Two Cd(II) Coordination Polymers Based on a Flexible Tricarboxylate Ligand: Syntheses, Structures, and Photoluminescence and Catalytic Properties①
ZHU En-Wei SUN Jun-JieJIA Yu QIAO Yu ZHU Yu CHE Gung-Bo
Two Cd(II) Coordination Polymers Based on a Flexible Tricarboxylate Ligand: Syntheses, Structures, and Photoluminescence and Catalytic Properties①
ZHU En-WeiaSUN Jun-JiebJIA YuaQIAO YuaZHU Yub②CHE Guang-Boa②
a(()130103)b(225300)
Studies on the synthesis and design of coordination polymers (CPs) with flexible ligands are of great interest owing to their dynamic structures and promising applications. The title coordination polymers, {[Cd·(HTTTA)·(phen)]·2H2O}n(1) and [Cd·(HTTTA)·(phen)]n(2) (H3TTTA = 2,2΄,2΄΄-[1,3,5-triazine-2,4,6-triyltris(thio)] tris-acetic acid, phen = 1,10-phenanthroline) have been synthesized and characterized by elemental analysis, IR, powder XRD and single-crystal X-ray diffraction. Complex 1consists of one CdIIion, one HTTTA2-ligand and one phen co-ligand. The Cd(II) metal clusters were bridged by the tridentate HTTTA2-ligands into infinite 1D chains, which are further connected into the 3D architecture by abundant hydrogen bonds. In 2, three carboxyl groups of HTTTA2-ligand show different coordination directions because of the C–S–C flexible bond angle, which leads to different 1D chains of 2. The strong-stacking interaction and four C–H···O hydrogen bonds connect the 1D chains into a 3D framework. The solid-state photoluminescence and catalytic properties were studied as well.
crystal structure, H3TTTA, cadmium (II) complex, photoluminescence, catalytic property;
1 INTRODUCTION
Coordination Polymers (CPs) with well-defined supramolecular architectures have been one of the most attractive increasing targets owing to the remarkable potential applications such as gas adsorption and separation, chemical sensing, biome- dical imaging, magnetic and catalytic materials and so on[1-6]. In the construction of CPs, metal ions ‘joints΄ connect organic ‘struts΄ to generate multi- dimensional metal-organic structures through the coordinated bonds and supramolecular interac- tions[7-9]. Compared to the rigid ligands which constructed the predicting frameworks directly and predictably, CPs based on flexible and multi-dentate ligands are quite challenging as they can adopt multiple conformations and self-adaption with rota- tions of ligands in self-assembly[10-13]. Though it is difficult to predict and control the frameworks, flexible CPs can bring more opportunities to explore unpredictable chiral, helical frameworks and high-nuclear networks for understanding and controlling the internal factor on crystallization[14]. H3TTTA (2, 2΄,2΄΄-[1,3,5-triazine-2,4,6-triyltris-(thio)]tris-acetic acid) owns a planar triazine ring with three metal-compounding mercaptodiacetate flexible arms, which leads to abundant frameworks by self-adapted to meet the geometrical require- ments and the different protonations of carboxyl groups. Besides, free sulfur and nitrogen atoms in CPs will act as both electron donors or acceptors of interesting hydrogen bonds and the free functional organic sites (FOSs) which should be an important strategy for metal ions or molecules sorption and post-synthetizations of CPs[15, 16]. Several coordina- tion polymers related to H3TTTA ligand have been reported, displaying versatile coordination modes and architectures along with magnetic, fluorescent and photocatalytic properties[17-20]. Recently, we have also explored CPs for chemical sensor and heterogeneous catalyst with this flexible and func- tional tripodal ligand H3TTTA[21-23].
2 EXPERIMENTAL
The ligand was prepared according to the previous procedure[24]. All other reagents were commercially available and used as purchased. The elemental analyses were carried out with a Perkin-Elmer 240 elemental analyzer. The FT-IR spectra were recor-ded from KBr pellets in the range of 400~4000 cm-1on a Nicolet spectrometer. Powder X-ray diffraction (PXRD) patterns were collected on a Rigaku D/max2500VB3+/PC diffractometer equipped with Cu-radiation (= 1.5406 Å).The fluorescence spectra were performed on a QuantaMaster TM 40 &TimeMaster spectrophotometer with slit width of 2 nm.
