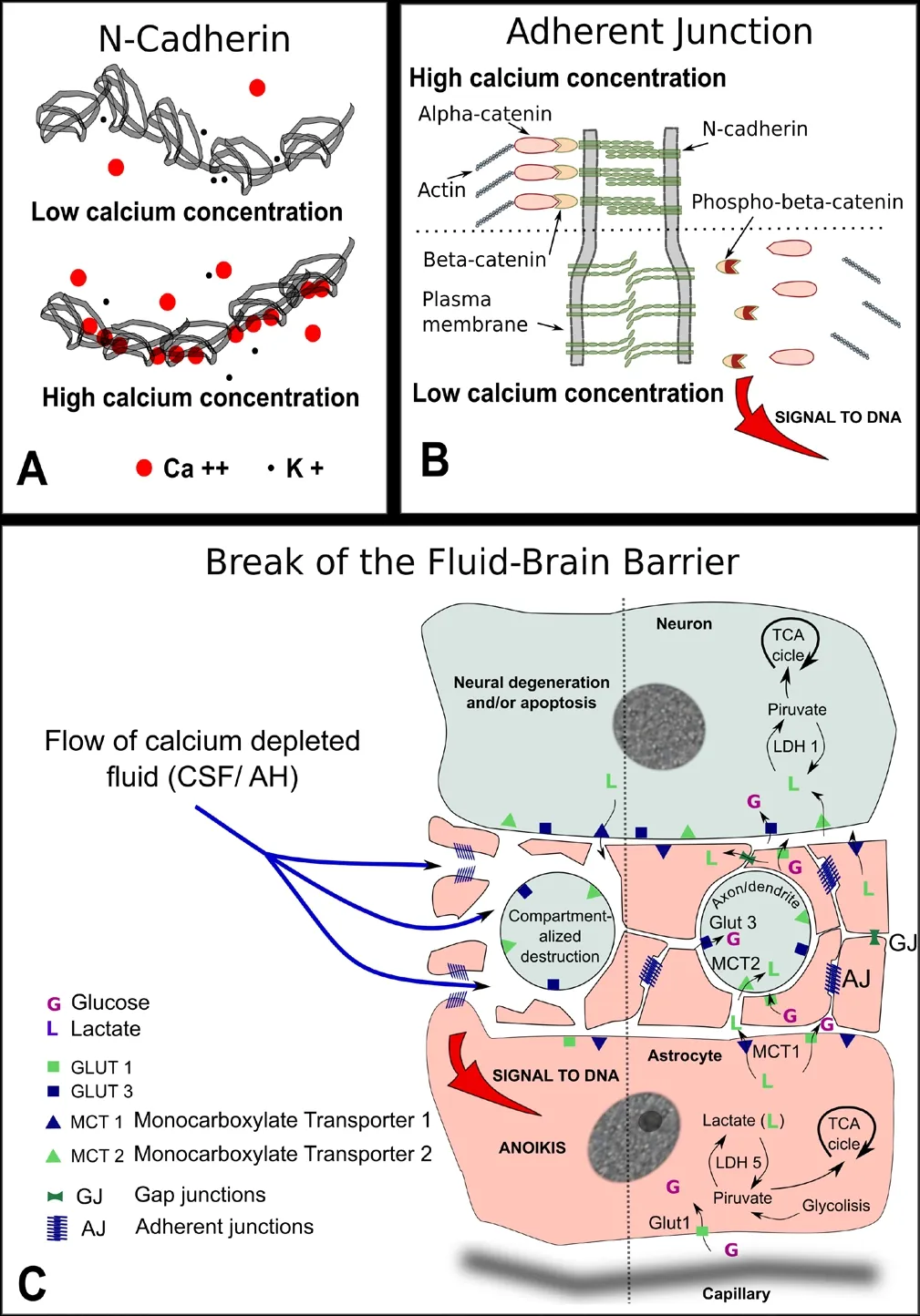
Figure 1 Pathogeny of the break of the fluid-brain barrier (FBB).