2. 1 Synthesis of {[Cd·(HTTTA)·- (phen)]·2H2O}n (1)
A mixture containing H3TTTA (0.0087 g, 0.025 mmol), phen (0.0045 g, 0.0025 mmol), Cd(NO3)2·6H2O (0.0030 g, 0.0125 mmol) and 1 M NaOH (40 μL) in 5 mL water was sealed in a Teflon-lined autoclave and heated to 85oC under autogenous pressure for three days and then allowed to cool to room temperature. After filtration, the colorless prism crystals were washed with water and dried in air. Anal. Calcd. for C21H19CdN5O8S3(M= 678.00): C, 37.20; H, 2.82; N, 10.33%. Found: C, 36.99; H, 2.93; N, 10.07%. IR (cm-1): 3419(w), 3068(w), 2990(w), 2913(w), 1658(m), 1587(s), 1521(m), 1472(s), 1384(s), 1266(s), 1218(s), 1099(w), 937(w), 838(m), 778(w), 729(m), 690(m), 640(w).
2. 2 Synthesis of [Cd·(HTTTA)·(phen)]n (2)
A mixture containing H3TTTA (0.0087 g, 0.025 mmol), phen (0.0045 g, 0.0025 mmol), Cd(NO3)2·6H2O (0.0030 g, 0.0125 mmol) and 1 M NaOH (400 μL) in 5 mL water was sealed in a Teflon-lined autoclave and heated to 85oC under autogenous pressure for three days and then allowed to cool to room temperature. After filtration, the colorless block crystals were washed with water and dried in air. Anal. Calcd. for C21H15CdN5O6S3(M= 641.96): C, 39.29; H, 2.36; N, 10.91%. Found: C, 38.98; H, 2.43; N, 11.07%. IR (cm-1): 3068(w), 2990(w), 2913(w), 1658(m), 1587(s), 1521(m), 1472(s), 1384(s), 1266(s), 1218(s), 1099(w), 937(w), 838(m), 778(w), 729(m), 690(m), 640(w).
2. 3 Structure determination
For X-ray diffraction analyses,single crystals of dimensions 0.28 × 0.25 × 0.20 mm3for 1 and 0.26 × 0.24 × 0.22 mm3for 2 were mounted on a glass rod. The crystal data were collected with a Rigaku Saturn 724+ CCD area detector systemusing gra- phite-monochromated Moα radiation (= 0.071073 nm) at 293 K. The numbers of observed and unique reflections are 10538 and 3819 (int= 0.0202) for 1 and 20183 and 3823 (int= 0.1295) for 2. Absorption corrections were applied using the multi-scan technique[25]. The structure was solved by direct methods of SHELXS-97[26]and refined by full-matrix least-squares methods on2using the SHELXL-97 program package[27]. The hydrogen atoms were generated geometrically and refined isotropically using the riding model. Intensity data were corrected for factors and empirical absorption. Crystal data, as well as details of data collection and refinement, for the complexes are summarized in Table 1, and selected bond lengths and bond angles are listed in Table S1-S2.

Table 1. Crystal Data and Structure Refinement for Complexes 1 and 2
3 RESULTS AND DISCUSSION
3. 1 Structural description
The asymmetric unit of complex 1 consists of one CdIIion, one HTTTA2-ligand and one phen co-ligand (Fig. 1). Each six-coordinated Cd(II) ion is bound by two N atoms from one phen molecule, four oxygen atoms from two HTTTA2-ligands and one H2O molecule, adopting a distorted octahedral geometry. The axial position is occupied by O(2) and O(3) atoms with Cd(1)–O(2) and Cd(1)–O(3) bond lengths of 2.2886(14) and 2.5911(15) Å, while the equatorial plane is formed by two nitrogen atoms (N(1), N(3)) and two oxygen atoms (O(1), O(5) withbond distances Cd(1)–N(1) = 2.3478(16) Å, Cd(1)–N(2) = 2.3264(18) Å, Cd(1)–O(1) = 2.2520(14) Å and Cd(1)–O(5) = 2.2392(14) Å. The bond length of Cd(1)–O(3) is longer than the common Cd–O bond lengths, indicating the weaker interactions between CdIIions and the O atoms from unprotonated carboxyl groups. Two carboxyl groups of H3TTTA are deprotonated and further connect the metal clusters with2-1:1and1coordination modes, respectively. H3TTTA is more flexible because of the adjustable directions (one is coplanar and the other two are verticalplanar of the triazine rings) and angles of the -S–CH2- groups. As shown in Fig. 2, the metal clusters with two CdIIions were bridged by the tridentate HTTTA2–ligands into 1D infinite chains. Strong O–H···O hydrogen bonds (O(4)–H(4)···O(15), O(13)–H(131)···O(2), O(13)–H(132)···O(6), O(15)–H(151)···O(13), O(15)–H(152)···O(5)) existed in the structure between water molecules and coordinated carboxyl groups (Fig. S1). Besides the classical hydrogen bonds, weak hydrogen bonds such as C–H···O (C(1)–H(1)···O(5), C(3)–H(3)···O(13) and C(31)–H(31B)···O(3)) and C–H···S(C(8)–H(8)···S(3)) help to build a 3D architecture (Fig. 3).
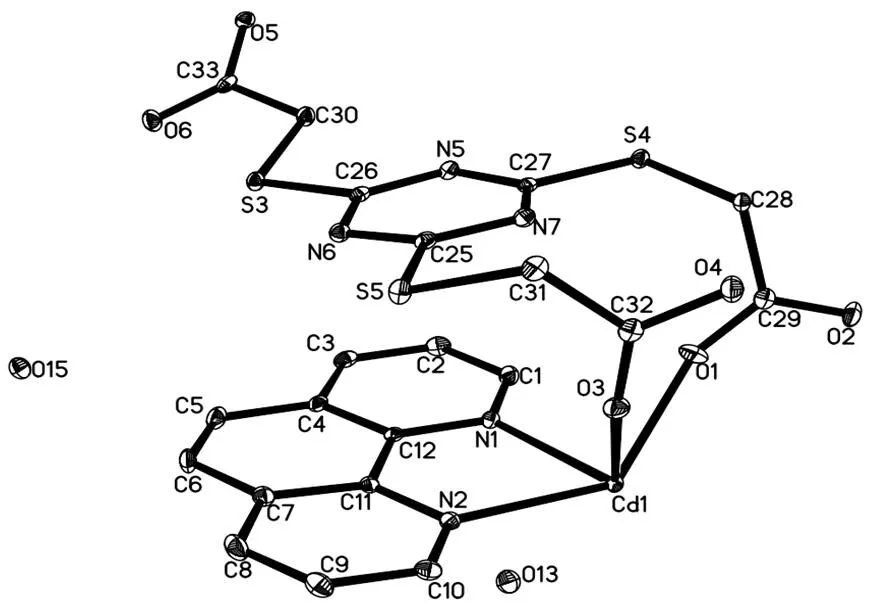
Fig. 1. ORTEP representation of the symmetry expanded local structure of complex 1. Displacement ellipsoids are drawn at 30% probability level
Fig. 2. View of the 1D metal chain connected by chelating the carboxylate group