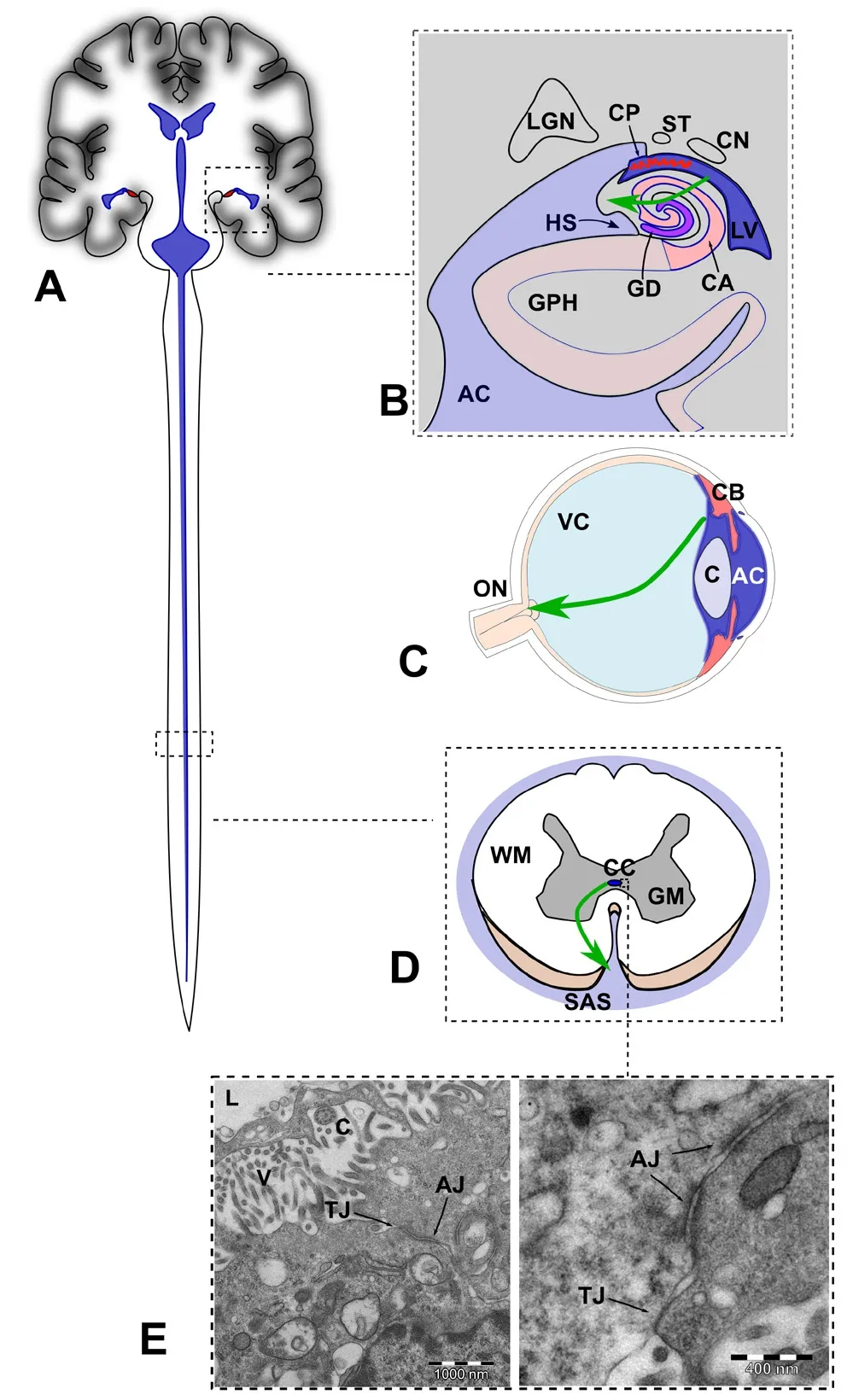
Figure 2 Sites of focal rupture of the fluid-brain barrier (FBB) in the central nervous system (not at scale).
Conclusion
The main lesson from glaucoma might be that the cerebral fluids are not innocuous. They can penetrate the brain parenchyma and carve its way out of it creating a current of ionic stress that disposes of any cell in its course. This fact merits to be underscored in a classification of the brain circulatory barriers that recognizes the potential deleterious effect of a break in any of those barriers. Such a classification is tentatively proposed in Figure 3. A break in the brain/cerebral fluid barrier, a part of the central nerve tissue fluid/parenchyma barrier may hold the key to the understanding of a group of diseases characterized by the in flow of calcium depleted fluids in areas of the central nervous system tissue leading to the unfastening of the adherent junctions, and,eventually, anoikis of astrocytes along with secondary neural damage. The group of potential diseases depending in this pathogenic mechanism first described in glaucoma, might include amyotrophic lateral sclerosis and Alzheimer's disease based on circumstantial but suggestive evidence. The pathology of these diseases is elusive as the first implicated,the astrocyte, disappears by silent apoptotic mechanisms. To this one, a number of adjacent difficulties, including a lack of awareness on the role of fluid-brain barriers, could be added. The issue must be resolved experimentally.

Figure 3 Fluid barriers of the central nervous system.
Acknowledgments:The author would like to thank Carmen Ruiz and Serafín Velez from the Department of Surgery, Faculty of Medicine and Juan Bueno and David Porcel from the Center of Scientific Instrumentation of the University of Granada, Spain.
Author contributions:Conception and design of the present communication, as well as the carrying out the analysis and interpretation of data and the writing of the manuscript in all its stages: FJC.
Conflicts of interest:The author has no actual or potential conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by the author before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Jérôme Braudeau, AgenT, France.
Additional file:Open peer review report 1.
- 中国神经再生研究(英文版)的其它文章
- Muscle secretion of toxic factors,regulated by miR126-5p, facilitates motor neuron degeneration in amyotrophic lateral sclerosis
- Comparative study of microarray and experimental data on Schwann cells in peripheral nerve degeneration and regeneration: big data analysis
- Busting the myth: more good than harm in transgenic cells
- Characteristics and advantages of adenoassociated virus vector-mediated gene therapy for neurodegenerative diseases
- Gene expression changes in dorsal root ganglia following peripheral nerve injury: roles in in flammation, cell death and nociception
- Nicotinamide adenine dinucleotide phosphate oxidase activation and neuronal death after ischemic stroke