Fig. 3. View of the 3D supramolecular network showing hydrogen bonds (dashed lines)
Complex 2crystallizes in the orthorhombic space group. Theasymmetric unit of 2consists of one Cd(II) ion, one HTTTA2-and phen ligands (Fig. 4). The six-coordinated Cd(II) center issurrounded by two nitrogen atoms from one phen ligand (Cd–N = 2.303(10) and 2.316(11)Å), and four carboxylic oxygen atoms from three HTTTA2-ligands (Cd–O = 2.188(9)~2.519(9) Å), forming adistorted CdN2O4octahedral geometry. The bond length between Cd(1) and O(1) is 2.754 Å, and we did not assign it as coordination bond which is longer than others in the structure, indicating weak interactions between Cd(1) and O(1). Each HTTTA2–ligand connects to threeCd(II) ions by the three carboxyl groups, showing two coordination modes of2-1:1and2-2:1. Three carboxyl groups of the HTTTA2–ligand which connect the Cd(II) ions into a 1D infinite chain show different spatial orientations, one on the plane of the ligand core and the other two perpendicular with the opposite directions (Fig. 5). Auxiliary phen ligand here not only acts as a coordination donor to finish the seven-coordinated environment, but also contributes to the 2D network by strongstacking interactions between Cg1 rings with a centroid···centroid distance of 3.5811 Å (Cg1i= C(13)~C(16), C(20),C(21); (i= –,, 3/2–)) along with the intermolecular C(15)– H(15)···O(5) hydrogen bond (Fig. 6). Furthermore, the 2D network is then connected into a 3D frame- work by the other three intermolecular C–H···O hydrogen bonds (C(4)–H(4B)···O(6), C(8)– H(8B)···O(2) and C(19)–H(19)···O(3) (Fig. S2).

Fig. 4. ORTEP representation of the symmetry expanded local structure of complex 2. Displacement ellipsoids are drawn at 30% probability level

Fig. 5. View of the 1D metal chain connected by chelating the carboxylate group
Fig. 6. View of the 2D network connected by hydrogen bonds and π-π stacking interactions
3. 2 PXRD pattern
The experimental PXRD patterns of complexes 1 and 2were measured at room temperature (Figs. 7 and 8). The peak positions of the experimental and simulated XRPD patterns are in good agreement, which demonstrates that the complex was obtained successfully as pure crystalline phase.
3. 3 FT-IR spectrum
In the FT-IR spectrum of 1 (Fig. 9), the wide band at 3419 cm-1can be assigned to the O–H group of solvent water molecules. Strong absorption observed at 1587 cm-1corresponds to the vibration of O–H group of coordination water molecules. The asymmetric stretching vibrationsas(COO−) are observed at about 1658 cm−1and symmetric stretching vibrationss(COO−) at 1384 cm−1. The stretching vibration of C–S bond is at 719 cm-1. The peak of C=N bonds in phen molecules is shown at 1472 cm-1and the stretching vibration of C=C bonds is at 1658 cm-1. The shifts of the two peaks in phen molecule indicate the coordination effect between the metal centers and phen molecules. The difference between the two complexes is the wide bands at 3419 cm-1because no H2O solvent mole- cule appears in complex 2.
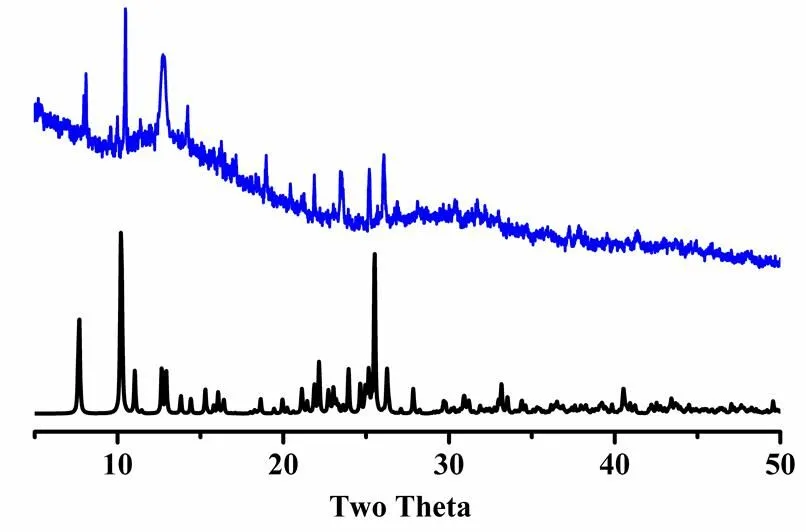
Fig. 7. PXRD patterns of complex 1 (black: simulated; blue: experimental)
Fig. 8. PXRD patterns of complex 2 (black: simulated; blue: experimental)
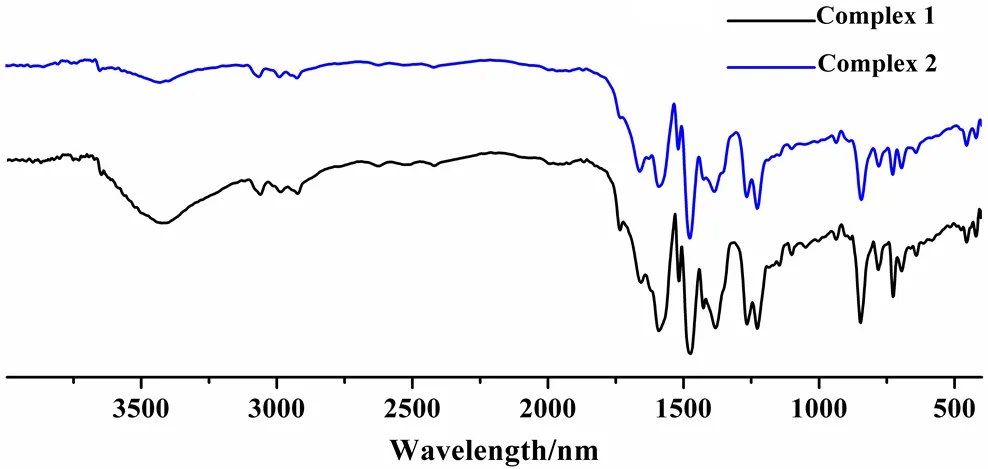
Fig. 9. FT-IR spectra of complexes 1 and 2
3. 5 Catalytic activity
The catalytic activities of complexes 1 and 2 in the cyanosilylation of benzaldehyde and aceto- phenone have been investigated in sonication without solvent (Scheme 1). The results are summa- ri-zed in Table 2 with complex 1, a higher conver- sion of 88.13 % of benzaldehyde is reached in 1 h reaction time while 61.29 % conversion of aceto- phenone. Meanwhile, 2 can only catalyze benzal- dehyde and acetophenone with conversions of 84.15% and 55.66%, respectively. The catalytic yields of acetophenone are less than that of ben- zaldehyde because of the less active substrates. The possible catalytic mechanism which happened in the coordinated metal centers for the cyanosilylation reaction in the case of complexes 1 and 2 has been reasonably shown in Scheme 2.
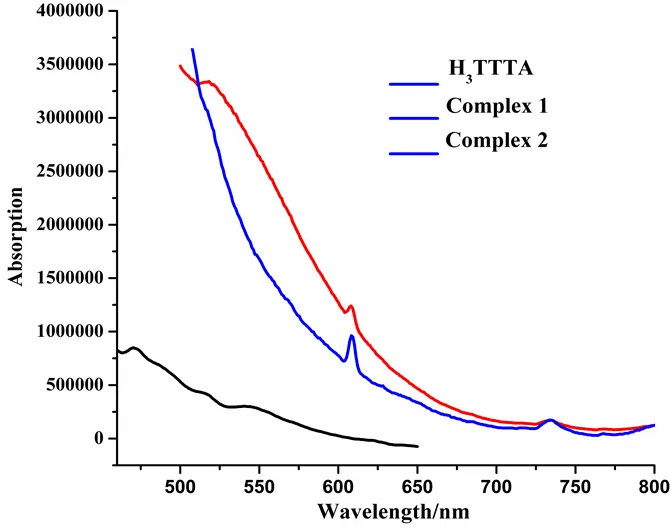
Fig. 10. Emission spectra of H3TTTA and complexes in solid sample
Scheme 1. Cyanosilylation reaction in the presence of complexes 1 and 2

Table 2. Catalytic Activities for Two Complexes in 2 h ( yielda and TOFb)
aYield determined by GC.bTOF = (yield)/(mol% cat)/(t)
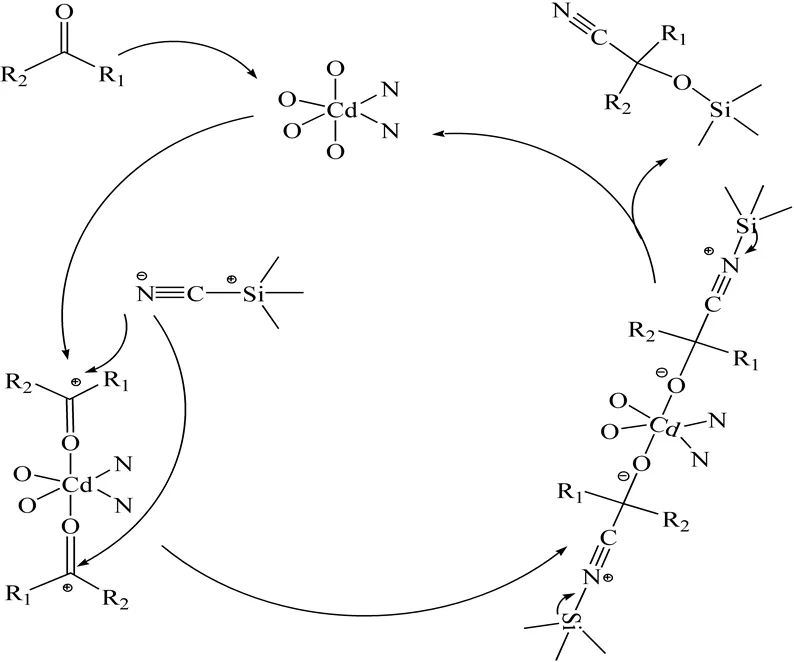
Scheme 2. Possible catalytic mechanism for the cyanosilylation reaction with 1 and 2
4 CONCLUSION
Two complexes based on flexible ligand H3TTTA have been constructed successfully. The different directions of the carboxyl groups in H3TTTA make the 1D chains of 1 and 2 different. Both the 3D frameworks are connected by supramolecular inter- actions such as hydrogen bonds andstacking interactions. Emissions of both complexesare blue shifted and enhanced because of charge transfer between the metal and ligands,10electronic confi- vguration of Cd(II).1 and 2 show medium catalytic activities in the cyanosilylation of benzaldehyde and acetophenone.
(1) Trickett, C. A.; Helal, A.; Al‑Maythalony, B. A.; Yamani, Z. H.; Cordova, K. E.; Yaghi, O. M. This review details the structural and chemical features of state-of-the-art metal-organic frameworks for their application in the carbon cycle of capturing, purifying and transforming CO2into valuable products.2017, 2, 17045.
(2) Guo, Z.; Song, X.; Lei, H.; Wang, H.; Su, S.; Xu, H.; Qian, G.; Zhang, H.; Chen, B. A ketone functionalized luminescent terbium metal-organic framework for sensing of small molecules2015, 51, 376–379.
(3) Zhu, Y.; Wang, Y. M.; Zhao, S. Y.; Liu, P.; Wei, C.; Wu, Y. L.; Xia, C. K.;Xie. J. M. Three N-H functionalized metal-organic frameworks with selective CO2uptake, dye capture, and catalysis.. 2014, 53, 7692–7699.
(4) Xu, M.; Yuan, S.; Chen, X. Y.; Chang, Y. J.; Day, G. S.; Gu, Z. Y.; Zhou, H. C.Two-dimensional metal-organic framework nanosheets as an enzyme Inhibitor: modulation of the-chymotrypsin activity.. 2017, 139, 8312–8319.
(5) Yang, J.; Trickett, C. A.; Alahmadi, S. B.; Alshammari, A.; Yaghi, O. M.Calcium l-lactate frameworks as naturally degradable carriers for pesticides..2017, 139, 8118–8121.
(6) Sawano, T.; Lin, Z.; Boures, D.; An, B.; Wang, C.; Lin, W.Metal-organic frameworks stabilize mono(phosphine)-metal complexes for broad-scope catalytic reactions.2016, 138, 9783–9786.
(7) O'Keeffe, M.; Peskov, M. A.; Ramsden, S. J.; Yaghi, O. M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets.2008, 41, 1782–1789.
(8) Rao, K. P.; Higuchi, M.; Sumida, K.; Furukawa, S.; Duan, J. G.; Kitagawa, S. Design of superhydrophobic porous coordination polymers through the introduction of external surface corrugation by the use of an aromatic hydrocarbon building unit.. 2014, 53, 8225–8230.
(9) Zhang, L.; Zou, C.; Zhao, M.; Jiang, K.; Lin, R. B.; He, Y. B.; Wu, C. D.; Cui, Y. Y.; Chen, B. L.; Qian, G. D. Doubly interpenetrated metal-organic framework for highly selective C2H2/CH4and C2H2/CO2separation at room temperature.. 2016, 16, 7194–7197.
(10) Zhang, S. Q.; Jiang, F. L.; Wu, M. Y.; Ma, J.; Bu, Y.; Hong, M. C.Assembly of discrete one-, two-, and three-dimensional Zn(II) complexes containing semirigid V-shaped tricarboxylate ligands.. 2012, 12, 1452–1463.
(11) Zhu, Y.; Wang, Y. M.; Liu, P.; Xia, C. K.; Wu, Y. L.; Lu, X. Q.; Xie, J. M. Two chelating-amino-functionalized lanthanide metal-organic frameworks for adsorption and catalysis.. 2015, 44, 1955–1961.
(12) Gomez, G. E.; Brusau, E. V.; Kaczmarek, A. M.; Mellot-Draznieks, C.; Sacanell, J.; Rousse, G.; Deun, R. V.; Sanchez, C. Narda, G. E.; Soler Illia, G. J. A. A.Flexible ligand based lanthanide three-dimensional metal-organic frameworks with tunable solid-state photoluminescence and OH-solvent-sensing properties.. 2017, 17, 2321–2331.
(13) Guo, Z. J.; Yu, J. M.; Zhang, Y. Z.; Zhang, J.; Chen, Y.; Wu, Y. F.; Xie, L. H.; Li, J. R. Water-stable In(III)-based metal-organic frameworks with rod-shaped secondary building units: single-crystal to single-crystal transformation and selective sorption of C2H2over CO2and CH4.2017, 56, 2188–2197.
(14) Liu, C. L.; Huang, Q. Y.; Meng, X. R. A one-dimensional zinc(II) coordination polymer with a three-dimensional supramolecular architecture incorporating 1-[(1H-benzimidazol-2-yl)methyl]-1H-tetrazole and adipate.. 2016, C72, 1002–1006.
(15) Zhang, Y. B.; Furukkawa, H.; Zhang, Y. B.; Yaghi, O. M.High methane storage working capacity in metal-organic frameworks with acrylate links.. 2016, 138, 10244–10251.
(16) Zhang, J. W.; Jiang, Y.; Xie, Y. R.; Chu, J.; Liu, B. Q. Syntheses, structures, photoluminescence, and magnetism of a series of discrete heavy lanthanide complexes based on a tricarboxylic acid.2016, 453, 257–262.
(17) Wang, S. N.; Sun, R.; Wang, X. S.; Li, Y. Z.; Pan, Y.; Bai, J. F.; Scheer, M.; You, X. Z. Versatile lanthanide coordination assemblies due to the synergistic effect of lanthanide contraction and flexibility of a flexible tricarboxylate ligand.. 2007, 9, 1051–1061.
(18) Zhu, Y.; Zhu, M.; Liu, P.; Xia, L.; Wu, Y. L.; Xie, J. M. Two Gd(III) coordination polymers based on a flexible tricarboxylate: syntheses, structures, luminescence and catalytic properties.. 2017, 1130, 26–32.
(19) Zhu, Y.; Wang, Y. M.; Xu, J.; Liu, P.; Weththasinha, H. A. B. M. D.; Xia, L.; Wu, Y. L.; Xie, J. M.Syntheses, structures, molecular and cationic recognitions and catalytic properties of two lanthanide coordination polymers based on a flexible tricarboxylate..2014, 219, 259–264.
(20) Chi, C. J.; Peng, Y. Q.; Zeng, S. Y.; Sun, D, Z. Poly[[(1,10-phenanthroline){3-2,2΄,2΄΄-[1,3,5-triazine-2,4,6-triyltris(sulfanediyl)]triacetato}cadmium]0.42-hydrate].2011, E67, m826–m827.
(21) Wang, Y. M.; Zhu, Y.; Xu, J.; Wei, C.; Liu, P.; Wu, Y. L.; Xie, J. M. Structures, photoluminescence and heterogeneous catalysis of five metal complexes constructed by a flexible tricarboxylate ligand.2014, 81, 32–38.
(22) Zhu, Y.; Zhu, M.; Xia, L.; Wu, Y. L.; Hua, H.; Xie, J. M. Lanthanide metal-organic frameworks with six-coordinated Ln(III) ions and free functional organic sites for adsorptions and extensive catalytic activities.. 2016, 6, 29728
(23) Saha, R.; Joarder, B.; Roy, A. S.; Islam, S. M.; Kumar, S. Simultaneous presence of both open metal sites and free functional organic sites in a noncentrosymmetric dynamic metal-organic framework with bimodal catalytic and sensing activities.2013, 19, 16607–16614.
(24) Li, B. Y.; Chrzanowski, M.; Zhang, Y. M.;Ma, S. Q. Applications of metal-organic frameworks featuring multi-functional sites.2016, 307, 106–129.
(25) Higashi, T.. Rigaku Corporation, Tokyo, Japan 1995.
(26) Sheldrick, G. M.. University of Goettingen, Germany 1997.
(27) Sheldrick, G. M.. University of Goettingen, Germany 1997.
(28) Liyanage, P. S.; de Silva, R. M. de Silva, K. M. N. Nonlinear optical (NLO) properties of novel organometallic complexes: high accuracy density functional theory (DFT) calculations.2003, 639, 195–201.
(29) Aliprandi, A.; Genovese, D.; Mauro, M.; Cola, L. D. Recent advances in phosphorescent Pt(II) complexes featuring metallophilic interactions: properties and applications.2015,44, 1152–1169.
22 March 2018;
10 July 2018 (CCDC 1565078 for 1 and 1830616 for 2)
① This project was supported by the National Natural Science Foundation of China (51603086), Natural Science Research in Colleges and Universities in Jiangsu Province (17KJB150037), Science & Technology Development of Jilin Province (20160520131JH), Technology Support Program (Social Development) of Taizhou (TS201628), and Undergraduate Training Program for Innovation and Entrepreneurship (201612917021X)
Zhu Yu, lecturer, majoring in coordination chemistry. E-mail: zhuyu0905@sohu.com Che Guang-Bo, professor, majoring in coordination chemistry. E-mail: guangboche@jlnu.edu.cn
10.14102/j.cnki.0254-5861.2011-2014
- 结构化学的其它文章
- Blue-emissions Modulated by Packing Forces in Alkaline-earth Metal Organic Frameworks Based on Thiophene-2,5-dicarboxylic: Structures and Theoretical Calculations①
- A Selective Luminescent Acetone Sensing Coordination Polymer Constructed from Zinc(II) Ions and Imidazolyl-bearing Ligands①
- Syntheses, Crystal Structures and DNA-binding Properties of Zn(II), Ni(II) and Co(II) Compounds Containing Thiazole Derivatives①
- A New Luminescent Cd(II) Coordination Polymer Constructed with 2-(Carboxymethoxy)benzoic Acid①
- Synthesis, Single-crystal Structure and Fluorescence Property of a New Boron Compound Based on 2-(2΄-Hydroxyphenyl)-1H-benzimidazole①
- Theoretical Investigations on the Structural and Electronic Properties of WO3Polymorphs①

